Stability and Retention of Nanoemulsion Formulations Incorporating Lavender Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Preparation of Conventional Emulsions (CEs) and Nanoemulsions (NEs)
2.2.2. Preparation of Conventional Emulsions with Lavender Essential Oil (CELs) and Nanoemulsions with Lavender Essential Oil (NELs)
2.3. Physicochemical Characterization
2.3.1. Droplet Size Distribution of CE and CEL
2.3.2. Droplet Size distribution of NE and NEL
2.3.3. ζ-Potential Distribution of NE and NEL
2.4. Measurement of LEO Incorporation in CEL and NEL
2.5. Colloidal Stability Assessment
- i.
- Centrifugation: samples were subjected to centrifugation at 3000 rpm for 30 min.
- ii.
- Accelerated aging: samples underwent three consecutive 24 h cycles of storage at 45 °C and 25 °C.
- iii.
- Storage under various conditions: samples were stored at 4 °C, 25 °C, and 45 °C for a period of 60 days.
2.6. Release Kinetics of LEO from CEL and NEL
2.7. Study of the Effect of CEL and NEL on Skin Parameters
2.8. Statistical Analysis
3. Results
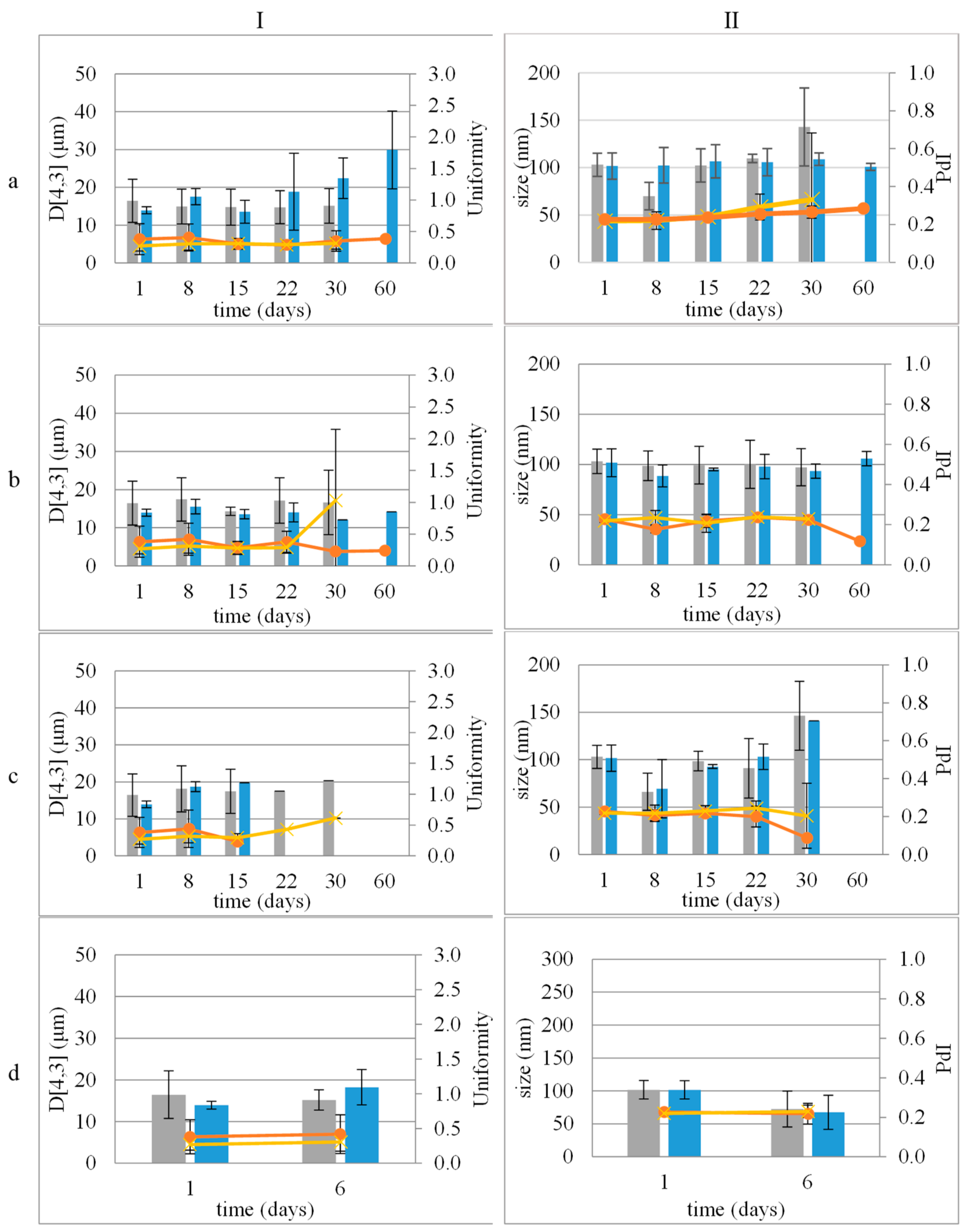
3.1. Droplet Size Distribution of the Dispersed Phase of CE and CEL
3.2. Droplet Size Distribution and ζ-Potential of the Dispersed Phase of NE and NEL
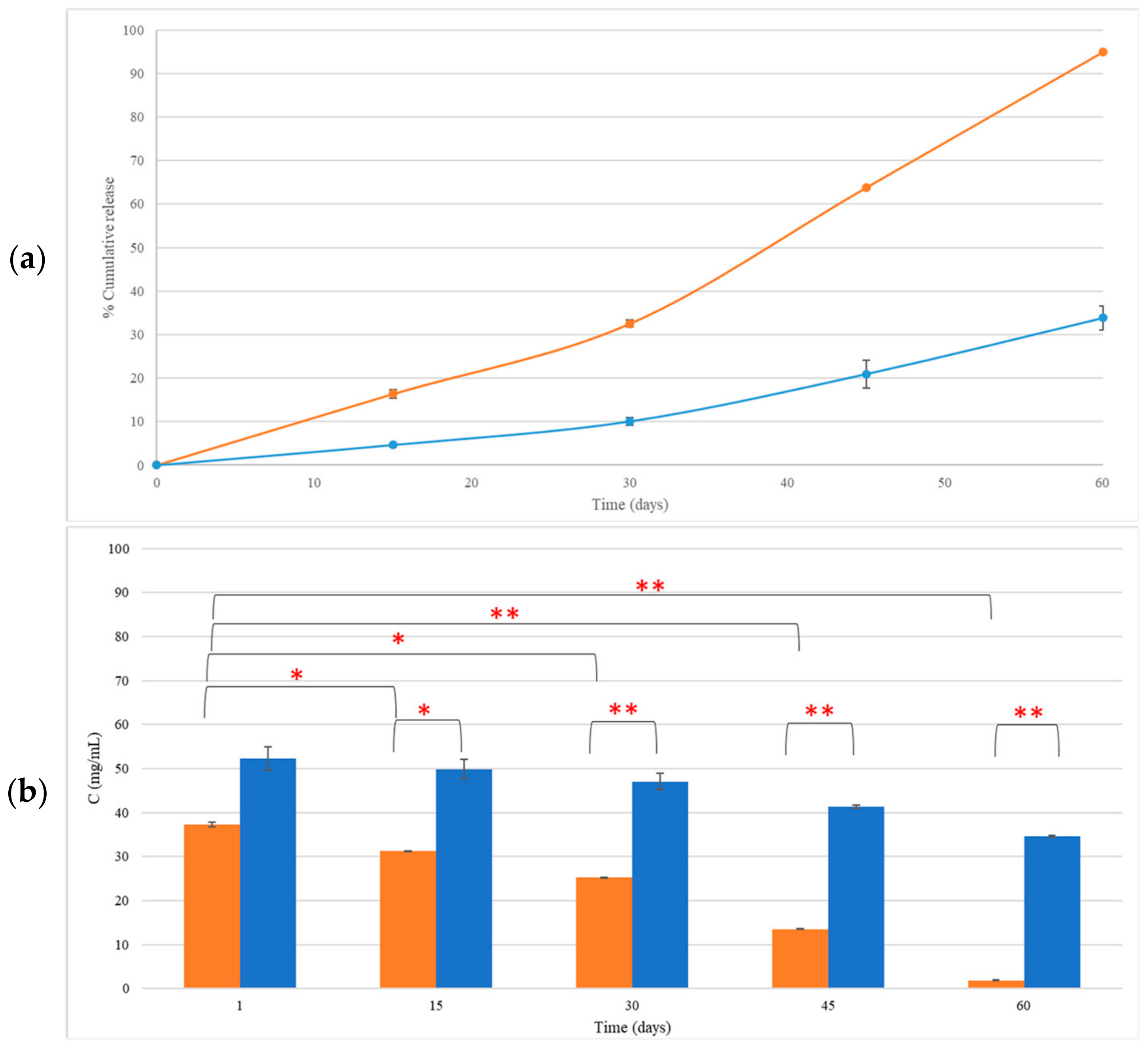
3.3. LEO Content in CEL and NEL
3.4. Stability Study
3.4.1. Colloidal Stability of Nanocarriers
3.4.2. LEO Retention in CEL and NEL
3.5. Release Mechanism of Ingredients of LEO Incorporated in CEL and NEL
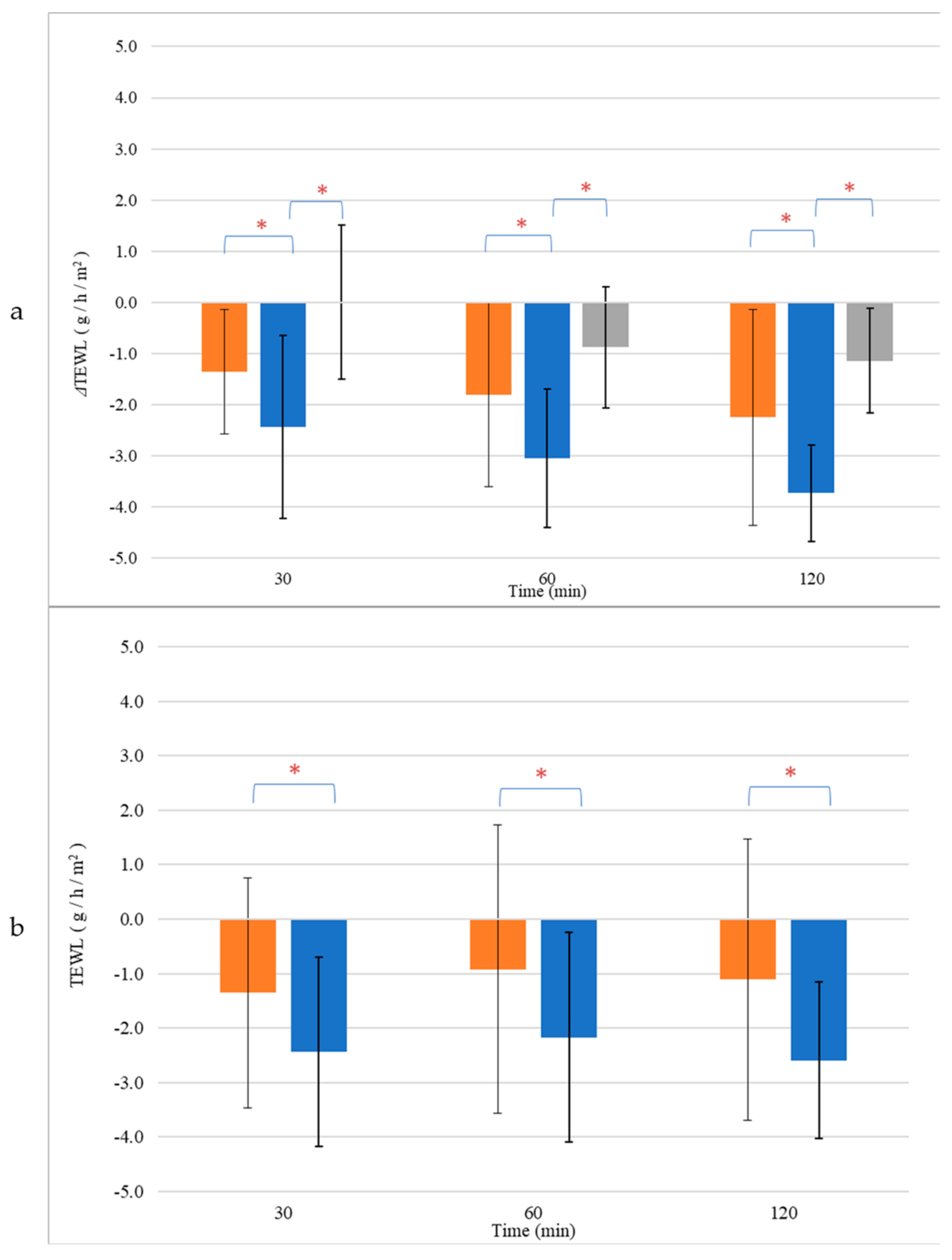
3.6. Skin Hydration and TEWL Alterations
4. Discussion
- i.
- The zero-order model that describes the release of incorporated ingredients as a function of time, at a constant rate that is independent of its concentration [29].
- ii.
- The Korsmeyer–Peppas model that is employed to characterize the release of incorporated ingredients in systems where non-Fickian mechanisms occur [30]. This model proves especially valuable in situations where the release mechanism is not known or when multiple types of drug release phenomena occur. This model is most suitable for analyzing the initial 60% of the release curve [29,31].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M. Chapter 6—Nanoemulsion preparation, characterization, and application in the field of biomedicine. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 169–188. [Google Scholar] [CrossRef]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. Essential Oils as Multicomponent Mixtures and Their Potential for Human Health and WellBeing. Front. Pharmacol. 2022, 13, 956541. [Google Scholar] [CrossRef]
- de Matos, S.P.; Lucca, L.G.; Koester, L.S. Essential oils in nanostructured systems: Challenges in preparation and analytical methods. Talanta 2019, 1, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Hatziantoniou, S.; Kapetanstratakis, I.S.; Drakoulis, N. Cosmetics and Personal Care Products. In Encyclopedia of Toxicology, 4th ed.; Philip, J.W., Ed.; Academic Press: Cambridge, MA, USA, 2024. [Google Scholar] [CrossRef]
- de Groot, A.; Schmidt, E. Essential Oils, Part V: Peppermint Oil, Lavender Oil, and Lemongrass Oil. Dermatitis 2016, 27, 325–332. [Google Scholar] [CrossRef]
- Cruz Sánchez, E.; García, M.T.; Pereira, J.; Oliveira, F.; Craveiro, R.; Paiva, A.; Gracia, I.; García-Vargas, J.M.; Duarte, A.R.C. Alginate–Chitosan Membranes for the Encapsulation of Lavender Essential Oil and Development of Biomedical Applications Related to Wound Healing. Molecules 2023, 28, 3689. [Google Scholar] [CrossRef]
- Batiha, G.E.; Teibo, J.O.; Wasef, L.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; Papadakis, M. A review of the bioactive components and pharmacological properties of Lavandula species. Naunyn Schmiedeb. Arch. Pharmacol. 2023, 396, 877–900. [Google Scholar] [CrossRef]
- Dobros, N.; Zawada, K.D.; Paradowska, K. Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules 2022, 28, 256. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Yusoff, K.; Thomas, W.; Akseer, R.; Alhosani, M.S.; Abushelaibi, A.; Lim, S.H.; Lai, K.S. Lavender essential oil induces oxidative stress which modifies the bacterial membrane permeability of carbapenemase producing Klebsiella pneumoniae. Sci. Rep. 2020, 10, 819. [Google Scholar] [CrossRef]
- Sayout, A.; Ouarhach, A.; Rabie, R.; Dilagui, I.; Soraa, N.; Romane, A. Evaluation of Antibacterial Activity of Lavandulapedunculata subsp. atlantica (Braun-Blanq.) Romo Essential Oil and Selected Terpenoids against Resistant Bacteria Strains-Structure-Activity Relationships. Chem. Biodivers. 2020, 17, e1900496. [Google Scholar] [CrossRef]
- D’Auria, F.D.; Tecca, M.; Strippoli, V.; Salvatore, G.; Battinelli, L.; Mazzanti, G. Antifungal activity of Lavandula angustifolia essential oil against Candida albicans yeast and mycelial form. Med. Mycol. 2005, 43, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Minooeianhaghighi, M.H.; Sepehrian, L.; Shokri, H. Antifungal effects of Lavandula binaludensis and Cuminum cyminum essential oils against Candida albicans strains isolated from patients with recurrent vulvovaginal candidiasis. J. Mycol. Med. 2017, 27, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Białoń, M.; Krzyśko-Łupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P.P. Chemical Composition of Two Different Lavender Essential Oils and Their Effect on Facial Skin Microbiota. Molecules 2019, 24, 3270. [Google Scholar] [CrossRef] [PubMed]
- Ravi, T.P.U.; Padma, T. Nanoemulsions for drug delivery through different routes. Res. Biotechnol. 2011, 2, 1–13. [Google Scholar]
- Ashaolu, T.J. Nanoemulsions for health, food, and cosmetics: A review. Environ. Chem. Lett. 2021, 19, 3381–3395. [Google Scholar] [CrossRef] [PubMed]
- Solè, I.; Solans, C. Nanoemulsions. In Encyclopedia of Colloid and Interface Science; Tadros, T., Ed.; Springer: Berlin, Germany, 2013; pp. 733–747. [Google Scholar] [CrossRef]
- Rabelo, C.A.S.; Taarji, N.; Khalid, N.; Kobayashi, I.; Nakajima, M.; Neves, M.A. Formulation and characterization of water-in-oil nanoemulsions loaded with açaí berry anthocyanins: Insights of degradation kinetics and stability evaluation of anthocyanins and nanoemulsions. Food Res. Int. 2018, 106, 542–548. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Miastkowska, M.; Sikora, E.; Kulawik-Pióro, A.; Kantyka, T.; Bielecka, E.; Kałucka, U.; Kamińska, M.; Szulc, J.; Piasecka-Zelga, J.; Zelga, P.; et al. Bioactive Lavandula angustifolia essential oil-loaded nanoemulsion dressing for burn wound healing. In vitro and in vivo studies. Biomater. Adv. 2023, 148, 213362. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Mohammadifar, M.; Aghadavoud, E.; Vakili, Z.; Aarabi, M.H.; Talaei, S.A. Deep skin wound healing potential of lavender essential oil and licorice extract in a nanoemulsion form: Biochemical, histopathological and gene expression evidences. J. Tissue Viability 2020, 29, 116–124. [Google Scholar] [CrossRef]
- Liakopoulou, A.; Mourelatou, E.; Hatziantoniou, S. Exploitation of traditional healing properties, using the nanotechnology’s advantages: The case of curcumin. Toxicol. Rep. 2021, 28, 1143–1155. [Google Scholar] [CrossRef]
- Wolf, M.; Klang, V.; Stojcic, T.; Fuchs, C.; Wolzt, M.; Valenta, C. NLC versus nanoemulsions: Effect on physiological skin parameters during regular in vivo application and impact on drug penetration. Int. J. Pharm. 2018, 549, 343–351. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Miladinović, D.L.; Dimitrijević, M.V.; Mihajilov-Krstev, T.M.; Marković, M.S.; Ćirić, V.M. The significance of minor components on the antibacterial activity of essential oil via chemometrics. LWT 2021, 136, 110305. [Google Scholar] [CrossRef]
- Bera, B.; Khazal, R.; Schroën, K. Coalescence dynamics in oil-in-water emulsions at elevated temperatures. Sci. Rep. 2021, 11, 10990. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Hydrodynamic diameter and zeta potential of nanostructured lipid carriers: Emphasizing some parameters for correct measurements. Colloids Surf. A Physicochem. Eng. 2021, 620, 126610. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, MA, USA, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelke, R.E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. Available online: https://europepmc.org/article/MED/4011621 (accessed on 3 October 2023).
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release I. Fickian and Non-Fickian Release from NonSwellable Devices in the Form of Slabs, Spheres, Cylinders or Discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Vaz, S.; Silva, R.; Amaral, M.H.; Martins, E.; Sousa Lobo, J.M.; Silva, A.C. Evaluation of the biocompatibility and skin hydration potential of vitamin E-loaded lipid nanosystems formulations: In vitro and human in vivo studies. Colloids Surf. B Biointerfaces 2019, 1, 242–249. [Google Scholar] [CrossRef]
- Infante, V.H.P.; Maia Campos, P.M.B.G.; Gaspar, L.R.; Darvin, M.E.; Schleusener, J.; Rangel, K.C.; Meinke, M.C.; Lademann, J. Safety and efficacy of combined essential oils for the skin barrier properties: In vitro, ex vivo and clinical studies. Int. J. Cosmet. Sci. 2022, 44, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A. Status of terpenes as skin penetration enhancers. Drug Discov. Today 2007, 12, 1061–1067. [Google Scholar] [CrossRef] [PubMed]


| A | CEL | |||||
|---|---|---|---|---|---|---|
| Time (days) | 1 | 15 | 30 | 45 | 60 | |
| Components | ||||||
| eucalyptol | 0.440 ± 0.044 | 0.403 ± 0.025 | 0.342 ± 0.010 | 0.220 ± 0.009 | 0.097 ± 0.001 | |
| cis-linalool oxide (furanoid) | 0.063 ± 0.001 | 0.060 ± 0.001 | 0.055 ± 0.007 | 0.033 ± 0.006 | 0.011 ± 0.000 | |
| linalool | 24.237 ± 0.976 | 20.610 ± 0.372 | 17.198 ± 0.069 | 9.185 ± 0.035 | 1.276 ± 0.042 | |
| camphor | 2.893 ± 0.110 | 2.587 ± 0.064 | 2.159 ± 0.257 | 1.171 ± 0.142 | 0.144 ± 0.005 | |
| isoborneol | 0.142 ± 0.008 | 0.130 ± 0.001 | 0.107 ± 0.001 | 0.062 ± 0.001 | 0.020 ± 0.000 | |
| borneol | 0.342 ± 0.013 | 0.262 ± 0.006 | 0.219 ± 0.028 | 0.143 ± 0.016 | 0.060 ± 0.001 | |
| terpinen-4-ol | 0.206 ± 0.004 | 0.193 ± 0.012 | 0.165 ± 0.006 | 0.107 ± 0.019 | 0.030 ± 0.001 | |
| α-terpineol | 0.186 ± 0.012 | 0.164 ± 0.009 | 0.138 ± 0.004 | 0.082 ± 0.001 | 0.027 ± 0.001 | |
| linalool acetate | 8.760 ± 0.429 | 6.756 ± 0.378 | 4.770 ± 0.218 | 2.485 ± 0.035 | 0.217 ± 0.004 | |
| lavandulyl acetate | 0.042 ± 0.001 | 0.033 ± 0.005 | 0.029 ± 0.001 | 0.017 ± 0.001 | 0.006 ± 0.000 | |
| caryophyllene-oxide | 0.042 ± 0.002 | 0.039 ± 0.005 | 0.028 ± 0.003 | 0.015 ± 0.001 | 0.005 ± 0.000 | |
| Total | 37.355 ± 0.517 | 31.235 ± 0.075 | 25.209 ± 0.048 | 13.517 ± 0.107 | 1.893 ± 0.055 | |
| B | NEL | |||||
| Time (days) | 1 | 15 | 30 | 45 | 60 | |
| Components | ||||||
| eucalyptol | 1.605 ± 0.248 | 1.385 ± 0.219 | 0.984 ± 0.255 | 0.730 ± 0.069 | 0.481 ± 0.129 | |
| cis-linalool oxide (furanoid) | 0.096 ± 0.001 | 0.094 ± 0.002 | 0.092 ± 0.001 | 0.092 ± 0.003 | 0.092 ± 0.003 | |
| linalool | 31.530 ± 2.995 | 30.854 ± 2.384 | 30.053 ± 2.995 | 27.222 ± 0.322 | 23.371 ± 0.346 | |
| camphor | 4.711 ± 0.062 | 4.700 ± 0.057 | 4.602 ± 0.062 | 4.154 ± 0.037 | 3.706 ± 0.038 | |
| isoborneol | 0.182 ± 0.010 | 0.176 ± 0.001 | 0.181 ± 0.010 | 0.162 ± 0.004 | 0.145 ± 0.003 | |
| borneol | 1.316 ± 0.047 | 1.149 ± 0.021 | 1.001 ± 0.027 | 0.715 ± 0.028 | 0.408 ± 0.001 | |
| terpinen-4-ol | 0.271 ± 0.028 | 0.270 ± 0.029 | 0.269 ± 0.028 | 0.254 ± 0.019 | 0.239 ± 0.007 | |
| α-terpineol | 0.241 ± 0.019 | 0.237 ± 0.021 | 0.225 ± 0.019 | 0.223 ± 0.019 | 0.221 ± 0.003 | |
| linalyl acetate | 12.244 ± 0.584 | 10.892 ± 0.328 | 9.531 ± 0.060 | 7.671 ± 0.004 | 5.853 ± 0.047 | |
| lavandulyl acetate | 0.055 ± 0.002 | 0.052 ± 0.002 | 0.047 ± 0.000 | 0.040 ± 0.000 | 0.033 ± 0.001 | |
| caryophyllene-oxide | 0.060 ± 0.004 | 0.055 ± 0.001 | 0.047 ± 0.003 | 0.039 ± 0.004 | 0.031 ± 0.001 | |
| Total | 52.311 ± 2.603 | 49.862 ± 2.249 | 47.032 ± 1.897 | 41.302 ± 0.381 | 34.580 ± 0.245 | |
| Kinetic Model | Para-meter | Eucalyptol | cis-Linalool Oxide (furanoid) | Linalool | Camphor | Isoborneol | Borneol | Terpinen-4-ol | α-Terpineol | Linalool Acetate | Lavandulyl Acetate | Caryophyllene-Oxide | LEO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | |||||||||||||
| Zero-order | R2 | 0.947 | 0.889 | 0.960 | 0.940 | 0.951 | 0.991 | 0.928 | 0.957 | 0.999 | 0.975 | 0.972 | 0.973 |
| K0 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| First-order | R2 | 0.040 | 0.050 | 0.065 | 0.072 | 0.060 | 0.019 | 0.007 | 0.047 | 0.078 | 0.031 | 0.080 | 0.066 |
| K1 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Higuchi | R2 | 0.758 | 0.671 | 0.790 | 0.747 | 0.763 | 0.900 | 0.722 | 0.782 | 0.899 | 0.849 | 0.805 | 0.817 |
| KH | 0.243 | 0.248 | 0.296 | 0.293 | 0.271 | 0.261 | 0.267 | 0.267 | 0.318 | 0.271 | 0.291 | 0.299 | |
| Hixson–Crowell | R2 | 0.617 | 0.638 | 0.634 | 0.646 | 0.629 | 0.587 | 0.640 | 0.618 | 0.627 | 0.601 | 0.635 | 0.631 |
| KHC | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Korsmeyer–Peppas 1 | R2 | 0.875 | 0.787 | 0.940 | 0.895 | 0.873 | 0.986 | 0.799 | 0.924 | 0.988 | 0.973 | 0.827 | 0.950 |
| KKP | 1.079 | 1.096 | 1.056 | 1.074 | 1.083 | 1.003 | 1.103 | 1.062 | 1.015 | 1.034 | 1.010 | 1.052 | |
| n | 0.297 | 0.272 | 0.328 | 0.314 | 0.307 | 0.344 | 0.291 | 0.316 | 0.339 | 0.337 | 0.318 | 0.335 | |
| Kopcha | R2 | 0.171 | 0.053 | 0.349 | 0.174 | 0.158 | 0.820 | 0.070 | 0.295 | 0.680 | 0.668 | 0.128 | 0.423 |
| A | 0.059 | 0.036 | 0.101 | 0.071 | 0.063 | 0.157 | 0.043 | 0.083 | 0.162 | 0.138 | 0.064 | 0.112 | |
| B | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| A/B | 195.67 | 119.00 | 335.00 | 238.00 | 211.00 | 786.50 | 108.25 | 277.67 | 539.33 | 692.00 | 160.00 | 374.00 | |
| b | |||||||||||||
| Zero-order | R2 | 0.989 | 0.771 | 0.880 | 0.852 | 0.794 | 0.947 | 0.831 | 0.870 | 0.994 | 0.963 | 0.995 | 0.952 |
| K0 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | |
| First-order | R2 | 0.017 | 0.771 | 0.008 | 0.004 | 0.034 | 0.011 | 0.026 | 0.140 | 0.000 | 0.002 | 0.000 | 0.008 |
| K1 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Higuchi | R2 | 0.908 | 0.928 | 0.662 | 0.613 | 0.591 | 0.823 | 0.600 | 0.873 | 0.872 | 0.801 | 0.874 | 0.773 |
| KH | 0.235 | 0.014 | 0.075 | 0.063 | 0.057 | 0.217 | 0.034 | 0.030 | 0.168 | 0.127 | 0.159 | 0.104 | |
| Hixson–Crowell | R2 | 0.583 | 0.771 | 0.582 | 0.620 | 0.570 | 0.591 | 0.582 | 0.539 | 0.572 | 0.576 | 0.572 | 0.573 |
| KHC | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Korsmeyer–Peppas 1 | R2 | 0.936 | 0.997 | 0.568 | 0.156 | 0.354 | 0.945 | 0.172 | 0.636 | 0.927 | 0.833 | 0.909 | 0.799 |
| KKP | 1.086 | 1.003 | 1.177 | 1.033 | 1.133 | 1.051 | 1.228 | 1.107 | 1.085 | 1.122 | 1.095 | 1.125 | |
| n | 0.346 | 0.123 | 0.206 | 0.151 | 0.168 | 0.307 | 0.111 | 0.161 | 0.313 | 0.276 | 0.306 | 0.255 | |
| Kopcha | R2 | 0.525 | 0.975 | 0.075 | 0.000 | 0.187 | 0.468 | 0.018 | 0.397 | 0.594 | 0.306 | 0.509 | 0.284 |
| A | 0.108 | 0.018 | 0.013 | 0.001 | 0.018 | 0.086 | 0.003 | 0.015 | 0.077 | 0.040 | 0.067 | 0.032 | |
| B | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| A/B | 538.50 | 17,800.00 | 138.89 | 6.00 | 354.00 | 431.00 | 77.50 | 490.00 | 772.00 | 403.00 | 336.50 | 318.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flekka, K.; Dimaki, V.D.; Mourelatou, E.; Avgoustakis, K.; Lamari, F.N.; Hatziantoniou, S. Stability and Retention of Nanoemulsion Formulations Incorporating Lavender Essential Oil. Cosmetics 2024, 11, 65. https://doi.org/10.3390/cosmetics11030065
Flekka K, Dimaki VD, Mourelatou E, Avgoustakis K, Lamari FN, Hatziantoniou S. Stability and Retention of Nanoemulsion Formulations Incorporating Lavender Essential Oil. Cosmetics. 2024; 11(3):65. https://doi.org/10.3390/cosmetics11030065
Chicago/Turabian StyleFlekka, Konstantina, Virginia D. Dimaki, Elena Mourelatou, Konstantinos Avgoustakis, Fotini N. Lamari, and Sophia Hatziantoniou. 2024. "Stability and Retention of Nanoemulsion Formulations Incorporating Lavender Essential Oil" Cosmetics 11, no. 3: 65. https://doi.org/10.3390/cosmetics11030065








