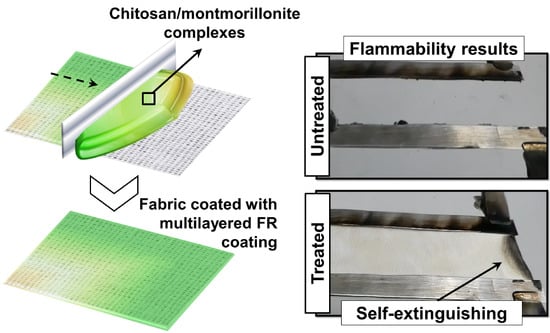
Flame Retardant Multilayered Coatings on Acrylic Fabrics Prepared by One-Step Deposition of Chitosan/Montmorillonite Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
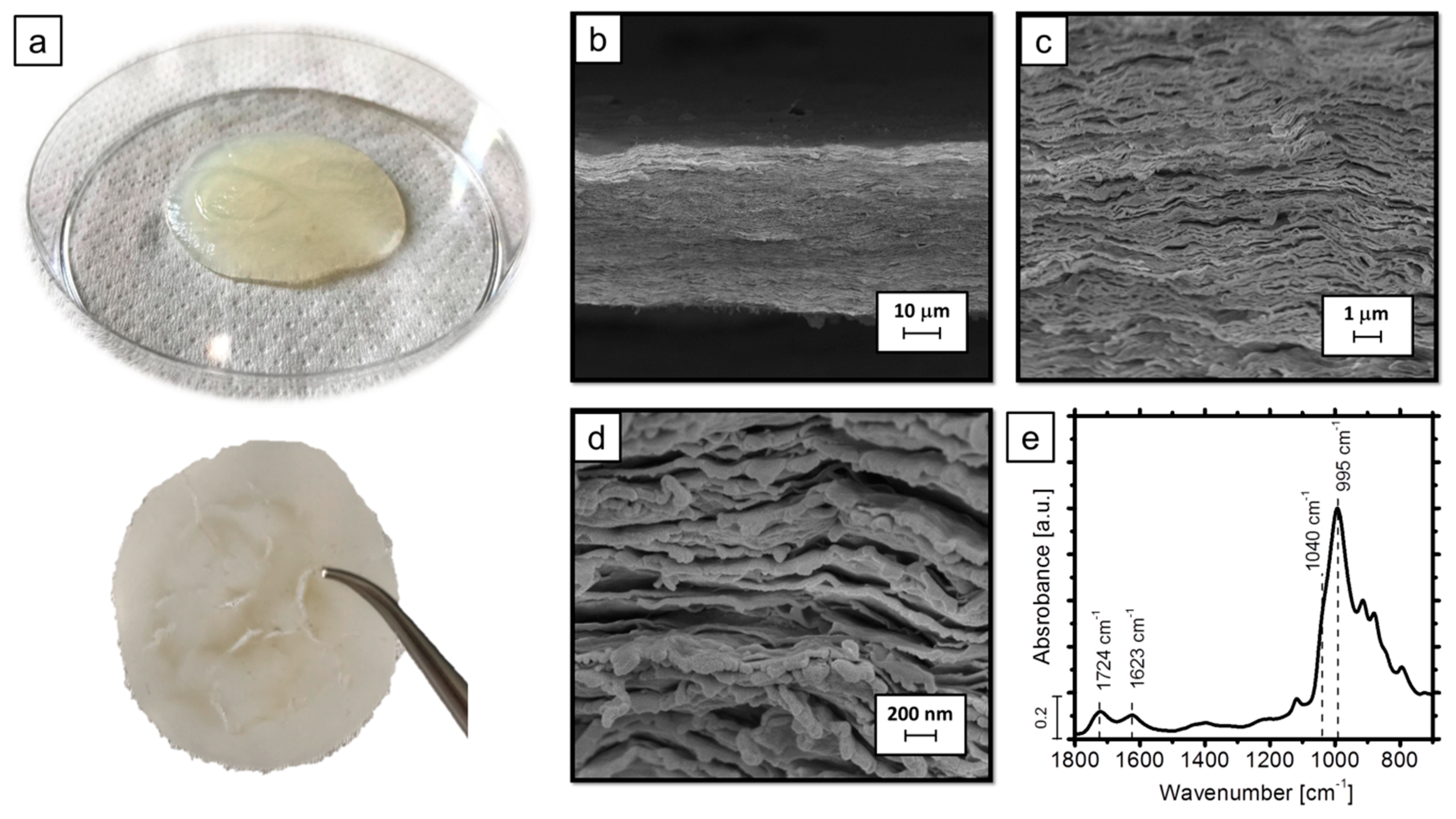
2.2. Compacted Complexes: Preparation and Deposition on Fabrics
2.3. Characterization Techniques
3. Results
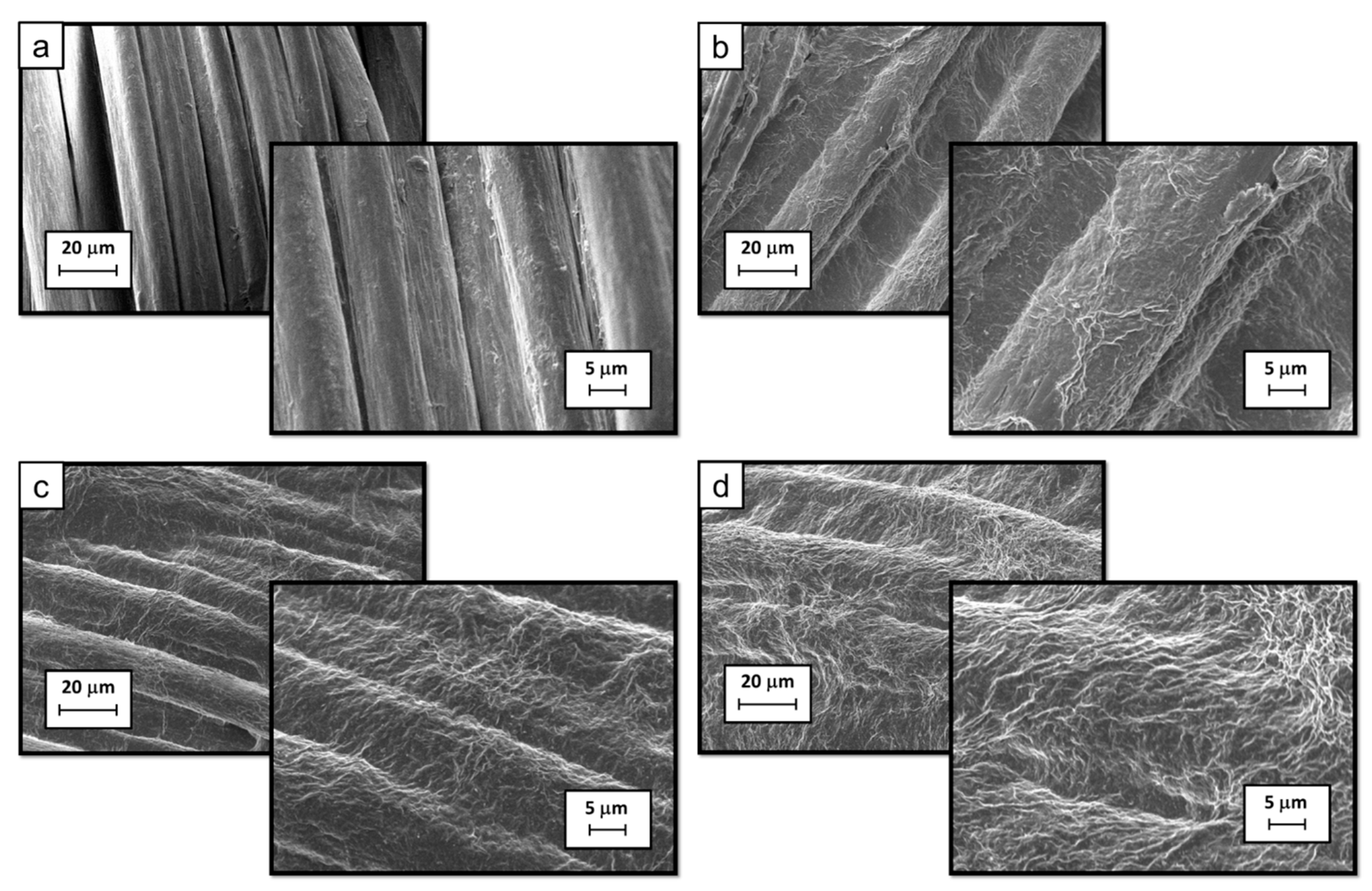
3.1. Morphological and Chemical Characterization
3.2. Flammability
3.3. Forced Combustion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sen, K.; Bahrami, S.H.; Bajaj, P. High-performance acrylic fibers. J. Macromol. Sci. Part C Polym. Rev. 1996, 36, 1–76. [Google Scholar] [CrossRef]
- Alongi, J.; Ra, H.; Carosio, F.; Malucelli, G. Update on Flame Retardant Textiles: State of the Art, Environmental Issues and Innovative Solutions; Smithers Rapra Technology Ltd.: Shawbury, UK, 2013. [Google Scholar]
- Bajaj, P.; Kumari, S. Modification of acrylic fibers: An overview. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 1987, 27, 181–217. [Google Scholar] [CrossRef]
- Tsai, J.-S. The effect of flame-retardants on the properties of acrylic and modacrylic fibres. J. Mater. Sci. 1993, 28, 1161–1167. [Google Scholar] [CrossRef]
- Hall, M.E.; Zhang, J.; Richard Horrocks, A. The flammability of polyacrylonitrile and its copolymers III. Effect of flame retardants. Fire Mater. 1994, 18, 231–241. [Google Scholar] [CrossRef]
- Hall, M.E.; Horrocks, A.R.; Zhang, J. The flammability of polyacrylonitrile and its copolymers. Polym. Degrad. Stabil. 1994, 44, 379–386. [Google Scholar] [CrossRef]
- Chou, S.; Wu, C.-J. Effect of brominated flame retardants on the properties of acrylonitrile/vinyl acetate copolymer fibers. Text. Res. J. 1995, 65, 533–539. [Google Scholar] [CrossRef]
- Malucelli, G.; Carosio, F.; Alongi, J.; Fina, A.; Frache, A.; Camino, G. Materials engineering for surface-confined flame retardancy. Mater. Sci. Eng. R 2014, 84, 1–20. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Kiekens, P. Recent advances in the design of water based-flame retardant coatings for polyester and polyester-cotton blends. Polymers 2016, 8, 357. [Google Scholar] [CrossRef] [Green Version]
- Holder, K.M.; Smith, R.J.; Grunlan, J.C. A review of flame retardant nanocoatings prepared using layer-by-layer assembly of polyelectrolytes. J. Mater. Sci. 2017, 52, 12923–12959. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Kabanov, V.A. Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012; p. 1112. [Google Scholar]
- Patra, D.; Vangal, P.; Cain, A.A.; Cho, C.; Regev, O.; Grunlan, J.C. Inorganic nanoparticle thin film that suppresses flammability of polyurethane with only a single electrostatically-assembled bilayer. ACS Appl. Mater. Interfaces 2014, 6, 16903–16908. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Di Blasio, A.; Alongi, J.; Malucelli, G. Layer by layer nanoarchitectures for the surface protection of polycarbonate. Eur. Polym. J. 2013, 49, 397–404. [Google Scholar] [CrossRef]
- Cain, A.A.; Plummer, M.G.B.; Murray, S.E.; Bolling, L.; Regev, O.; Grunlan, J.C. Iron-containing, high aspect ratio clay as nanoarmor that imparts substantial thermal/flame protection to polyurethane with a single electrostatically-deposited bilayer. J. Mater. Chem. A 2014, 2, 17609–17617. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Cain, A.A.; Grunlan, J.C. Clay-chitosan nanobrick walls: Completely renewable gas barrier and flame-retardant nanocoatings. ACS Appl. Mater. Interfaces 2012, 4, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Fontaine, G.; Alongi, J.; Bourbigot, S. Starch-based layer by layer assembly: Efficient and sustainable approach to cotton fire protection. ACS Appl. Mater. Interfaces 2015, 7, 12158–12167. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Negrell-Guirao, C.; Alongi, J.; David, G.; Camino, G. All-polymer layer by layer coating as efficient solution to polyurethane foam flame retardancy. Eur. Polym. J. 2015, 70, 94–103. [Google Scholar] [CrossRef]
- Koklukaya, O.; Carosio, F.; Wãgberg, L. Superior flame-resistant cellulose nanofibril aerogels modified with hybrid layer-by-layer coatings. ACS Appl. Mater. Interfaces 2017, 9, 29082–29092. [Google Scholar] [CrossRef] [PubMed]
- Guin, T.; Krecker, M.; Milhorn, A.; Grunlan, J.C. Maintaining hand and improving fire resistance of cotton fabric through ultrasonication rinsing of multilayer nanocoating. Cellulose 2014, 21, 3023–3030. [Google Scholar] [CrossRef]
- Carosio, F.; Negrell-Guirao, C.; Di Blasio, A.; Alongi, J.; David, G.; Camino, G. Tunable thermal and flame response of phosphonated oligoallylamines layer by layer assemblies on cotton. Carbohydr. Polym. 2015, 115, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Di Pierro, A.; Alongi, J.; Fina, A.; Saracco, G. Controlling the melt dripping of polyester fabrics by tuning the ionic strength of polyhedral oligomeric silsesquioxane and sodium montmorillonite coatings assembled through layer by layer. J. Colloid Interface Sci. 2018, 510, 142–151. [Google Scholar] [CrossRef] [PubMed]
- van der Gucht, J.; Spruijt, E.; Lemmers, M.; Stuart, M.A.C. Polyelectrolyte complexes: Bulk phases and colloidal systems. J. Colloid Interface Sci. 2011, 361, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Negrell-Guirao, C.; Carosio, F.; Boutevin, B.; Cottet, H.; Loubat, C. Phosphonated oligoallylamine: Synthesis, characterization in water, and development of layer by layer assembly. J. Polym. Sci. Pol. Phys. 2013, 51, 1244–1251. [Google Scholar] [CrossRef]
- Ghanadpour, M.; Carosio, F.; Wågberg, L. Ultrastrong and flame-resistant freestanding films from nanocelluloses, self-assembled using a layer-by-layer approach. Appl. Mater. Today 2017, 9, 229–239. [Google Scholar] [CrossRef]
- Cain, A.A.; Murray, S.; Holder, K.M.; Nolen, C.R.; Grunlan, J.C. Intumescent nanocoating extinguishes flame on fabric using aqueous polyelectrolyte complex deposited in single step. Macromol. Mater. Eng. 2014, 299, 1180–1187. [Google Scholar] [CrossRef]
- Haile, M.; Fincher, C.; Fomete, S.; Grunlan, J.C. Water-soluble polyelectrolyte complexes that extinguish fire on cotton fabric when deposited as ph-cured nanocoating. Polym. Degrad. Stabil. 2015, 114, 60–64. [Google Scholar] [CrossRef]
- Schaaf, P.; Schlenoff, J.B. Saloplastics: Processing compact polyelectrolyte complexes. Adv. Mater. 2015, 27, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Haile, M.; Sarwar, O.; Henderson, R.; Smith, R.; Grunlan, J.C. Polyelectrolyte coacervates deposited as high gas barrier thin films. Macromol. Rapid Commun. 2017, 38. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Kochumalayil, J.; Cuttica, F.; Camino, G.; Berglund, L. Oriented clay nanopaper from biobased components--mechanisms for superior fire protection properties. ACS Appl. Mater. Interfaces 2015, 7, 5847–5856. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies—Table and Charts, 3rd ed.; Wiley: Weinheim, Germany, 2006. [Google Scholar]
- Silva, S.M.; Braga, C.R.; Fook, M.V.; Raposo, C.M.; Carvalho, L.H.; Canedo, E.L. Application of infrared spectroscopy to analysis of chitosan/clay nanocomposites, infrared spectroscopy. In Infrared Spectroscopy-Materials Science, Engineering and Technology; InTech: London, UK, 2012. [Google Scholar]
- Dalton, S.; Heatley, F.; Budd, P.M. Thermal stabilization of polyacrylonitrile fibres. Polymer 1999, 40, 5531–5543. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J. Influence of layer by layer coatings containing octapropylammonium polyhedral oligomeric silsesquioxane and ammonium polyphosphate on the thermal stability and flammability of acrylic fabrics. J. Anal. Appl. Pyrol. 2016, 119, 114–123. [Google Scholar] [CrossRef]
- Carosio, F.; Cuttica, F.; Medina, L.; Berglund, L.A. Clay nanopaper as multifunctional brick and mortar fire protection coating—Wood case study. Mater. Des. 2016, 93, 357–363. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]


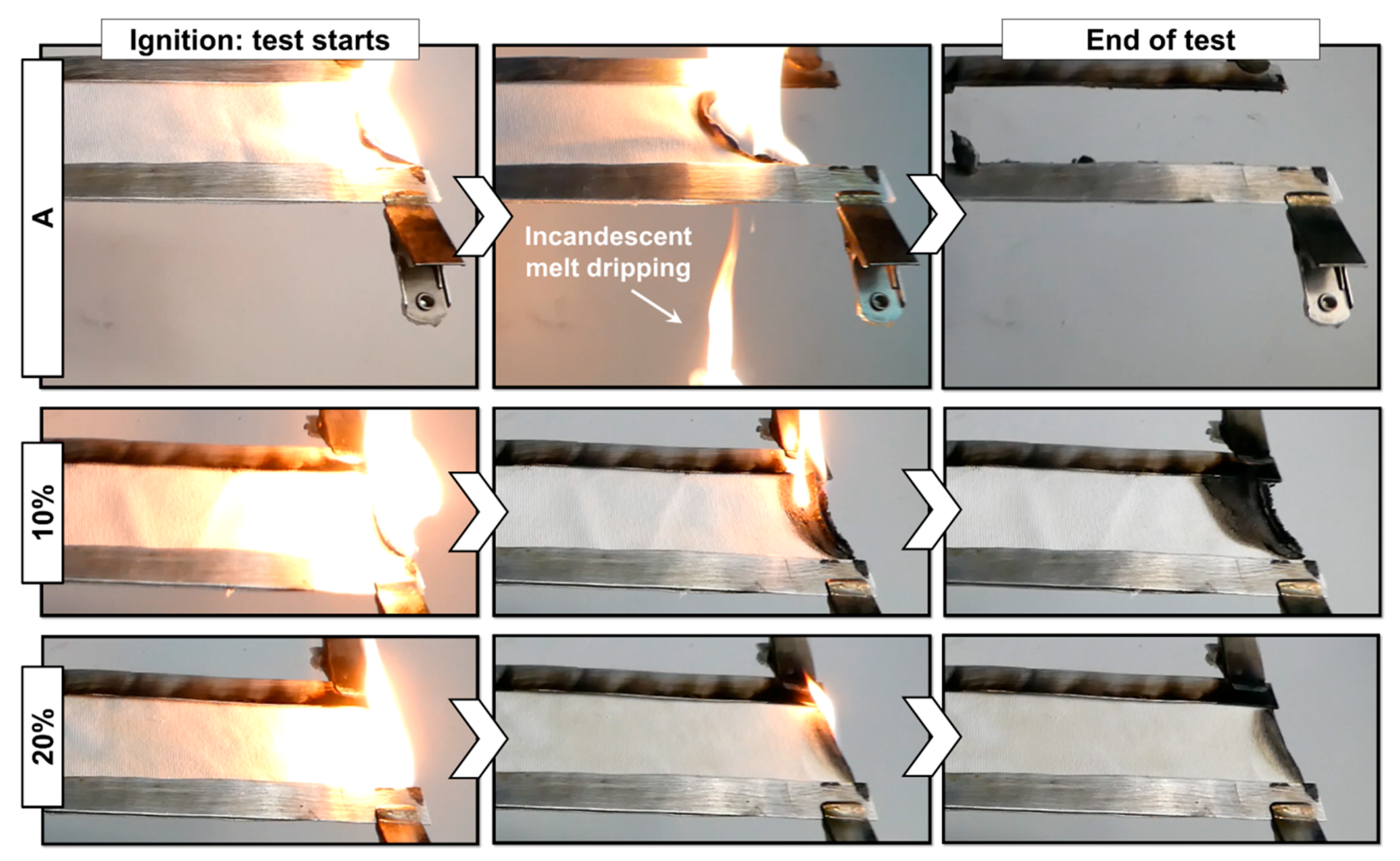
| Sample | Flame Spread Rate (mm/s) | Dripping | Residue (%) |
|---|---|---|---|
| A | 1.2 ± 0.1 | YES | 10 ± 1 |
| 5% | 1.0 ± 0.2 | NO | 20 ± 2 |
| 10% | 0.6 ± 0.1 | NO | 77 ± 11 |
| 20% | N.A. | NO | 93 ± 3 |
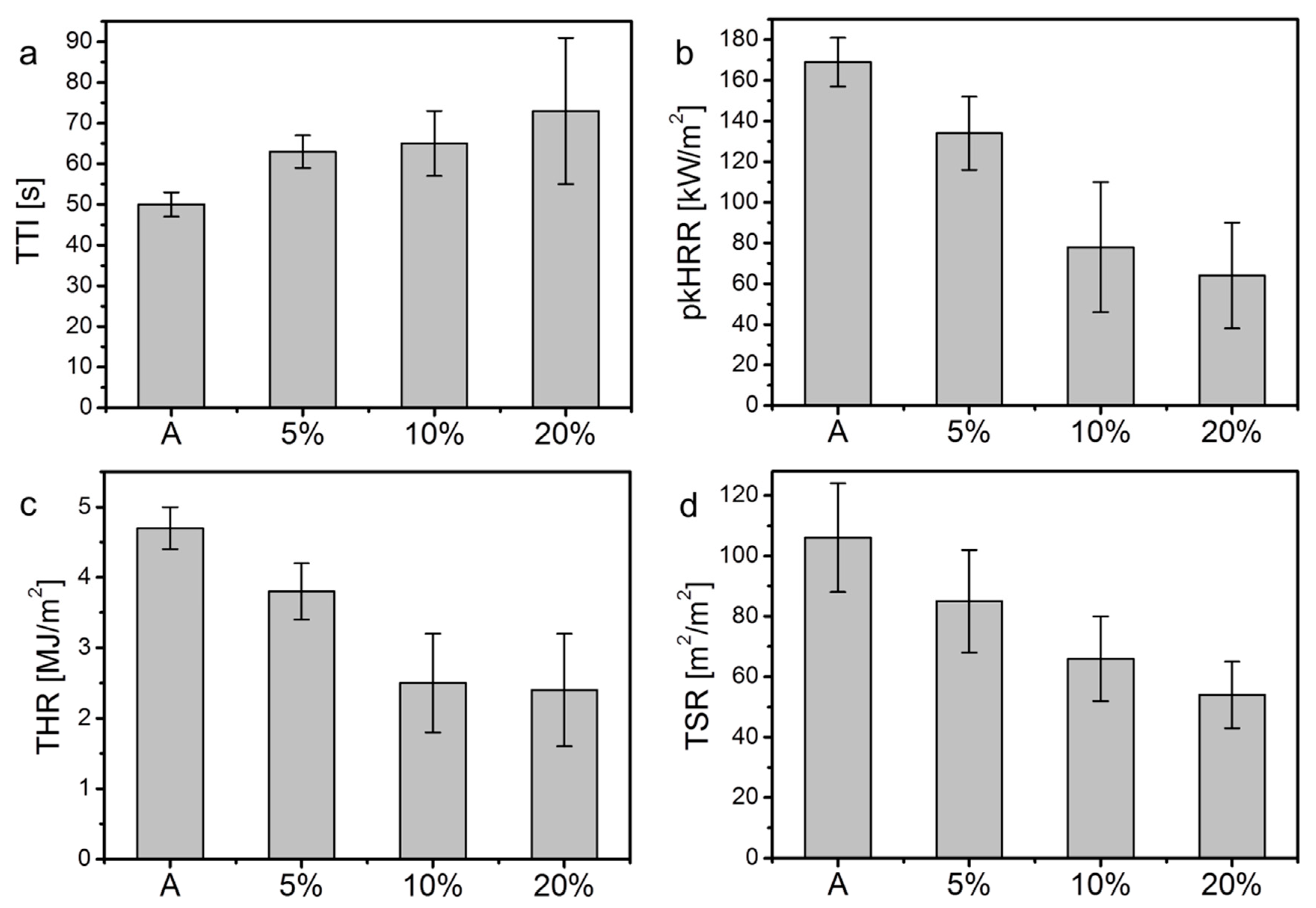
| Sample | TTI ± σ [s] | pkHRR ± σ [kW/m2] (Reduction, %) | FIGRA [kW/s m2] | THR ± σ [MJ/m2] (Reduction, %) | EHC ± σ [MJ/kg] (Reduction, %) | TSR ± σ [m2/m2] (Reduction, %) | Residue ± σ [%] |
|---|---|---|---|---|---|---|---|
| A | 50 ± 3 | 169 ± 12 | 2.7 ± 0.2 | 4.7 ± 0.3 | 25.6 ± 2.6 | 106 ± 18 | 28 ± 1 |
| 5% | 63 ± 4 | 134 ± 18 (21) | 1.8 ± 0.4 | 3.8 ± 0.4 (20) | 22.1 ± 2.8 (13) | 85 ± 17 (19) | 42 ± 4 |
| 10% | 65 ± 8 | 78 ± 32 (55) | 0.9 ± 0.5 | 2.5 ± 0.7 (48) | 18.0 ± 5.0 (30) | 66 ± 14. (37) | 52 ± 6 |
| 20% | 73 ± 18 | 64 ± 26 (62) | 0.7 ± 0.3 | 2.4 ± 0.8 (49) | 17.0 ± 2.2 (32) | 54 ± 11 (49) | 55 ± 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carosio, F.; Alongi, J. Flame Retardant Multilayered Coatings on Acrylic Fabrics Prepared by One-Step Deposition of Chitosan/Montmorillonite Complexes. Fibers 2018, 6, 36. https://doi.org/10.3390/fib6020036
Carosio F, Alongi J. Flame Retardant Multilayered Coatings on Acrylic Fabrics Prepared by One-Step Deposition of Chitosan/Montmorillonite Complexes. Fibers. 2018; 6(2):36. https://doi.org/10.3390/fib6020036
Chicago/Turabian StyleCarosio, Federico, and Jenny Alongi. 2018. "Flame Retardant Multilayered Coatings on Acrylic Fabrics Prepared by One-Step Deposition of Chitosan/Montmorillonite Complexes" Fibers 6, no. 2: 36. https://doi.org/10.3390/fib6020036