Surface-Initiated Graft Atom Transfer Radical Polymerization of Methyl Methacrylate from Chitin Nanofiber Macroinitiator under Dispersion Conditions
Abstract
:1. Introduction
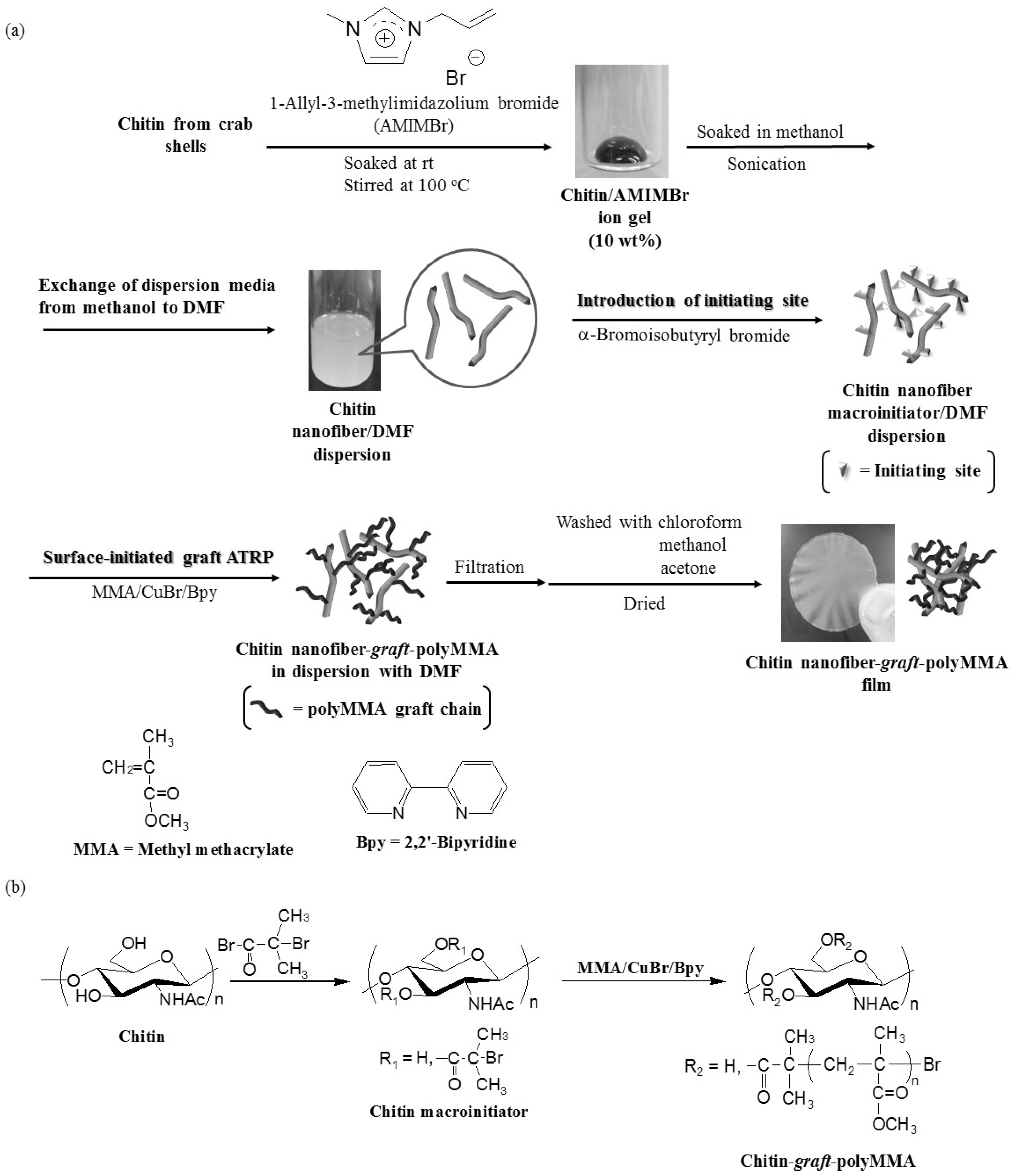
2. Experimental Section
2.1. Materials
2.2. Preparation of CNF Dispersion with DMF [21]
2.3. Preparation of CNF Macroinitiator in Dispersion with DMF
2.4. Surface-Initiated Graft ATRP of MMA from CNF Macroinitiator in Dispersion with DMF
2.5. Methods
3. Results and Discussion
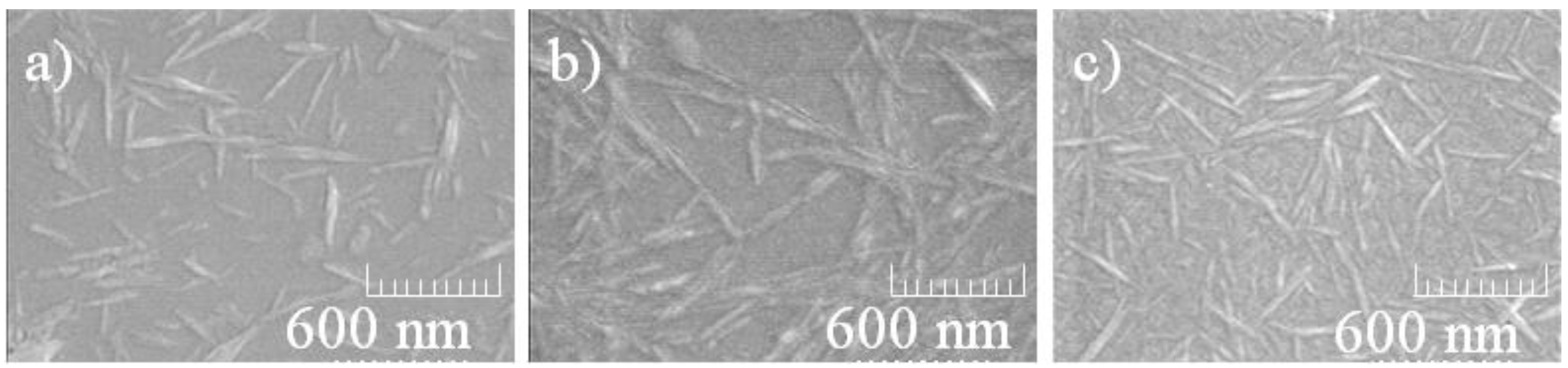
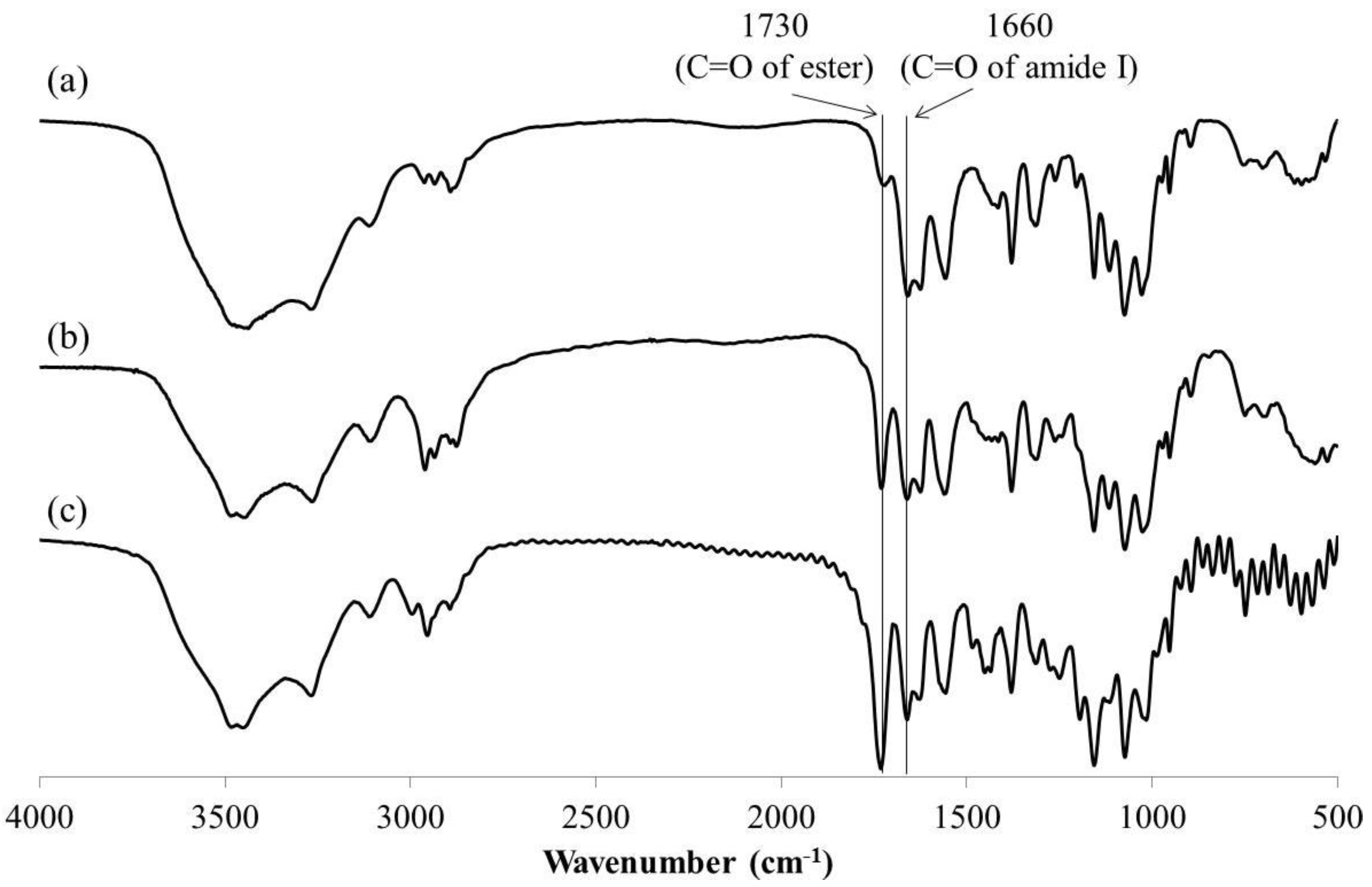
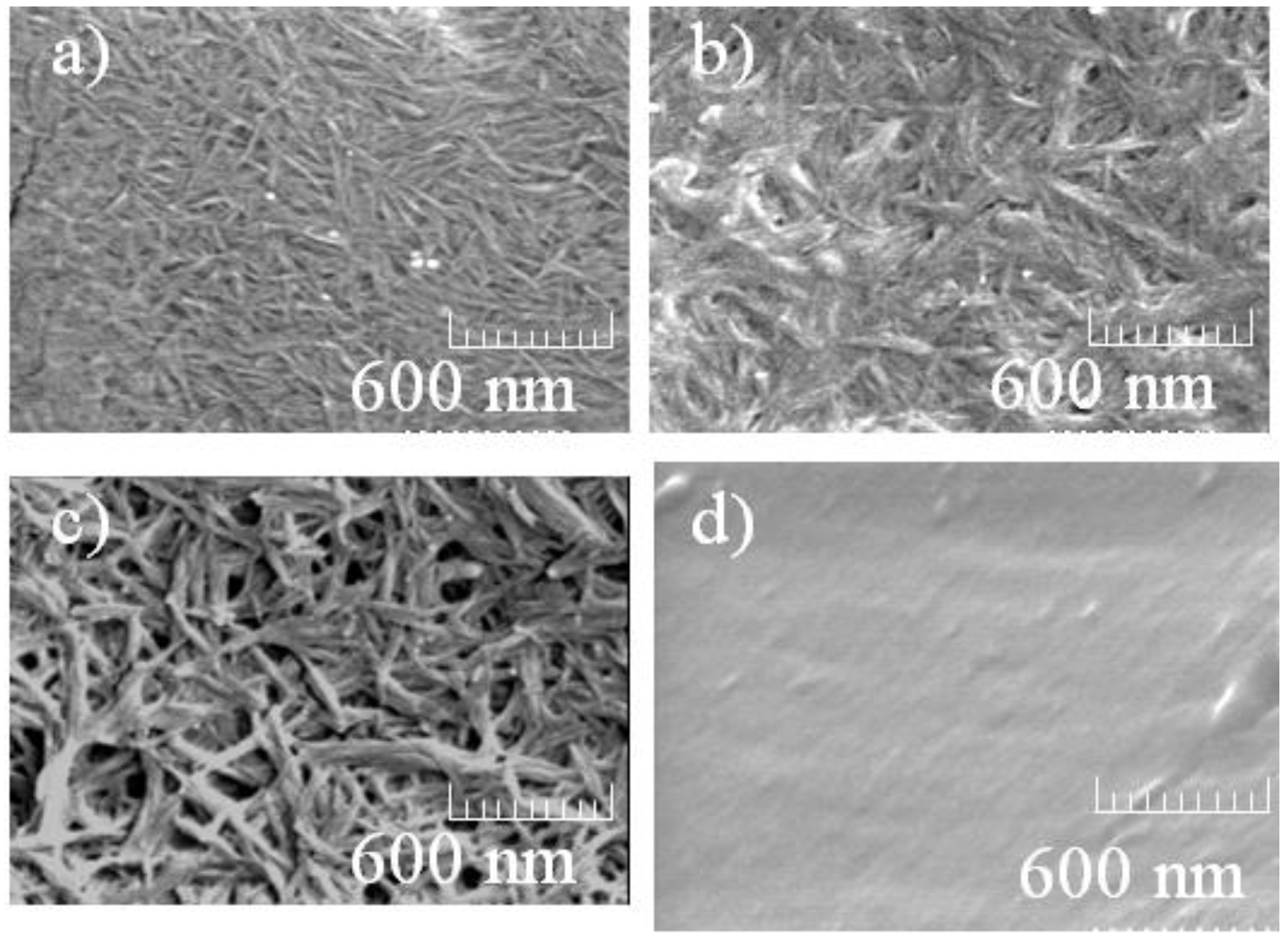
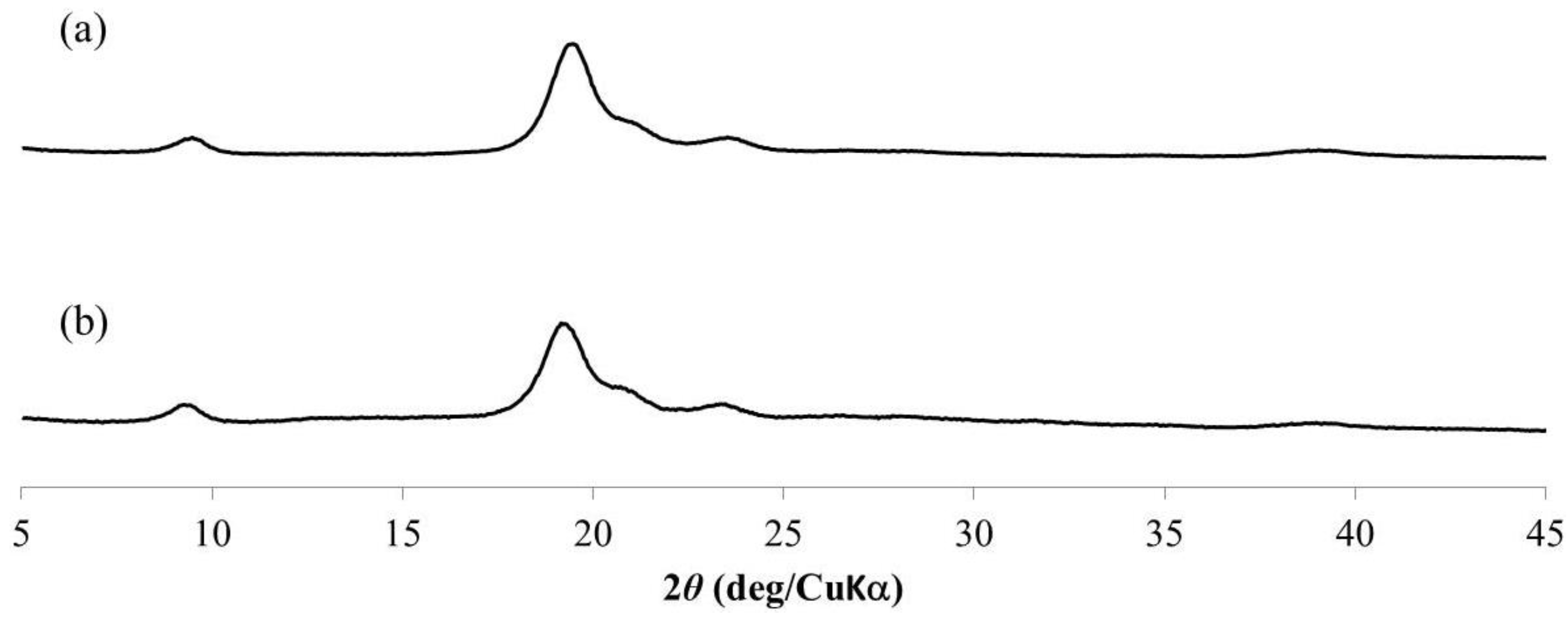
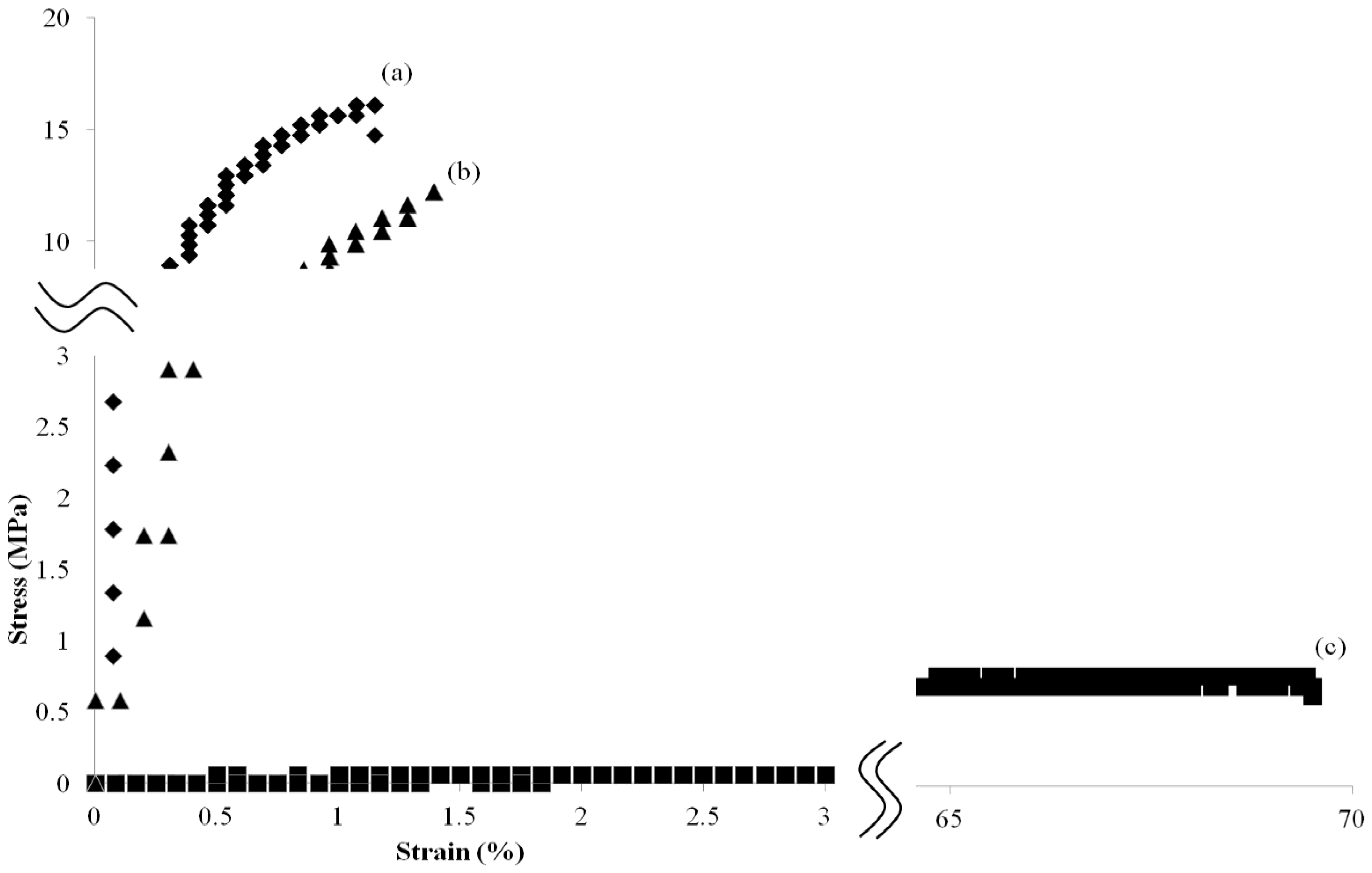
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. New techniques for optimization of surface area and porosity in nanochitins and nanochitosans. Adv. Polym. Sci. 2011, 244, 167–186. [Google Scholar]
- Muzzarelli, R.A.A. Biomedical exploitation of chitin and chitosan via mechano-chemical disassembly, electrospinning, dissolution in imidazolium ionic liquids, and supercritical drying. Mar. Drugs 2011, 9, 1510–1533. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Mattioli-Belmonte, M. Emerging biomedical applications of nano-chitins and nano-chitosans obtained via advanced eco-friendly technologies from marine resources. Mar. Drugs 2014, 12, 5468–5502. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. Fabrication of nanostructured and microstructured chitin materials through gelation with suitable dispersion media. RSC Adv. 2015, 5, 12736–12746. [Google Scholar] [CrossRef]
- Liebert, T.; Heinze, T. Interaction of ionic liquids with polysaccharides 5. Solvents and reaction media for the modification of cellulose. Bioresources 2008, 3, 576–601. [Google Scholar]
- Feng, L.; Chen, Z.I. Research progress on dissolution and functional modification of cellulose in ionic liquids. J. Mol. Liq. 2008, 142, 1–5. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.N.; Pang, S.S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Fardim, P.; Heinze, T. Ionic liquids—Promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 2012, 17, 7458–7502. [Google Scholar] [CrossRef] [PubMed]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- El Seoud, O.A.; Koschella, A.; Fidale, L.C.; Dorn, S.; Heinze, T. Applications of ionic liquids in carbohydrate chemistry: A window of opportunities. Biomacromolecules 2007, 8, 2629–2647. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Solubility of carbohydrates in ionic liquids. Energy Fuels 2010, 24, 737–745. [Google Scholar] [CrossRef]
- Wang, W.T.; Zhu, J.; Wang, X.L.; Huang, Y.; Wang, Y.Z. Dissolution behavior of chitin in ionic liquids. J. Macromol. Sci. B Phys. 2010, 49, 528–541. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Kozlecki, T.; Gorak, A. Review of the application of ionic liquids as solvents for chitin. J. Polym. Eng. 2012, 32, 67–69. [Google Scholar] [CrossRef]
- Kadokawa, J. Ionic liquid as useful media for dissolution, derivatization, and nanomaterial processing of chitin. Green Sustain. Chem. 2013, 3, 19–25. [Google Scholar] [CrossRef]
- Yamazaki, S.; Takegawa, A.; Kaneko, Y.; Kadokawa, J.; Yamagata, M.; Ishikawa, M. An acidic cellulose-chitin hybrid gel as novel electrolyte for an electric double layer capacitor. Electrochem. Commun. 2009, 11, 68–70. [Google Scholar] [CrossRef]
- Prasad, K.; Murakami, M.; Kaneko, Y.; Takada, A.; Nakamura, Y.; Kadokawa, J. Weak gel of chitin with ionic liquid, 1-allyl-3-methylimidazolium bromide. Int. J. Biol. Macromol. 2009, 45, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Takegawa, A.; Mine, S.; Prasad, K. Preparation of chitin nanowhiskers using an ionic liquid and their composite materials with poly(vinyl alcohol). Carbohydr. Polym. 2011, 84, 1408–1412. [Google Scholar] [CrossRef]
- Tajiri, R.; Setoguchi, T.; Wakizono, S.; Yamamoto, K.; Kadokawa, J. Preparation of self-assembled chitin nanofibers by regeneration from ion gels using calcium halide dihydrate/methanol solutions. J. Biobased Mater. Bioenergy 2013, 7, 655–659. [Google Scholar] [CrossRef]
- Setoguchi, T.; Yamamoto, K.; Kadokawa, J. Preparation of chitin nanofiber-graft-poly(l-lactide-co-e-caprolactone) films by surface-initiated ring-opening graft copolymerization. Polymer 2012, 53, 4977–4982. [Google Scholar] [CrossRef]
- Kadokawa, J.; Setoguchi, T.; Yamamoto, K. Preparation of highly flexible chitin nanofiber-graft-poly(g-l-glutamic acid) network film. Polym. Bull. 2013, 70, 3279–3289. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yoshida, S.; Mine, S.; Kadokawa, J. Synthesis of chitin-graft-polystyrene via atom transfer radical polymerization initiated from a chitin macroinitiator. Polym. Chem. 2013, 4, 3384–3389. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yoshida, S.; Kadokawa, J. Surface-initiated atom transfer radical polymerization from chitin nanofiber macroinitiator film. Carbohydr. Polym. 2014, 112, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.; Charlet, G. Chitin fractionation and characterization in n,n-dimethylacetamide/lithium chloride solvent system. Carbohydr. Polym. 2002, 50, 363–370. [Google Scholar] [CrossRef]
- Zhao, D.B.; Fei, Z.F.; Geldbach, T.J.; Scopelliti, R.; Laurenczy, G.; Dyson, P.J. Allyl-functionalised ionic liquids: Synthesis, characterisation, and reactivity. Helv. Chim. Acta. 2005, 88, 665–675. [Google Scholar] [CrossRef]
- Ando, T.; Kataoka, S. Acylation of chitin with acid anhydrides in trichloroacetic acid systems. Kobunshi Ronbunshu 1980, 37, 1–7. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endo, R.; Yamamoto, K.; Kadokawa, J.-i. Surface-Initiated Graft Atom Transfer Radical Polymerization of Methyl Methacrylate from Chitin Nanofiber Macroinitiator under Dispersion Conditions. Fibers 2015, 3, 338-347. https://doi.org/10.3390/fib3030338
Endo R, Yamamoto K, Kadokawa J-i. Surface-Initiated Graft Atom Transfer Radical Polymerization of Methyl Methacrylate from Chitin Nanofiber Macroinitiator under Dispersion Conditions. Fibers. 2015; 3(3):338-347. https://doi.org/10.3390/fib3030338
Chicago/Turabian StyleEndo, Ryo, Kazuya Yamamoto, and Jun-ichi Kadokawa. 2015. "Surface-Initiated Graft Atom Transfer Radical Polymerization of Methyl Methacrylate from Chitin Nanofiber Macroinitiator under Dispersion Conditions" Fibers 3, no. 3: 338-347. https://doi.org/10.3390/fib3030338






