Synthesis of Co-Electrospun Lead Selenide Nanostructures within Anatase Titania Nanotubes for Advanced Photovoltaics
Abstract
:1. Introduction
2. Results and Discussion
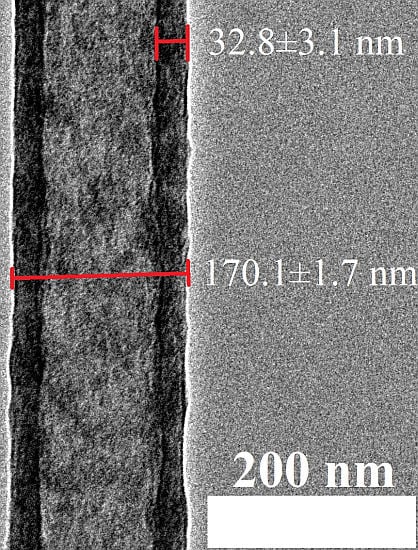
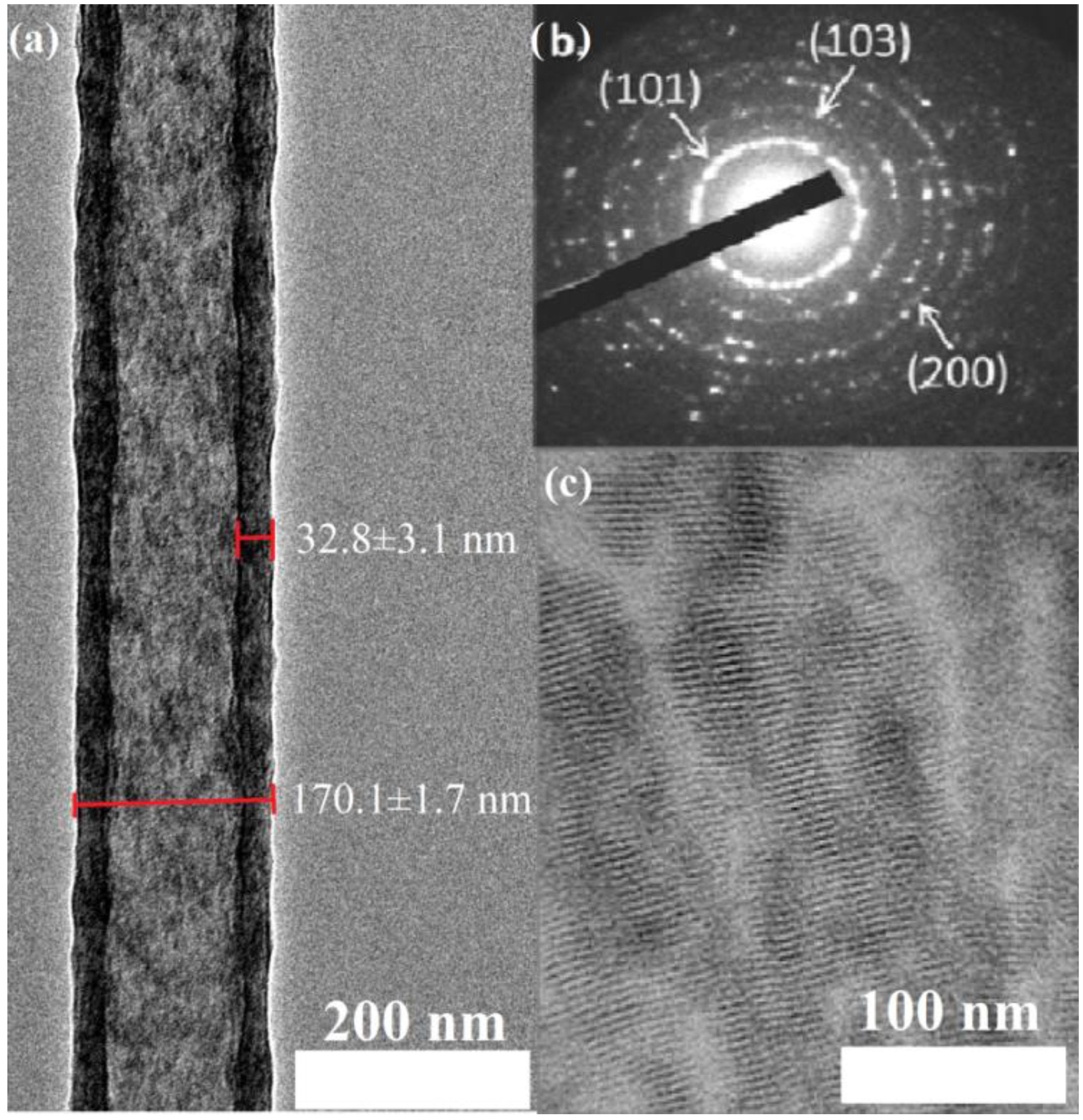
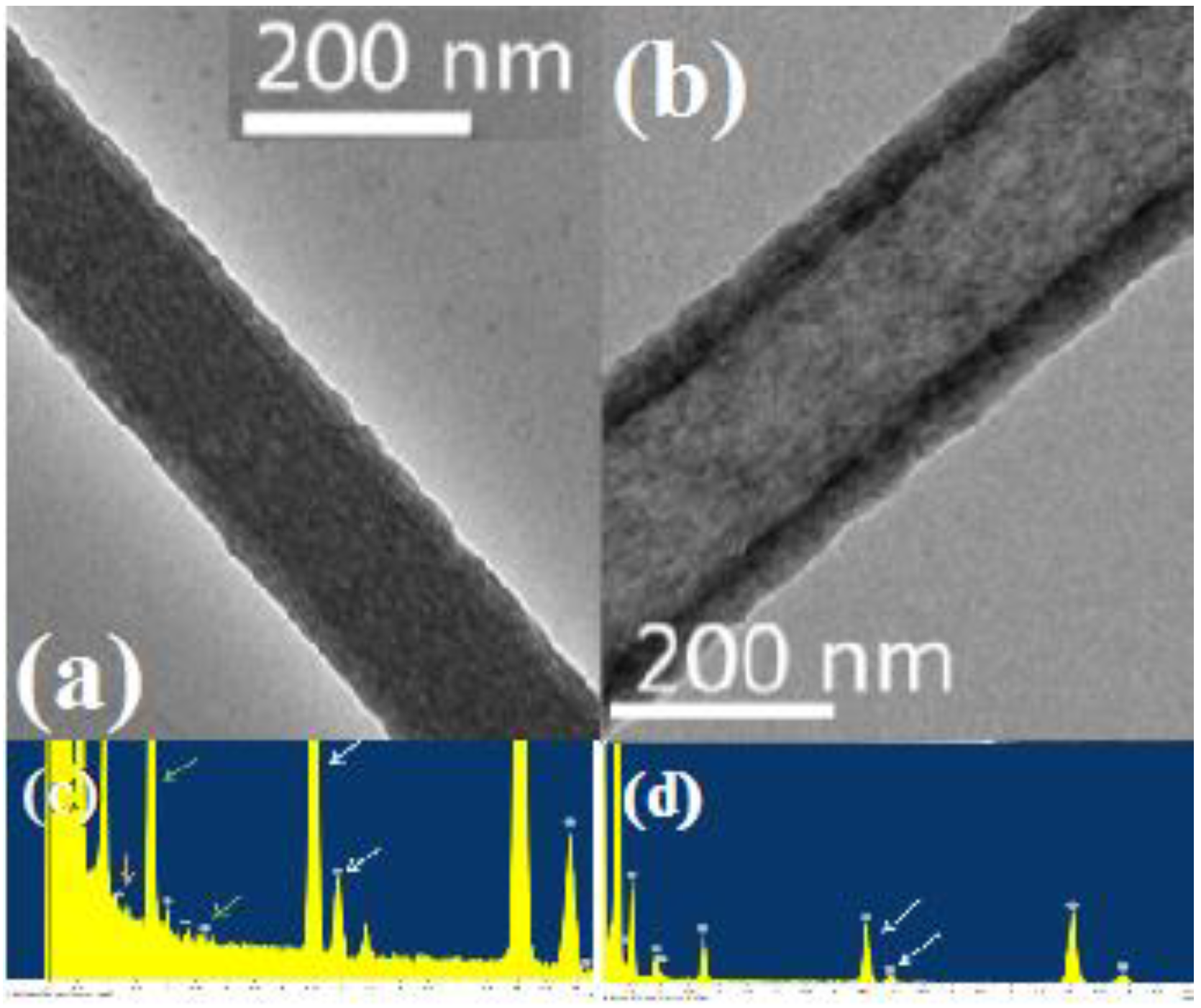
2.1. Characterization of Morphology and Material Composition
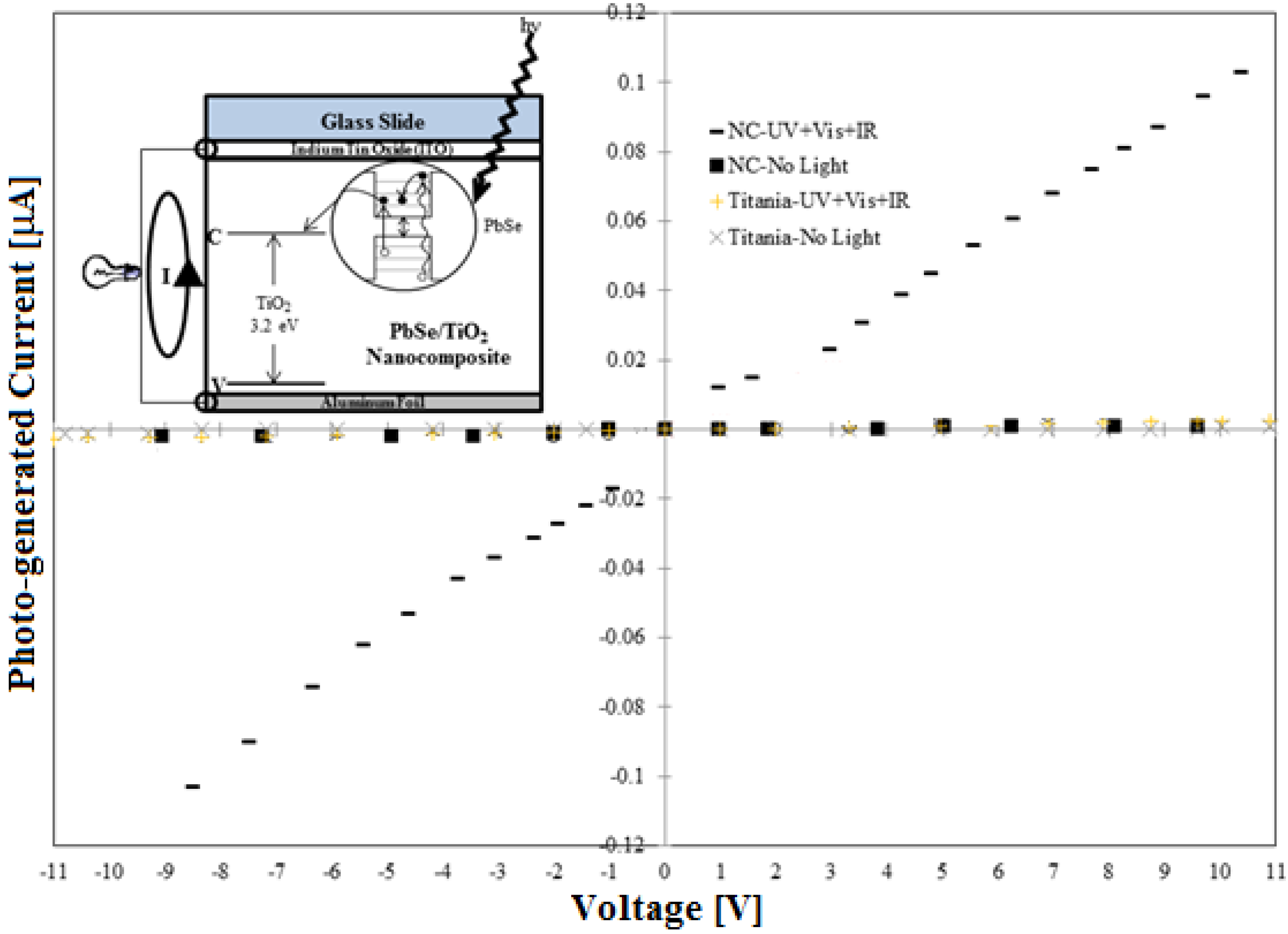
2.2. Photovoltaic Characterization
3. Materials and Methods
3.1. Chemicals
3.2. PbSe Nanostructure Preparation
3.3. TiO2 Nanotube Preparation
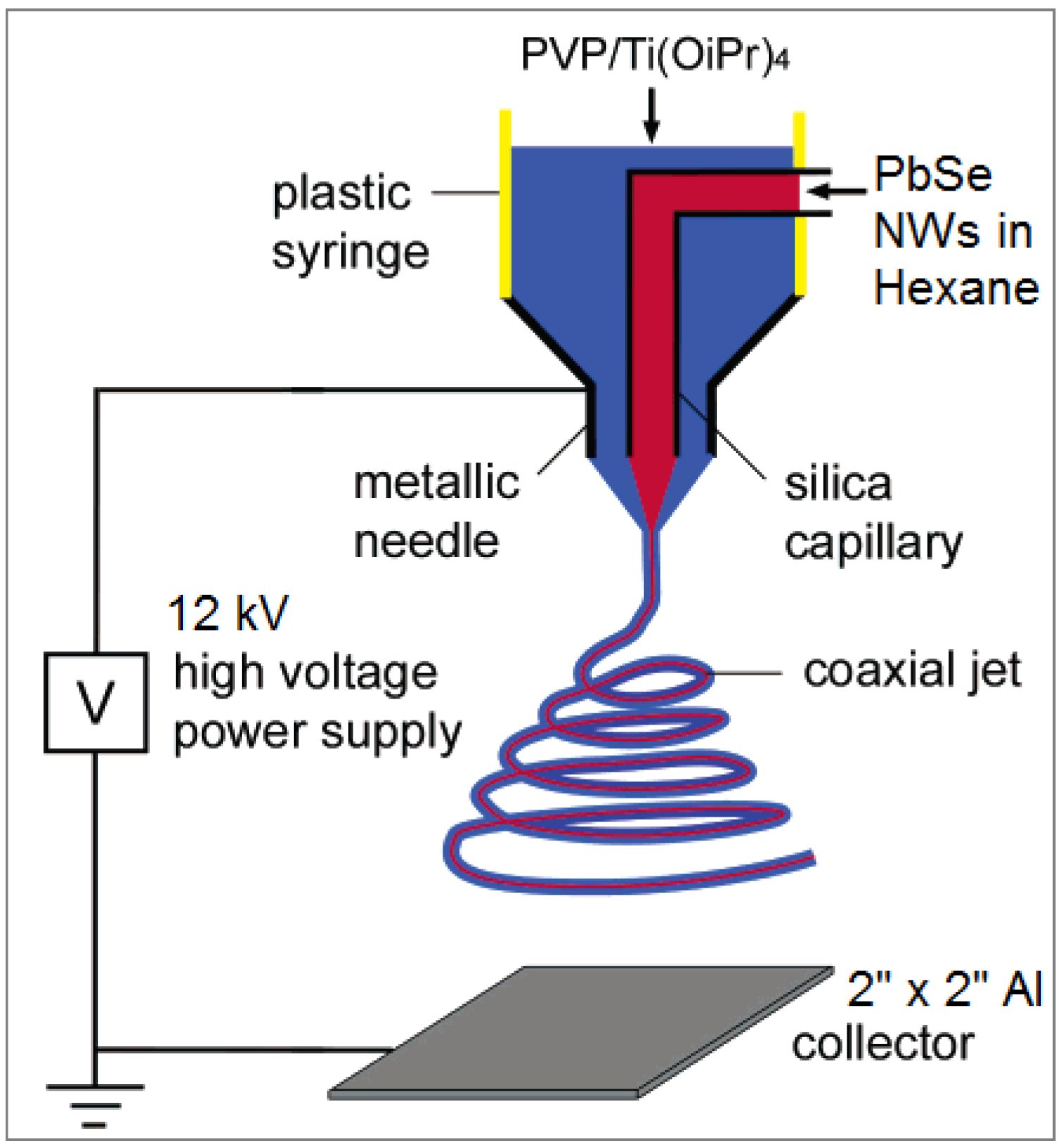
3.4. Electrospinning Setup and Conditions
3.5 Characterization
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maniyali, Y.; Almansoori, A.; Fowler, M.; Elkamel, A. Energy hub based on nuclear energy and hydrogen energy storage. Ind. Eng. Chem. Res. 2013, 52, 7470–7481. [Google Scholar] [CrossRef]
- Thavasi, V.; Singh, G.; Ramakrishna, S. Electrospun nanofibers in energy and environmental applications. Energ. Environ. Sci. 2008, 1, 205–221. [Google Scholar] [CrossRef]
- ASTM International. ASTM G173-03(2012). Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Pohekar, S.D.; Kumar, D.; Ramachandran, M. Dissemination of cooking energy alternatives in India—A review. Renew. Sustain. Energ. Rev. 2005, 9, 379–393. [Google Scholar] [CrossRef]
- Nault, R.M. Basic Research Needs for Solar Energy Utilization; United States Department of Energy: Washington, DC, USA, 2005.
- EL-Shimy, M. Viability analysis of PV power plants in Egypt. Renew. Energ. 2009, 34, 2187–2196. [Google Scholar] [CrossRef]
- Mankins, J.C. New directions for space solar power. Acta Astronaut. 2009, 65, 146–156. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Wu, H.-C.; Chiu, Y.-C.; Chen, W.-C. Plasmon-enhanced polymer photovoltaic device performance using different patterned Ag/PVP electrospun nanofibers. Adv. Energ. Mater. 2014, 4. [Google Scholar] [CrossRef]
- Börjesson, K.; Lennartson, A.; Moth-Poulsen, K. Efficiency limit of molecular solar thermal energy collecting devices. ACS Sustain. Chem. Eng. 2013, 1, 585–590. [Google Scholar] [CrossRef]
- Kim, J.-U.; Park, S.-H.; Choi, H.-J.; Lee, W.-K.; Lee, J.-K.; Kim, M.-R. Effect of electrolyte in electrospun poly (vinylidene fluoride-co-hexafluoropropylene) nanofibers on dye-sensitized solar cells. Solar Energ. Mater. Solar Cell. 2009, 93, 803–807. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, J.-W.; Park, K.H.; Kim, T.-Y.; Yim, S.-H.; Zhao, X.G.; Gu, H.-B.; Jin, E.M. Dye-sensitized solar cells based on electrospun poly (vinylidenefluoride-co-hexafluoropropylene) nanofibers. Polym. Bull. 2013, 70, 507–515. [Google Scholar] [CrossRef]
- Grätzel, M. Solar Energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kim, B.-G.; Park, H.J. Novel conjugated polymer for organic photovoltaics: Synthesis and device optimization. Synth. Met. 2015, 199, 280–283. [Google Scholar] [CrossRef]
- Pathak, D.; Wagner, T.; Adhikari, T.; Nunzi, J.M. Photovoltaic performance of AgInSe2-conjugated polymer hybrid system bulk heterojunction solar cells. Synth. Met. 2015, 199, 87–92. [Google Scholar] [CrossRef]
- Peumans, P.; Uchida, S.; Forrest, S.R. Efficient bulk heterojunction photovoltaic cells using small-molecular-weight organic thin films. Nature 2003, 425, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, B.; Melianas, A.; Bergqvist, J.; Tress, W.; Bao, Q.; Qian, D.; Inganäs, O.; Zhang, F. A new fullerene-free bulk-heterojunction system for efficient high-voltage and high-fill factor solution-processed organic photovoltaics. Adv. Mater. 2015, 27, 1900–1907. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V. Quantum dot solar cells. Semiconductor nanocrystals as light harvesters. J. Phys. Chem. C 2008, 112, 18737–18753. [Google Scholar] [CrossRef]
- Yang, S.; Nair, S.; Ramakrishna, S. Morphology of the electrospun TiO2 on the photovoltaic properties of CdS quantum dot-sensitized solar cells. J. Nanosci. Nanotechnol. 2015, 15, 721–725. [Google Scholar]
- Nozik, A.J. Quantum dot solar cells. Phys. E Low dimens. Syst. Nanostruct. 2002, 14, 115–120. [Google Scholar] [CrossRef]
- Schaller, R.D.; Sykora, M.; Pietryga, J.M.; Klimov, V.I. Seven excitons at a cost of one: Redefining the limits for conversion efficiency of photons into charge carriers. Nano Lett. 2006, 6, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, J.; Wujcik, E.K.; Qiu, B.; Rutman, D.; Zhang, X.; Salazard, E.; Wei, S.; Guo, Z. Hydrophobic Electrospun Polyimide Nanofibers for Self-cleaning Materials. Macromol. Mate. Eng. 2015, 300, 358–368. [Google Scholar] [CrossRef]
- Wujcik, E.K. Synthesis of Lead Selenide-titania Heterostructures for High-efficiency Low-cost Solar Cells. Master’s Thesis, The University of Rhode Island, Kingston, RI, USA, 2009. [Google Scholar]
- Valiquette, D.; Pellerin, C. Miscible and core-sheath PS/PVME fibers by electrospinning. Macromolecules 2011, 44, 2838–2843. [Google Scholar] [CrossRef]
- Wujcik, E.K. Discovery of Nanostructured Material Properties for Advanced Sensing Platforms. Electronic Dissertation, The University of Akron, Akron, OH, USA, 2013. [Google Scholar]
- Sharma, J.; Lizu, M.; Stewart, M.; Zygula, K.; Lu, Y.; Chauhan, R.; Yan, X.; Guo, Z.; Wujcik, E.K.; Wei, S. Multifunctional Nanofibers towards active biomedical therapeutics. Polymers 2015, 7, 186–219. [Google Scholar] [CrossRef]
- Chen, M.; Qu, H.; Zhu, J.; Luo, Z.; Khasanov, A.; Kucknoor, A.S.; Haldolaarachchige, N.; Young, D.P.; Wei, S.; Guo, Z. Magnetic electrospun fluorescent polyvinylpyrrolidone nanocomposite fibers. Polymer 2012, 53, 4501–4511. [Google Scholar] [CrossRef]
- Peng, Q.; Sun, X.-Y.; Spagnola, J.C.; Hyde, G.K.; Spontak, R.J.; Parsons, G.N. Atomic layer deposition on electrospun polymer fibers as a direct route to Al2O3 microtubes with precise wall thickness control. Nano Lett. 2007, 7, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y. Direct fabrication of composite and ceramic hollow nanofibers by electrospinning. Nano Lett. 2004, 4, 933–938. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Blasdel, N.J.; Wujcik, E.K.; Carletta, J.E.; Lee, K.-S.; Monty, C.N. Fabric nanocomposite resistance temperature detector. IEEE Sens. J. 2015, 15, 300–306. [Google Scholar] [CrossRef]
- Monty, C.; Wujcik, E.K.; Blasdel, N. Flexible Electrode for Detecting Changes in Temperature, Humidity, and Sodium Ion Concentration in Sweat. US 20130197319 A1, 1 August 2013. [Google Scholar]
- Wujcik, E.K.; Blasdel, N.J.; Trowbridge, D.; Monty, C.N. Ion sensor for the quantification of sodium in sweat samples. IEEE Sens. J. 2013, 13, 3430–3436. [Google Scholar] [CrossRef]
- Wang, H.; Oey, C.C.; Djurišić, A.B.; Xie, M.H.; Leung, Y.H.; Man, K.K.Y.; Chan, W.K.; Pandey, A.; Nunzi, J.-M.; Chui, P.C. Titania bicontinuous network structures for solar cell applications. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- Shankar, K.; Paulose, M.; Mor, G.K.; Varghese, O.K.; Grimes, C.A. A study on the spectral photoresponse and photoelectrochemical properties of flame-annealed titania nanotube-arrays. J. Phys. D Appl. Phys. 2005, 38, 3543–3549. [Google Scholar] [CrossRef]
- Du, A.; Ng, Y.H.; Bell, N.J.; Zhu, Z.; Amal, R.; Smith, S.C. Hybrid graphene/titania nanocomposite: Interface charge transfer, hole doping, and sensitization for visible light response. J. Phys. Chem. Lett. 2011, 2, 894–899. [Google Scholar] [CrossRef]
- Silipas, T.D.; Indrea, E.; Dreve, S.; Suciu, R.-C.; Rosu, M.C.; Danciu, V.; Cosoveanu, V.; Popescu, V. TiO2––Based systems for photoelectrochemical generation of solar hydrogen. J. Phys. Conf. Ser. 2009, 182. [Google Scholar] [CrossRef]
- Masuda, Y.; Ohji, T.; Kato, K. Multineedle TiO2 nanostructures, self-assembled surface coatings, and their novel properties. Cryst. Growth Des. 2010, 10, 913–922. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, W.J.; Sahoo, Y.; Cartwright, A.N.; Prasad, P.N. Multiple exciton generation and electrical extraction from a PbSe quantum dot photoconductor. Appl. Phys. Lett. 2008, 92. [Google Scholar] [CrossRef]
- Schaller, R.; Klimov, V. High efficiency carrier multiplication in pbse nanocrystals: Implications for solar energy conversion. Phys. Rev. Lett. 2004, 92. [Google Scholar] [CrossRef]
- Ellingson, R.J.; Beard, M.C.; Johnson, J.C.; Yu, P.; Micic, O.I.; Nozik, A.J.; Shabaev, A.; Efros, A.L. Highly efficient multiple exciton generation in colloidal PbSe and PbS Quantum dots. Nano Lett. 2005, 5, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Beard, M.C.; Midgett, A.G.; Law, M.; Semonin, O.E.; Ellingson, R.J.; Nozik, A.J. Variations in the Quantum efficiency of multiple exciton generation for a series of chemically treated PbSe nanocrystal films. Nano Lett. 2009, 9, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.M.; Beard, M.C.; Song, Q.; Law, M.; Ellingson, R.J.; Nozik, A.J. Multiple exciton generation in films of electronically coupled PbSe quantum dots. Nano Lett. 2007, 7, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.; Delerue, C. Role of impact ionization in multiple exciton generation in PbSe nanocrystals. Phys. Rev. B 2006, 73, 205423. [Google Scholar] [CrossRef]
- Alcoutlabi, M.; McKenna, G.B. Effects of confinement on material behaviour at the nanometre size scale. J. Phys. Condens. Matter 2005, 17, R461–R524. [Google Scholar] [CrossRef]
- Kamperman, M.; Korley, L.T.J.; Yau, B.; Johansen, K.M.; Joo, Y.L.; Wiesner, U. Nanomanufacturing of continuous composite nanofibers with confinement-induced morphologies. Polym. Chem. 2010, 1, 1001–1004. [Google Scholar] [CrossRef]
- Yu, W.W.; Falkner, J.C.; Shih, B.S.; Colvin, V.L. Preparation and characterization of monodisperse PbSe semiconductor nanocrystals in a noncoordinating solvent. Chem. Mater. 2004, 16, 3318–3322. [Google Scholar]
- Cho, K.-S.; Talapin, D.V.; Gaschler, W.; Murray, C.B. Designing PbSe nanowires and nanorings through oriented attachment of nanoparticles. J. Am. Chem. Soc. 2005, 127, 7140–7147. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.-T.; Sahoo, Y.; Choudhury, K.R.; Swihart, M.T.; Minter, J.R.; Prasad, P.N. Shape control of PbSe nanocrystals using noble metal seed particles. Nano Lett. 2006, 6, 709–714. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wujcik, E.K.; Aceto, S.R.; Heskett, D.; Bose, A. Synthesis of Co-Electrospun Lead Selenide Nanostructures within Anatase Titania Nanotubes for Advanced Photovoltaics. Fibers 2015, 3, 173-183. https://doi.org/10.3390/fib3020173
Wujcik EK, Aceto SR, Heskett D, Bose A. Synthesis of Co-Electrospun Lead Selenide Nanostructures within Anatase Titania Nanotubes for Advanced Photovoltaics. Fibers. 2015; 3(2):173-183. https://doi.org/10.3390/fib3020173
Chicago/Turabian StyleWujcik, Evan K., Stephanie R. Aceto, David Heskett, and Arijit Bose. 2015. "Synthesis of Co-Electrospun Lead Selenide Nanostructures within Anatase Titania Nanotubes for Advanced Photovoltaics" Fibers 3, no. 2: 173-183. https://doi.org/10.3390/fib3020173