Multifunctional Gelcoats for Fiber Reinforced Composites
Abstract
:1. Introduction
2. Gelcoats and Multifunctional Gelcoats
2.1. Unsaturated Polyester, Epoxy, and Epoxy-Compatible Unsaturated Polyester Gelcoats
2.1.1. Unsaturated Polyesters
- hydroperoxide initiator with a heavy metal salt promotor (usually cobalt);
- acyl peroxide initiator with an amine type promotor (usually tertiary aromatic amine);
- photoinitiator with UV radiation.
2.1.2. Epoxy Resins
- Bisphenol F epoxy resins and phenol-novolac glycidylether
- Cresol-novolac glycidylether
- Cycloaliphatic glycidyl compounds (weather resistance)
- Epoxidized cycloolefins (cycloaliphatic epoxy resins; weather resistance)
- Glycidyl compounds containing bromine
- N-glycidyl compounds of hereocycles and amines
- Aliphatic glycidylethers (reactive diluents)
- Cycloaliphatic and aromatic glycidylethers, glycidylesters (reactive diluents)
2.1.3. Other Gelcoat Resin Systems
2.2. Multifunctional Gelcoats
2.2.1. Water Resistance
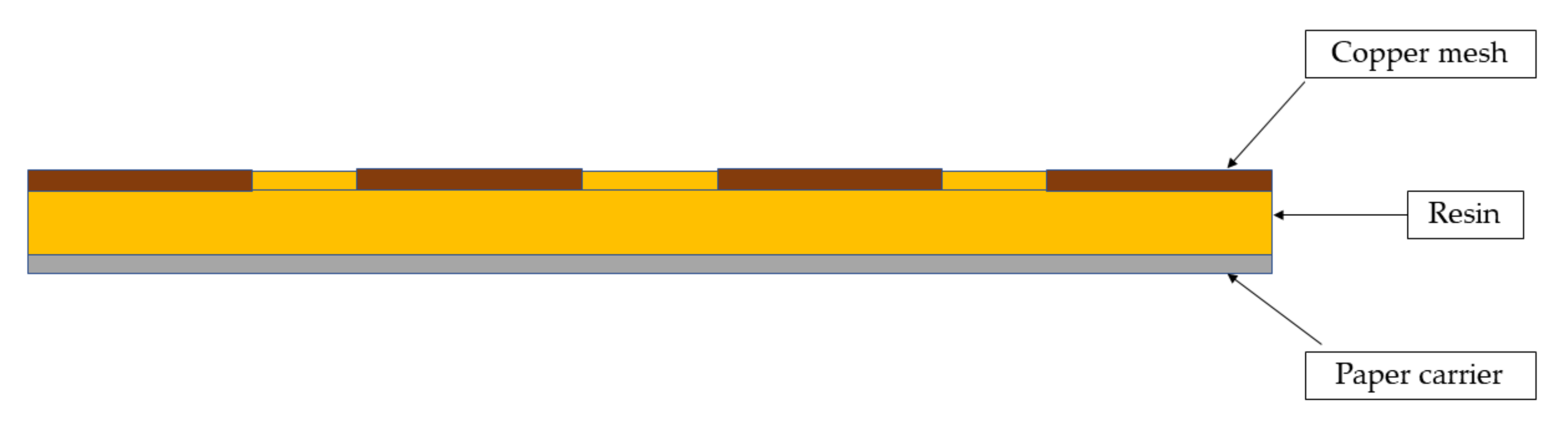
2.2.2. Electric Conductivity
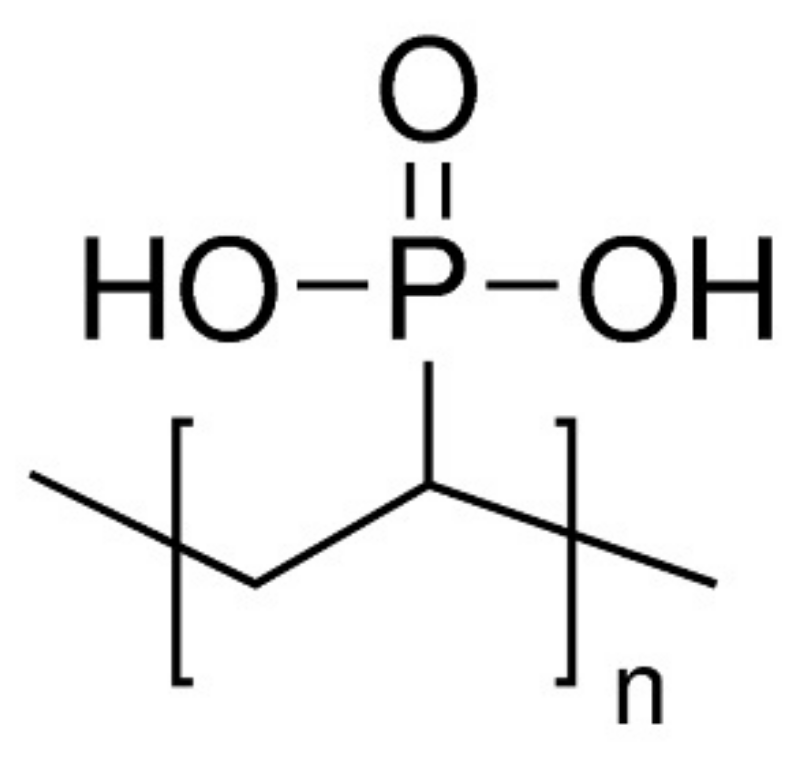
2.2.3. Flame Retardancy
3. Gelcoating Techniques and Common Defects
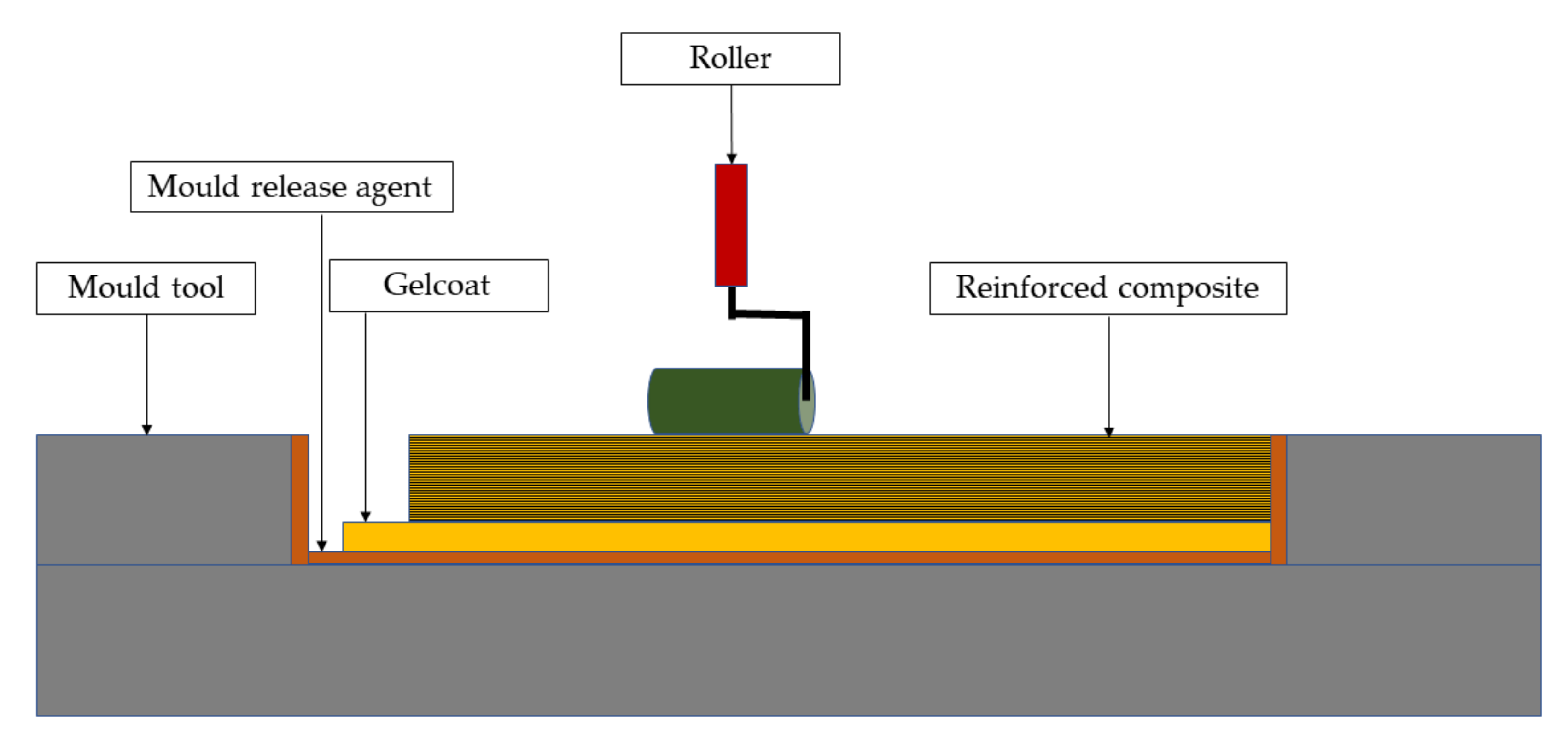
3.1. Classical Methods
3.2. Novel Methods
3.2.1. UV Curable Gelcoat Systems
3.2.2. In-Mould Gelcoating Processes
- Opening the mould a bit to create space for the injection of the gelcoat
- Using a third mould tool as the counter-face to create space
- Using a removable spacer material to define the space for the gelcoat
- Coating the fabric to minimize penetration of the gelcoat into the reinforcement
- Using a separator layer to keep the gelcoat and laminate apart
3.3. Common Defects
4. Testing Methods
4.1. Mechanical Testing Methods
4.2. Testing Methods for Multifunctional Gelcoats
5. Summary
Funding
Conflicts of Interest
References
- Laurenzi, S.; Marchetti, M. Advanced composite materials by resin transfer molding for aerospace applications. In Composites and Their Properties; Hu, N., Ed.; IntechOpen: London, UK, 2012; pp. 197–226. [Google Scholar]
- Groessing, H.; Becker, D.; Kaufmann, S.; Schledjewski, R.; Mitschang, P. An evaluation of the reproducibility of capacitive sensor based in-plane permeability measurements: A benchmarking study. Exp. Polym. Lett. 2015, 9, 129–142. [Google Scholar] [CrossRef]
- Pomázi, Á.; Toldy, A. Particle distribution of solid flame retardants in infusion moulded composites. Polymers 2017, 9, 250. [Google Scholar] [CrossRef]
- Toldy, A.; Szolnoki, B.; Marosi, G. Flame retardancy of fibre-reinforced epoxy resin composites for aerospace applications. Polym. Degrad. Stab. 2011, 96, 371–376. [Google Scholar] [CrossRef]
- Borsting, D.A.; Zhou, Q.; Van Der Zee, J.J.; Rajamani, R. Method of Applying Gelcoat and An Arrangement Performing Said Method. U.S. Patent 8,808,794 B2, 19 August 2014. [Google Scholar]
- Gascons, T.M. Assesment of Virtual Design and Manufacturing Techniques for Fibre Reinforced Composite Materials. Ph.D. Thesis, Universitat de Girona, Girona, Spain, February 2011. [Google Scholar]
- Fink, J.K. Reactive Polymers Fundamentals and Applications: A Concise Guide to Industrial Polymers; William Andrew Inc.: Norwich, NY, USA, 2006; ISBN 9780815515159. [Google Scholar]
- Stoye, D.; Werner, F. Resins for Coatings: Chemistry, Properties and Applications; Carl Hanser Verlag: Cincinnati, OH, USA, 1996; ISBN 3-446-18489-9. [Google Scholar]
- Airola, K.; Farm, O.; Mahbub, P.; Valtonen, E. Unsaturated Polyester Resin Compositions. U.S. Patent 6,617,417, 9 September 2003. [Google Scholar]
- Jones, F.R. Unsaturated polyester resins. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 743–772. [Google Scholar]
- Li, L.; Sun, X.; Lee, L.J. Low temperature cure of vinyl ester resins. Polym. Eng. Sci. 1999, 39, 646–661. [Google Scholar] [CrossRef]
- Li, L.; Lee, L.J. Effects of inhibitors and retarders on low temperature free radical crosslinking polymerization between styrene and vinyl ester resin. Polym. Eng. Sci. 2001, 41, 53–65. [Google Scholar] [CrossRef]
- Roskott, L.; Groenendaal, A.A.M. Residual styrene content—What does this mean to the polyester processor. In Proceedings of the 33rd Annual Conference of Reinforced Plastics/Composites Institute, The Society of Plastics Industry, Section 5B, Washington, DC, USA, 7–10 February 1978. [Google Scholar]
- Li, L.; Lee, L.J. Effect of a chelating agent-2,4-pentanedione on low temperature composite molding of vinyl ester and unsaturated polyester resins. Polym. Compos. 2002, 23, 971–990. [Google Scholar] [CrossRef]
- Xu, L.; Lee, L.J. Effect of nanoclay on shrinkage control of low profile unsaturated polyester (UP) resin cured at room temperature. Polymer 2004, 45, 7325–7334. [Google Scholar] [CrossRef]
- Fan, J.D.; Lee, L.J. Optimization of polyester sheet molding compound. Part II: Theoretical modelling. Polym. Compos. 1986, 7, 250–260. [Google Scholar] [CrossRef]
- Li, L.; Cao, X.; Lee, L.J. Effect of dual-initiator on low temperature curing of unsaturated polyester resins. Polymer 2004, 45, 6601–6612. [Google Scholar] [CrossRef]
- Ellis, B. Chemistry and Technology of Epoxy Resins, 1st ed.; Springer Science: Dordrecht, The Netherlands, 1993; ISBN 978-94-010-5302-0. [Google Scholar]
- Clayton, A.; May, I. Epoxy Resins, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 1988; ISBN 0-8247-7690-9. [Google Scholar]
- Konieczna, A.; Rutkowska, A.; Rachon, D. Health risk of exposure to Bisphenol A (BPA). Rocz. Panstw. Zakl. Hig. 2015, 66, 5–11. [Google Scholar] [PubMed]
- Kittel, H. Bindemittel für wasserverdünnbare Systeme. Lehrbuch der Lacke und Beschichtungen; Colomb: Berlin, Germany, 1976; Volume 3, p. 237. [Google Scholar]
- Scholz, W.; Kortmann, W. Paint additives. In Paints, Coatings and Solvents, 2nd ed.; Stoye, D., Freitag, W., Eds.; Wiley-VCH: Weinheim, Germany, 1998; pp. 159–173. ISBN 3-527-28863-5. [Google Scholar]
- Sudha, G.S.; Arun, K.V. Effect of gel coat on moisture absorption and mechanical behavior of jute-epoxy laminated composites. J. Eng. Res. Appl. 2018, 52–59. [Google Scholar]
- Firdosh, S.; Murthy, H.N.N.; Angadi, G.; Raghavendra, N. Investigation of water absorption characteristics of nano-gelcoat for marine application. Prog. Org. Coat. 2018, 114, 173–187. [Google Scholar] [CrossRef]
- Yardimci, A.I.; Tanoglu, M.; Selamet, Y. Development of electrically conductive and anisotropic gel-coat systems using CNTs. Prog. Org. Coat. 2013, 76, 963–965. [Google Scholar] [CrossRef] [Green Version]
- Powder Gelcoat Has Electrical Conductivity. Available online: https://www.materialstoday.com/composite-processing/products/powder-gelcoat-has-electrical-conductivity-/ (accessed on 2 March 2019).
- Surfacing Films Improve Safety, Appearance and Processing Time. Available online: https://www.compositesworld.com/articles/surfacing-films-improve-safety-appearance-and-processing-time (accessed on 2 March 2019).
- Jain, P.; Choudhary, V.; Varma, I.K. Flame retarding epoxies with phosphorous. J. Macromol. Sci. Part C Polym. Rev. 2002, 42, 139–183. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Thermal decomposition, combustion and flame-retardancy of epoxy resins—A review of the recent literature. Polym. Int. 2004, 53, 1901–1929. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Wagner, S.; Döring, M. Recent developments in halogen free flame retardants for epoxy resins for electrical and electronic applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef] [PubMed]
- Weil, E.D.; Levchik, S.V. Phosphorus flame retardants. In Krik-Othmer Encyclopedia of Chemical Technology; Kirk, R.E., Othmer, D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA.
- Wang, J.; Su, X.; Mao, Z. The flame retardancy and thermal property of poly(ethylene terephthalate)/cyclotriphosphazene modified montmorillonite system. Polym. Degrad. Stab. 2014, 109, 154–161. [Google Scholar] [CrossRef]
- Zhang, R.C.; Hong, S.M.; Koo, C.M. Flame retardancy and mechanical properties of polyamide 6 with melamine polyphosphate and ionic liquid surfactant-treated montmorillonite. J. Appl. Polym. Sci. 2014, 131, 40648. [Google Scholar] [CrossRef]
- Ramani, A.; Dahoe, A.E. On flame retardancy in polycaprolactam composites by aluminim diethylphosphinate and melamine polyphosphate in conjunction with organically modified montmorillonite nanoclay. Polym. Degrad. Stab. 2014, 105, 1–11. [Google Scholar] [CrossRef]
- Yu, T.; Jiang, N.; Li, Y. Functionalized multi-wall carbon nanotube for improving the flame retardancy of ramie/poly(lactic acid) composite. Compos. Sci. Technol. 2014, 104, 26–33. [Google Scholar] [CrossRef]
- Pandey, P.; Mohanty, S.; Nayak, S.K. Improved flame retardancy and thermal stability of polymer/clay nanocomposites, with the incorporation of multiwalled carbon nanotube as secondary filler: Evaluation of hybrid effect of nanofillers. High Perform. Polym. 2014, 26, 826–836. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, L.; Liang, G.; Gu, A.; Qiang, Z.; Chen, X. Unique hybridized carbon nanotubes and their high performance flame retarding composites with high smoke suppression, good toughness and low curing temperature. J. Mater. Chem. 2014, 2, 4975–4988. [Google Scholar] [CrossRef]
- Yu, B.; Shi, Y.; Yuan, B.; Qiu, S.; Xing, W.; Hu, W.; Song, L.; Lo, S.M.; Hu, Y. Enhanced thermal and flame retardant properties of flame-retardant-wrapped graphene/epoxy resin nanocomposites. J. Mater. Chem. 2015, 3, 8034–8044. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.A.; Mülhaup, R.; Shartel, B. Flame-retardancy properties of intumescent ammonium poly(phosphate) and mineral filler magnesium hydroxide in combination with graphene. Polymer 2014, 6, 2875–2895. [Google Scholar] [CrossRef]
- Ran, S.; Chen, C.; Guo, Z.; Fang, Z. Char barrier effect of graphene nanoplatelets on the flame retardancy and thermal stability of high-density polyethylene flame-retarded by brominated polystyrene. J. Appl. Sci. 2014, 131, 40520. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Y.; Shen, M.; Huang, X.; Zhu, J.; Zhang, X. Preparation and properties of polystyrene nanocomposites with graphite oxide and graphene as flame retardants. J. Mater. Sci. 2013, 48, 4214–4222. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Hammerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Huang, H.; Tian, M.; Liu, I.; Liang, W.; Zhang, I. Effect of particle size on flame retardancy of Mg(OH)2-filled ethylene vinyl acetate copolymer composites. J. Appl. Polym. Sci. 2006, 100, 4461–4469. [Google Scholar] [CrossRef]
- Du, L.; Xu, G.; Zhang, Y.; Qian, J.; Chen, J. Synthesis and properties of a novel intumescent flame retardant (IFR) and its application in halogen-free flame retardant ethylene propylene diene terpolymer (EPDM). Polym.-Plast. Technol. Eng. 2011, 50, 372–378. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Fire Safety with Specialty Coatings. Available online: http://www.tpr2.com/news/JCT-fire-suppressant-coatings.pdf (accessed on 5 March 2019).
- Qu, H.; Wu, W.; Zheng, Y.; Xie, J.; Xu, J. Synergistic effects of inorganic tin compounds and Sb2O3 on thermal properties and flame retardancy of flexible poly(vinyl chloride). Fire Saf. J. 2011, 46, 462–467. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Mouritz, A.P.; Gibson, A.G. Fire Properties of Polymer Composite Materials; Springer: Dordrecht, The Netherlands, 2006; pp. 236–284. ISBN 1-4020-5355-X. [Google Scholar]
- Knop, S.; Krieger, W. Flame retardant gelcoats on composite laminates. In Proceedings of the Composites in Fire, Newcastle upon Tyne, UK, 9–10 September 2003; pp. 29–34. [Google Scholar]
- Gelcoats, UP and Urethane Acrylic Resins, Fibers. Available online: https://www.madercomposites.com/ENG/Produkte.html (accessed on 30 January 2019).
- CCP Composites. Available online: http://www.feiplar.com.br/coberturas/2014/materiais/palestras/automotivo/CCP.pdf (accessed on 30 January 2019).
- New Technology Fire Retardant Gelcoats and Topcoats Protecting Composites from Fire. Available online: http://jp.scottbader.com/uploads/files/5496_crystic-fireguard-range-english.pdf (accessed on 30 January 2019).
- SGi 128/SD 228 Fire Resistant Epoxy Gel Coat. Available online: http://www.sicomin.com/datasheets/product-pdf1237.pdf (accessed on 30 January 2019).
- Fire Retardant (FR) Resins. Available online: https://www.ashland.com/file_source/Ashland/Product/Documents/Composites/Fire%20Retardant%20Linecard%20(2).pdf (accessed on 30 January 2019).
- Enguard™ FR Series Gelcoats Chemistry: Composites. Available online: https://www.ashland.com/industries/building-and-construction/fire-retardant-materials/enguard-fr-series-gelcoats (accessed on 30 January 2019).
- Fireblock. Available online: http://www.polynt.com/en/chemical-products/composites/gelcoats/fireblock/?product-name=FIREBLOCK%202330PAWK745 (accessed on 15 February 2019).
- Toldy, A. Flame retardancy of carbon fibre reinforced composites. Exp. Polym. Lett. 2018, 12, 186. [Google Scholar] [CrossRef]
- Luangtriratana, P. Thermal Insulation of Polymeric Composites Using Surface Treatments. Ph.D. Thesis, University of Bolton, Bolton, UK, January 2014. [Google Scholar]
- Price, D.; Pyrah, K.; Hull, T.R.; Milnes, G.J.; Ebdon, J.R.; Hunt, B.J.; Joseph, P.; Konkel, C.S. Flame retarding poly(methyl methacrylate) with phosphorous containing compounds: Comparison of an additive with a reactive approach. Polym. Degrad. Stab. 2001, 74, 441–447. [Google Scholar] [CrossRef]
- Kahraman, M.V.; Apohan, N.K.; Arsu, N.; Güngör, A. Flame retardance of epoxy acrylate resin modified with phosphorus containing compounds. Prog. Org. Coat. 2004, 51, 213–219. [Google Scholar] [CrossRef]
- Akmakc, E.C.; Mülazim, Y.; Kahraman, M.V.; Apohan, N.K. Flame retardant thiolene photocured coatings. React. Funct. Polym. 2011, 71, 36–41. [Google Scholar] [CrossRef]
- Imamoglu, T.; Yaggi, Y. Photocuring of acrylate oligomers in the presence of vinyl phosphonic acids as a flame retarding monomer and the properties of the cured films. Turk. J. Chem. 2001, 25, 1–9. [Google Scholar]
- Huang, Z.; Shi, W. Synthesis and properties of a novel hyperbranched polyphosphate acrylate applied to UV curable flame retardant coatings. Eur. Polym. J. 2007, 43, 1302–1312. [Google Scholar] [CrossRef]
- Yuharzi, M.Y.; Haeryip, S.; Muhammad Zaimi, Z.A.; Nilson, G.C.H. A review on gelcoat used in laminated composite structure. Int. J. Res. Eng. Technol. 2015, 4, 49–58. [Google Scholar] [CrossRef]
- Saltz, W.T. Bay Area 2005 Ozone Strategy. Control Measure SS-4B, BAAQMD Regulation 8, Rule 50: Polyester Resin Operations. Available online: http://www.baaqmd.gov/~/media/files/planning-and-research/rules-and-regs/workshops/0850_wsrpt_080309.pdf (accessed on 30 January 2019).
- Ratna, D. Handbook of Thermoset Resins; Smithers Rapra Technology: Akron, OH, USA, 2009; ISBN 1847354105. [Google Scholar]
- Burton, B.L. Amine curing of epoxy resins: Options and key formulation considerations. Paint Coat. Ind. 2006, 22, 68–77. [Google Scholar]
- Ren, S.-P.; Lan, Y.-X.; Zhen, Y.-Q.; Ling, Y.-D.; Lu, M.-G. Curing reaction characteristics and phase behaviors of biphenol type epoxy resins with phenol Novolac resins. Thermochim. Acta 2006, 440, 60–67. [Google Scholar] [CrossRef]
- Dodiuk, H.; Goodman, S. Handbook of Thermoset Plastics, 3rd ed.; William Andrew: Amsterdam, The Netherlands, 2013; ISBN 9781455731077. [Google Scholar]
- Cohen, G. UV/EB Market Trends. Available online: https://www.radtech.org/images/pdf_upload/UV-EBMarketTrends7.12.pdf (accessed on 5 March 2019).
- Mark, H.F. Encyclopedia of Polymer Science and Technology, 4th ed.; Wiley: London, UK, 2003; ISBN 978-1-118-63389-2. [Google Scholar]
- Boey, F.; Rath, S.K.; Ng, A.K.; Abadie, M.J.M. Cationic UV cure kinetics for multifunctional epoxies. J. Appl. Polym. Sci. 2002, 86, 518–525. [Google Scholar] [CrossRef]
- Davidson, R.S.; Holman, R.J. Developments and trends in radiation curing. Color. Technol. 2003, 33, 46–58. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-curing: Technology and applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Decker, C. UV radiation curing chemistry. Pigment Resin Technol. 2001, 30, 278–286. [Google Scholar] [CrossRef]
- Crivello, J.V. UV and electron beam.induced cationic polymerization. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1999, 151, 8–21. [Google Scholar] [CrossRef]
- Li, F.; Bao, J.; Chen, X.; Bao, H.; Wang, H. Factors influencing EB curing of epoxy matrix. Radiat. Phys. Chem. 2002, 63, 557–561. [Google Scholar] [CrossRef]
- Shi, S.; Croutxé-Barghorn, C.; Allonas, X. Photoinitiating systems for cationic photopolymerization: ongoing push toward long wavelengths and low light intensities. Prog. Polym. Sci. 2017, 65, 1–41. [Google Scholar] [CrossRef]
- ISO 21348 Definitions of Solar Irradiance Spectral Categories; ISO: Geneva, Switzerland, 2017.
- Liaw, I.I.; Boyd, I.W. The development and application of UV excimer lamps in nanofabrication. In NATO Science for Peace and Security Series B: Physics and Biophysics; Vaseashta, A., Mihailescu, I.N., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 61–76. [Google Scholar] [CrossRef]
- Gaidukovs, S.; Medvids, A.; Onufrijevs, P.; Grase, L. UV-light-induced curing of branched epoxy novolac resin for coatings. Exp. Polym. Lett. 2018, 12, 918–929. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, S.Y.; Zhang, X.L.; Zhang, T. Improving the performance of UV-curable coatings with carbon nanomaterials. Exp. Polym. Lett. 2018, 12, 628–639. [Google Scholar] [CrossRef]
- Occupational Exposure to Styrene. Available online: http://www.upresins.org/wp-content/uploads/2017/09/170731_UPR_SHG2_EN.pdf (accessed on 5 March 2019).
- Rogers, W.; Hoppins, C.; Gombos, Z.J.; Summerscales, J. In-mould gel-coating of polymer composites: a review. J. Clean. Prod. 2014, 70, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Harper, A.R. Production of Composite Mouldings. Patent WO2013132211, 2 September 2013. [Google Scholar]
- Gombos, Z.J.; Summerscales, J. In-mould Gel-Coating with A Separator Layer. Available online: https://slideplayer.com/slide/7252982/ (accessed on 5 March 2019).
- Landowski, M.; Budzik, M.; Imielinska, K. Water absorption and blistering of glass fibre-reinforced polymer marine laminates with nanoparticle modified coating. J. Comp. Mater. 2014, 48, 2805–2813. [Google Scholar] [CrossRef]
- Salit, M.S. Manufacturing techniques of tropical natural fibre composites. In Tropical Natural Fibre Composites; Springer: Singapore, 2014; pp. 103–118. [Google Scholar]
- Raghavendra, N.; Murthy, H.N.N.; Krishna, M.; Mahesh, K.R.V.; Sridhar, R.; Firdosh, S.; Angadi, G.; Sharma, S.C. Mechanical behavior of organo-modified Indian bentonite nanoclay fibre-reinforced plastic nanocomposites. Fron. Mater. Sci. 2013, 7, 396–404. [Google Scholar] [CrossRef]
- Gombos, Z.J.; Summerscales, J. In-mould gel-coating for polymer composites. Compos. Part A 2016, 91, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Plessis, H.D. Fiberglass Boats: Construction, Gel Coat, Stressing, Blistering, Repair, Maintenance, 5th ed.; Adlard Coles Nautical: London, UK, 2010; pp. 160–162. ISBN 978-1-4081-2274-7. [Google Scholar]
- Lubin, G.E. Handbook of Composites, 1st ed.; Van Nostrand Reinhold Company Inc.: New York, NY, USA, 1982; ISBN 978-1-4615-7141-4. [Google Scholar]
- Ashland Gelcoat Felhasználási Útmutató. Available online: http://www.novia.hu/downloads/download.php?file=../admin_upload/felh_tanacsok_item_docs/18_Gelcoatguide201009HUN.pdf (accessed on 30 January 2019).
- Gelcoat Application Guide. Available online: https://www.ashland.com/file_source/Ashland/links/Gelcoat%20Application%20Guide.pdf (accessed on 30 January 2019).
- Mechanical Properties. Available online: http://www.pra-world.com/technical_services/laboratory/testing/mechanical#iso4624 (accessed on 8 October 2013).
- BS EN ISO 2409:2013 Paints and Varnishes. Cross-Cut Test; International Organization for Standardization: Geneva, Switzerland, 2013.
- BS 3900-E6 Paint and Varnishes. Cross-Cut Test; British Standard Institution: London, UK, 1974.
- ASTM D3359-02 Standard Test Methods for Measuring Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2002.
- BS EN 24624:1993 Paints and Varnishes. Pull-off Test; British Standard Institution: London, UK, 1993.
- EN ISO 4624:2002 Paints and Varnishes—Pull-Off Test for Adhesion; International Organization for Standardization: Geneva, Switzerland, 2002.
- ASTM D4541-95E1 Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers; ASTM International: West Conshohocken, PA, USA, 2002.
- BS 5350: Part C14:1979 Methods of Test for Adhesives. Adhesively Bonded Joints: Mechanical Tests. 90° Peel Test for A Rigid-to-Rigid Assembly; British Standard Institution: London, UK, 1979.
- Storm, B.K. Surface protection and coatings for wind turbine rotor blades. In Advances in Wind Turbine Blade Design and Materials; Brøndsted, P., Nijssen, R., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2013; pp. 387–412. [Google Scholar] [CrossRef]
- DS/EN ISO 2813 Paints and Varnishes—Determination of Specular Gloss of Non-Metallic Paint Films at 20 °C, 60 °C and 85 °C; International Organization for Standardization: Geneva, Switzerland, 2000.
- Norsok Standard M-501: Surface Preparation and Protective Coating; Norwegian Petroleum Industry: Lysaker, Norway, 2004.
- ISO 12944-2:2017 Paints and Varnishes–Corrosion Protection of Steel Structures by Protective Paint Systems–Part 2: Classification of Environments; International Organization for Standardization: Geneva, Switzerland, 2017.
- ASTM E-84-18b Standard Test Method for Surface Burning Characteristics of Building Materials; ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM E119-18ce01 Standard Test Methods for Fire Tests of Building Construction and Materials; ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM E1529-16e1 Standard Test Methods for Determining Effects of Large Hydrocarbon Pool Fires on Structural Members and Assemblies; ASTM International: Conshohocken, PA, USA, 2016.
- Weil, E.D. Fire-protective and flame-retardant coatings—A State-of-the-Art Review. J. Fire Sci. 2011, 29, 259–296. [Google Scholar] [CrossRef]
- Jimenez, M.; Duquesne, S.; Bourbigot, S. High-throughput fire testing for intumescent coatings. Ind. Eng. Chem. Res. 2006, 45, 7475–7481. [Google Scholar] [CrossRef]
- Ataei, S.; Khorasani, S.N.; Neisiany, R.E. Biofriendly vegetable oil healing agents used for developing self-healing coatings: A review. Prog. Org. Coat. 2019, 129, 77–95. [Google Scholar] [CrossRef]


| Epoxy System | Typical Application |
|---|---|
| Two-component systems (curing at room temperature) | Primers with high adhesion; water and chemically resistant coating; internal protective lacquers |
| Air-drying paints | Primers of all types that dry in air or an oven |
| Systems containing a solvent | Non-pigmented, highly chemically resistant anticorrosion paints |
| Powder coatings | Automobile accessories, tubes, window profiles, construction equipment, agricultural machinery |
| Single-component system (curing at room temperature via UV/electron beam) | Coating of chipboards, hardboard, furnishing veneer, plastic and paper |
| Brand Name | Producer | Matrix | Flame Retardant | Mode of Application | Area of Use |
|---|---|---|---|---|---|
| Nuvopol, Giralithe, Nuvochryl [51] | Mäder Group | UP, urethane acrylate | halogen-free | brushing, spraying | railway, marine |
| FB 2220 [52] | CCP Composites | n.a. | halogen-free, antimone-free | brushing, spraying | automotive, aircraft, construction |
| FB 2330 [52] | CCP Composites | n.a. | halogen-free, antimone-free | brushing, spraying | automotive, aircraft, construction |
| Crystic Fireguard series [53] | Scott Bader | UP | halogen-free | brushing, spraying | automotive, railway, marine, construction |
| SGi 128/SD 228 [54] | Sicomin | EP | halogen-free | brushing | railway, construction |
| Hetron FR 1540 [55] | Ashland | UP | brominated | brushing, pultrusion | automotive, railway, construction |
| Enguard FR series [56] | Ashland | n.a. | halogenated, mineral fillers | brushing, spraying, RTM | automotive, railway, construction |
| FIREBLOCK 2330PAWK745 [57] | Polynt Composites | UP | halogen-free | spraying | automotive, railway, construction |
| Defect | Failure | Cause | Solution |
|---|---|---|---|
 | sagging | improper spray technique too thick gelcoat low viscosity vibration of the mould slow curing | proper parameter settings good compound selection spraying according to technical service recommendations |
 | wrinkles, alligatoring | too thin gelcoat low curing low temperature long gelation times too much/not enough catalyst | optimal parameter settings accurate material composition |
 | cracking | too thick gelcoat incorrect demolding weak adhesion | proper demoulding check resin selection for compatibility |
 | blistering | unreacted/undispersed catalyst undercured gelcoat entrapped air/solvent/water contaminations | clean equipments proper mixing ratio |
 | fisheyes | dust/dirt/other contaminations too thin gelcoat unreacted/undispersed catalyst | clean equipments painting the surface |
 | chalking | inappropriate catalyst level unreacted catalyst undercured gelcoat low mould temperature insufficient buffing of the tool improper material selection | wiping with solvents proper mixing ratio optimal parameter settings |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomázi, Á.; Toldy, A. Multifunctional Gelcoats for Fiber Reinforced Composites. Coatings 2019, 9, 173. https://doi.org/10.3390/coatings9030173
Pomázi Á, Toldy A. Multifunctional Gelcoats for Fiber Reinforced Composites. Coatings. 2019; 9(3):173. https://doi.org/10.3390/coatings9030173
Chicago/Turabian StylePomázi, Ákos, and Andrea Toldy. 2019. "Multifunctional Gelcoats for Fiber Reinforced Composites" Coatings 9, no. 3: 173. https://doi.org/10.3390/coatings9030173






