Experimental and Modeling Studies of Bond Coat Species Effect on Microstructure Evolution in EB-PVD Thermal Barrier Coatings in Cyclic Thermal Environments
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Coating Materials and Sample Preparation
2.2. Characterizations
2.3. Cyclic Furnace Thermal Fatigue, Flame Thermal Fatigue, and Thermal Shock Tests
3. Model Details
3.1. Finite Element Model
3.2. Properties of Materials Used in the FE Model
3.3. Boundary Conditions
4. Results and Discussion
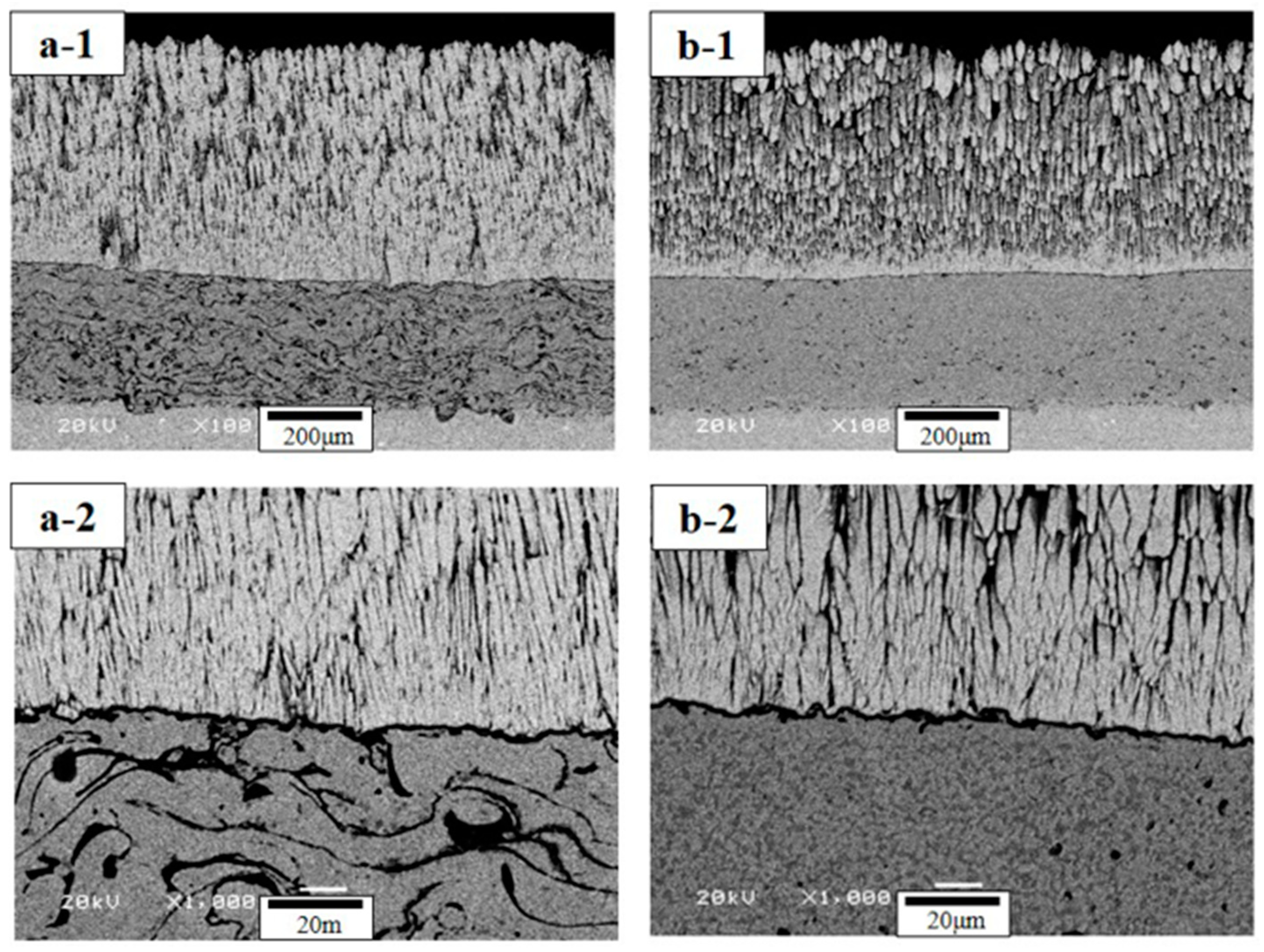
4.1. The Microstructure Analysis
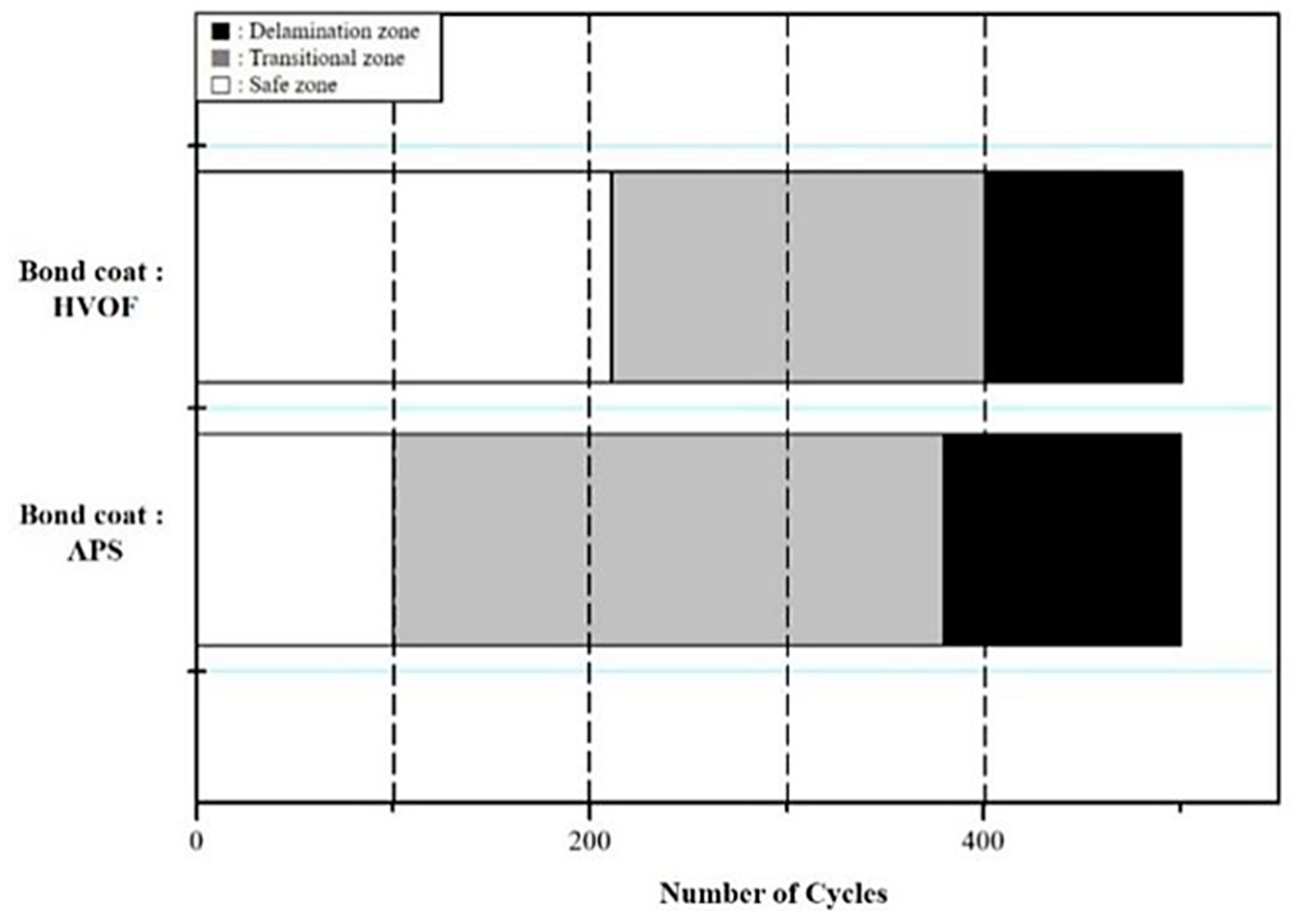
4.2. Service Life of TBC Systems
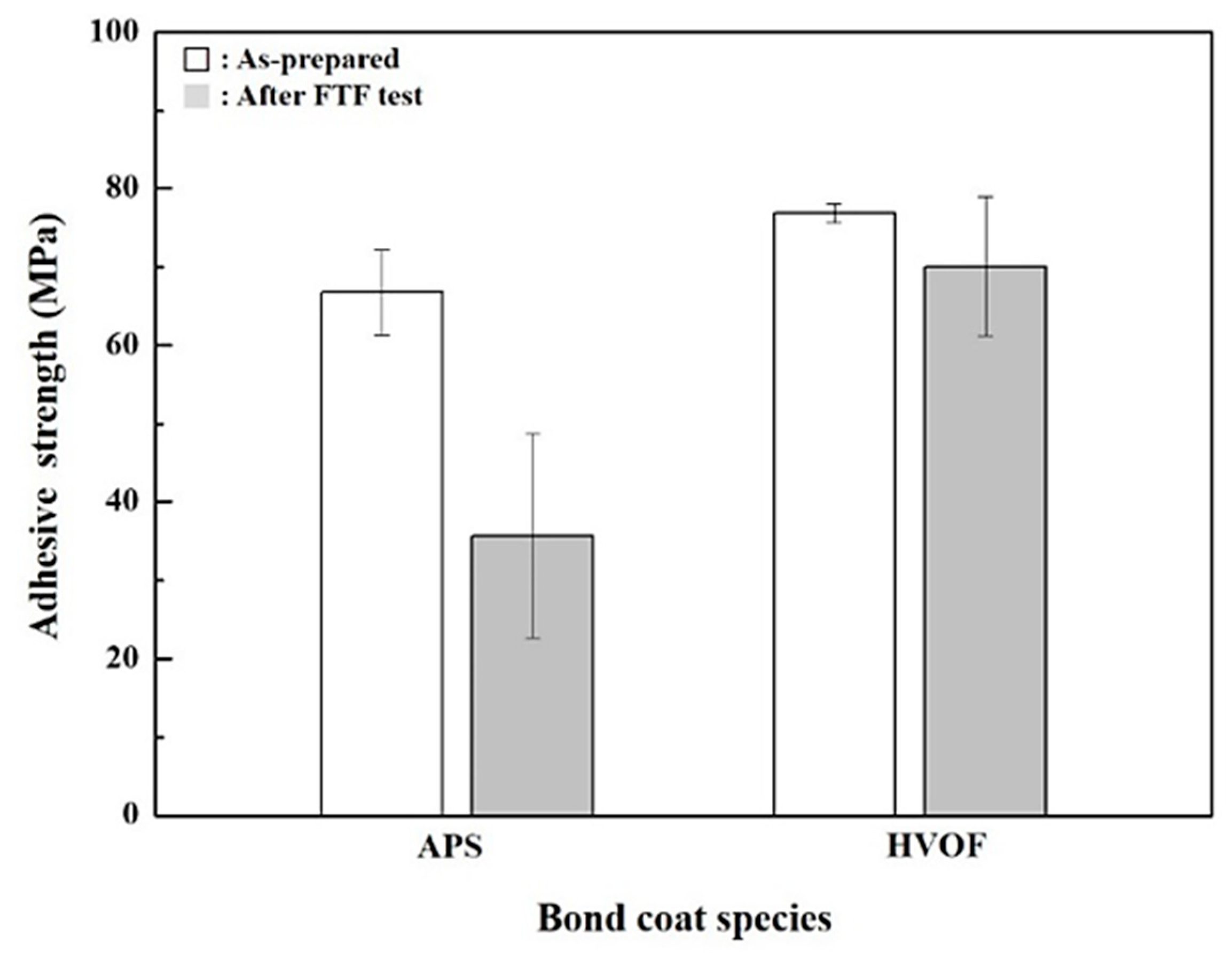
4.3. Mechanical Properties
4.4. Simulated Temperature Evolution
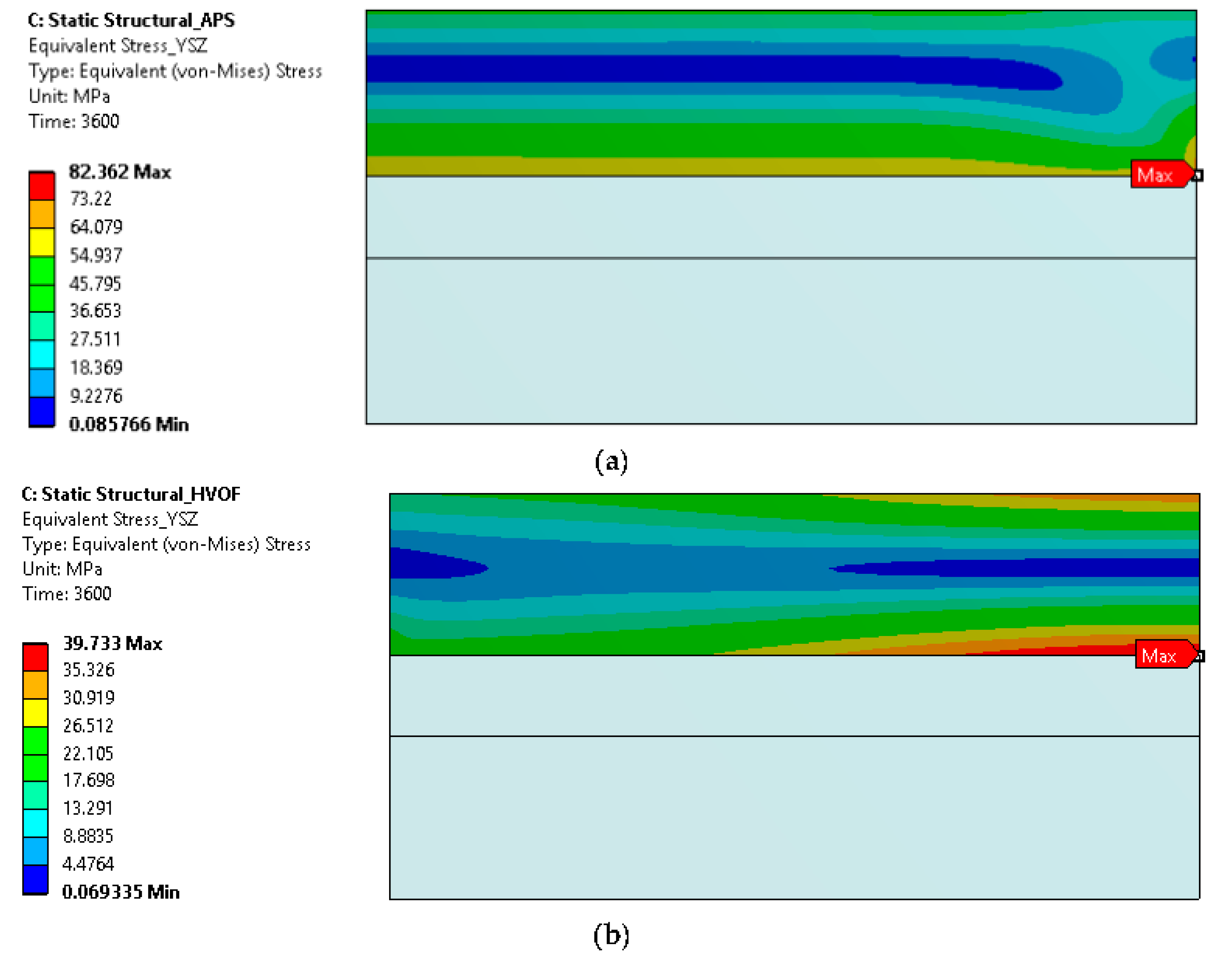
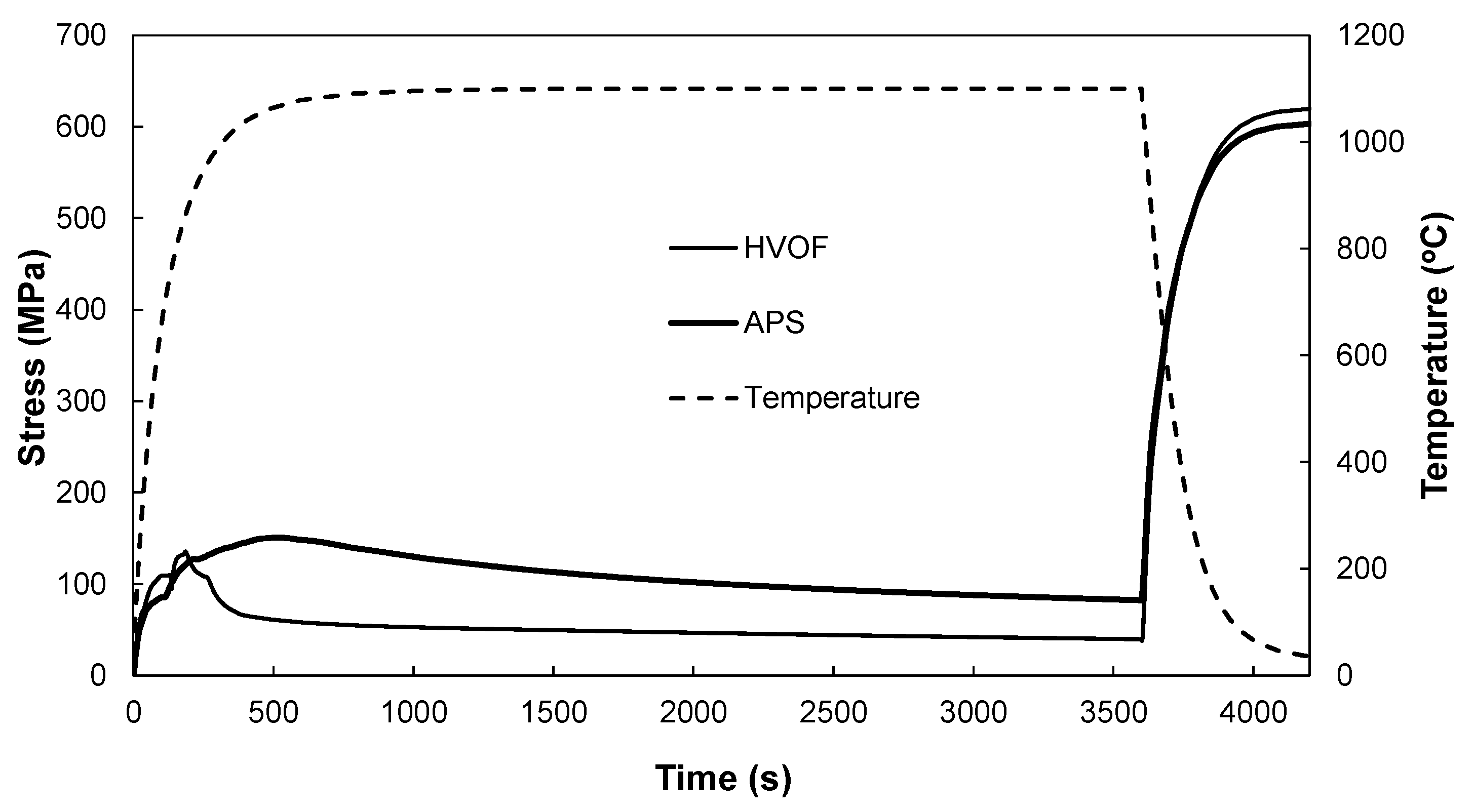
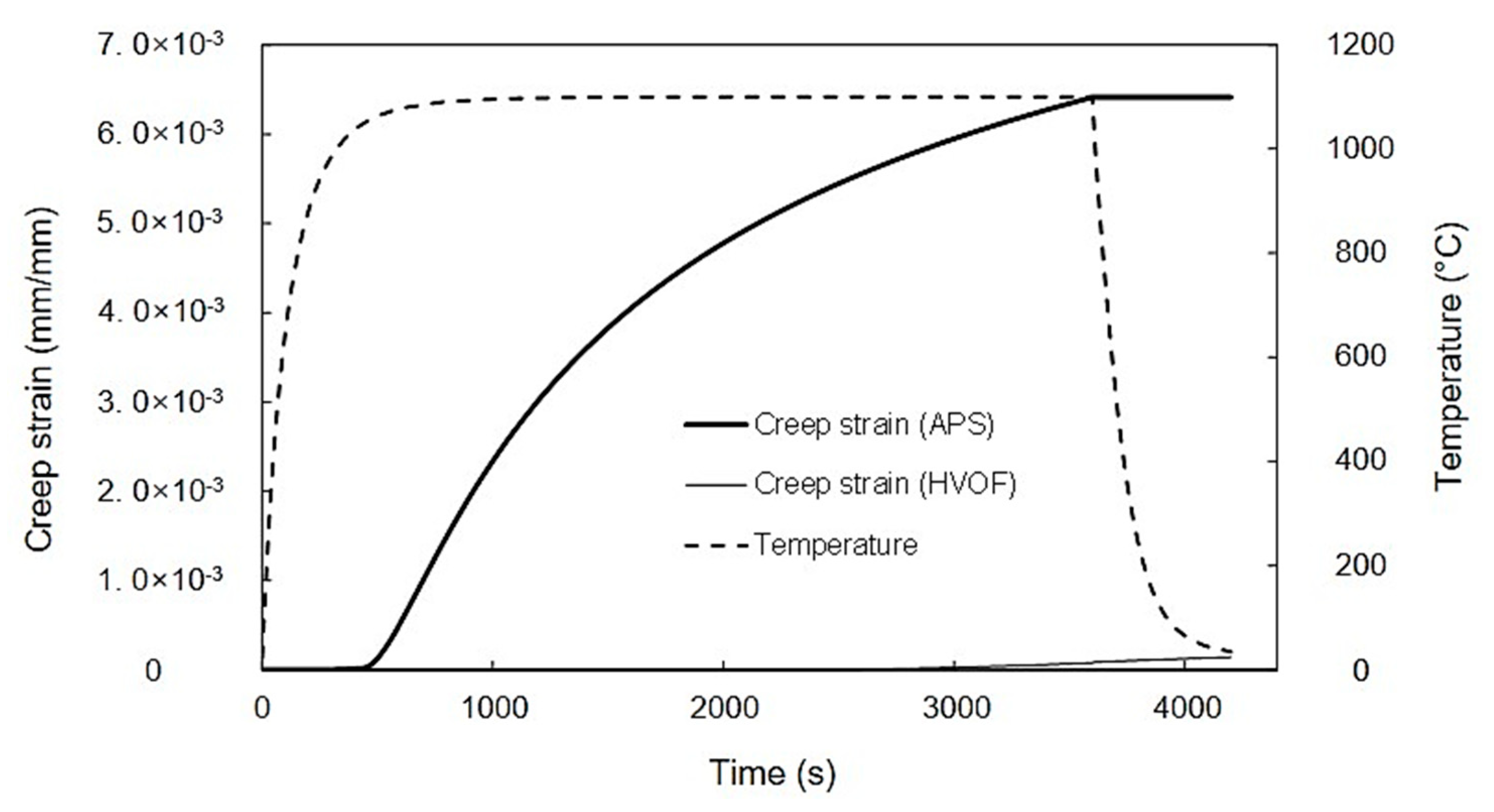
4.5. Simulated Stress and Creep Strain Evolutions
5. Conclusions
- After FTF tests, the TBC samples show a sound condition without cracking or delamination, independent of bond coat species. The interface microstructures show a thin TGO layer of 1–2 μm in thickness. The TGO layer is not fully developed in FTF tests, owing to the relatively short thermal exposure time.
- After FTF tests for 1429 cycles, the hardness values of top coats are slightly increased. The adhesive strength values are reduced, with a higher value for the TBCs with the HVOF bond coat than the APS ones.
- In CFTF tests, the TBCs with the APS and HVOF bond coats are delaminated in the range of 100–380 and 210–390 cycles, respectively.
- In TS tests, the TBCs with the bond coats prepared using the APS and HVOF processes are fully delaminated after 44–80 and 345–372 cycles, respectively, suggesting that HVOF bond coat is more effective in improving thermal durability than APS.
- The FE model simulation predicts a lower equivalent stress at the interface of the top coat and bond coat interface in TBCs prepared using the HVOF process compared with APS, suggesting a longer cyclic life of the coating with the HVOF bond coat, which is consistent with the experimental observations.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeMasi-Marcin, J.T.; Gupta, D.K. Protective coatings in the gas turbine engine. Surf. Coat. Technol. 1994, 68, 1–9. [Google Scholar] [CrossRef]
- Stecura, S. NASA Technical Memorandum; NASA Lewis Research Center: Cleveland, OH, USA, 1985. [Google Scholar]
- Strangman, T.E. Thermal barrier coatings for turbine airfoils. Thin Solid Films 1985, 127, 93–106. [Google Scholar] [CrossRef]
- Meier, S.M.; Gupta, D.K.; Sheffler, K.D. Ceramic thermal barrier coatings for commercial gas turbine engines. JOM 1991, 43, 50–53. [Google Scholar] [CrossRef]
- Łatka, L. Thermal barrier coatings manufactured by suspension plasma spraying—A review. Adv. Mater. Sci. 2018, 18, 95–117. [Google Scholar] [CrossRef]
- Sokołowski, P.; Nylen, P.; Musalek, R.; Łatka, L.; Kozerski, S.; Dietrich, D.; Lampke, T.; Pawłowski, L. The microstructural studies of suspension plasma sprayed zirconia coatings with the use of high-energy plasma torches. Surf. Coat. Technol. 2017, 318, 250–261. [Google Scholar] [CrossRef]
- Shillington, E.; Clarke, D. Spalling failure of a thermal barrier coating associated with aluminum depletion in the bond-coat. Acta Mater. 1999, 47, 1297–1305. [Google Scholar] [CrossRef]
- Rabiei, A. Failure mechanisms associated with the thermally grown oxide in plasma-sprayed thermal barrier coatings. Acta Mater. 2000, 48, 3963–3976. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, J.; Shao, F.; Zhong, X.; Zhao, H.; Zhuang, Y.; Ni, J.; Tao, S. Thermal stability of YSZ coatings deposited by plasma spray–physical vapor deposition. Coatings 2019, 9, 464. [Google Scholar] [CrossRef]
- Miller, R.A.; Lowell, C.E. Failure mechanisms of thermal barrier coatings exposed to elevated temperatures. Thin Solid Films 1982, 95, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Evans, A.; Mumm, D.; Hutchinson, J.; Meier, G.; Pettit, F. Mechanisms controlling the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 505–553. [Google Scholar] [CrossRef]
- Vaßen, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stöver, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Vassen, R.; Stuke, A.; Stöver, D. Recent developments in the field of thermal barrier coatings. J. Therm. Spray Technol. 2009, 18, 181–186. [Google Scholar] [CrossRef]
- Sidhu, T.S.; Prakash, S.; Agrawal, R.D. Investigations on role of HVOF sprayed Co and Ni based coatings to combat hot corrosion. Corros. Eng. Sci. Technol. 2008, 43, 335–342. [Google Scholar] [CrossRef]
- Jung, S.-H.; Jeon, S.-H.; Lee, J.-H.; Jung, Y.-G.; Kim, I.-S.; Choi, B.-G. Effects of composition, structure design, and coating thickness of thermal barrier coatings on thermal barrier performance. J. Korean Ceram. Soc. 2016, 53, 689–699. [Google Scholar] [CrossRef]
- Young Seok, S.; Jung, S.I.; Kwon, J.Y.; Lee, J.H.; Jung, J.G.; Paik, U.Y. Fracture behavior of plasma-sprayed thermal barrier coatings with different bond coats upon cyclic thermal exposure. Mater. Sci. Forum 2009, 620, 343–346. [Google Scholar]
- Lawn, B. Fracture of Brittle Solids; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- ASTM Standards C633 Standard Test Method for Adhesion or Cohesion Strength of Thermal Spray Coatings; American Society of Testing and Materials: Philadelphia, PA, USA, 2001.
- Tsantrizes, P.G.; Kim, G.G.; Brezinski, T.A. Thermal barrier coatings. In Proceedings of the AGARD Smp Meeting, Aalborg, Denmark, 15–16 October 1997. [Google Scholar]
- Knight, R.; Zhangxiong, D.; Kim, E.H.; Smith, R.W.; Sahoo, P.; Bucci, D. Influence of bond coat surface characteristics on the performance of Tbc systems. In Proceedings of the 15th International Thermal Spray Conference, ASM Thermal Spray Society, Nice, France, 25–29 May 1998. [Google Scholar]
- Chwa, S.O.; Ohmori, A. Microstructures of ZrO2–8wt.%Y2O3 coatings prepared by a plasma laser hybrid spraying technique. Surf. Coat. Technol. 2002, 153, 304–312. [Google Scholar] [CrossRef]
- Ma, X.; Takemoto, M. Quantitative acoustic emission analysis of plasma sprayed thermal barrier coatings subjected to thermal shock tests. Mater. Sci. Eng. A 2001, 308, 101–110. [Google Scholar] [CrossRef]
- Bednarz, P. Finite Element Simulation of Stress Evolution in Thermal Barrier Coating Systems; Forschungszentrum, Zentralbibliothek: Julich, Germany, 2006. [Google Scholar]
- Chen, H.; Hyde, T.H.; Voisey, K.T.; McCartney, D.G. Application of small punch creep testing to a thermally sprayed CoNiCrAlY bond coat. Mater. Sci. Eng. A 2013, 585, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Miller, R.A. Determination of creep behavior of thermal barrier coatings under laser imposed high thermal and stress gradient conditions. J. Mater. Res. 1999, 14, 146–161. [Google Scholar] [CrossRef]
- Wang, L.; Zhong, X.H.; Zhao, Y.X.; Tao, S.Y.; Zhang, W.; Wang, Y.; Sun, X.G. Design and optimization of coating structure for the thermal barrier coatings fabricated by atmospheric plasma spraying via finite element method. J. Asian Ceram. Soc. 2014, 2, 102–116. [Google Scholar] [CrossRef] [Green Version]
- Chang, G.C.; Phucharoen, W.; Miller, R.A. Behavior of thermal barrier coatings for advanced gas turbine blades. Surf. Coat. Technol. 1987, 30, 13–28. [Google Scholar] [CrossRef]
- Cui, Q.; Seo, S.-M.; Yoo, Y.-S.; Lu, Z.; Myoung, S.-W.; Jung, Y.-G.; Paik, U. Thermal durability of thermal barrier coatings with bond coat composition in cyclic thermal exposure. Surf. Coat. Technol. 2015, 284, 69–74. [Google Scholar] [CrossRef]








| Material | Temperature (°C) | A | n |
|---|---|---|---|
| YSZ | 750 | 2.00 × 10−22 | 4.5 |
| 850 | 2.00 × 10−20 | 4.32 | |
| 950 | 3.00 × 10−18 | 4.15 | |
| 1050 | 3.77 × 10−16 | 3.98 | |
| 1150 | 4.80 × 10−14 | 3.8 | |
| NiCrCoAlY (HVOF) | 750 | 1.25 × 10−14 | 4.5 |
| 850 | 1.40 × 10−11 | 3.8 | |
| 950 | 2.30 × 10−22 | 3.1 | |
| 1050 | 9.50 × 10−8 | 2.55 | |
| NiCrCoAlY (APS) | 750 | 6.00 × 10−20 | 7.9 |
| Inconel 718 | 10 | 4.85 × 10−36 | 1.0 |
| 1200 | 2.25 × 10−9 | 3.0 |
| Material | Temperature, T (°C) | Young’s Modulus, E (GPa) | Coefficient of Thermal Expansion, α (10–6/°C) | Poisson’s Ratio, ν | Thermal Conductivity, k (W/(m·°C)) | Specific Heat, C (J/(kg·°C)) |
|---|---|---|---|---|---|---|
| YSZ | 25 | 53 | 7.2 | 0.25 | 1.5 | 500 |
| 400 | 52 | 9.4 | 0.25 | 1.2 | 576 | |
| 800 | 46 | 16 | 0.25 | 1.2 | 637 | |
| NiCoCrAlY | 25 | 225 | 14 | 0.3 | 4.3 | 501 |
| 400 | 186 | 24 | 0.3 | 6.4 | 592 | |
| 800 | 147 | 47 | 0.3 | 10.2 | 781 | |
| Inconel 718 | 25 | 205 | 11.8 | 0.321 | 14.7 | 480 |
| 400 | 175.5 | 14.1 | 0.339 | 18.3 | 493.9 | |
| 500 | 168.5 | 14.4 | 0.344 | 19.6 | 514.8 | |
| 650 | 142 | 15.1 | 0.361 | 22 | 556.2 | |
| 750 | 130.5 | 16.2 | 0.381 | 23.2 | 594.35 |
| Specimen Species | TBC with APS Bond Coat | TBC with HVOF Bond Coat |
|---|---|---|
| Cyclic furnace thermal fatigue (CFTF) | 100–380 cycles | 210–390 cycles |
| Flame thermal fatigue (FTF) | 1429 cycles | 1429 cycles |
| Thermal shock (TS) | 44–80 cycles | 345–372 cycles |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Lyu, G.; Gulhane, A.; Park, H.-M.; Kim, J.S.; Jung, Y.-G.; Zhang, J. Experimental and Modeling Studies of Bond Coat Species Effect on Microstructure Evolution in EB-PVD Thermal Barrier Coatings in Cyclic Thermal Environments. Coatings 2019, 9, 626. https://doi.org/10.3390/coatings9100626
Lu Z, Lyu G, Gulhane A, Park H-M, Kim JS, Jung Y-G, Zhang J. Experimental and Modeling Studies of Bond Coat Species Effect on Microstructure Evolution in EB-PVD Thermal Barrier Coatings in Cyclic Thermal Environments. Coatings. 2019; 9(10):626. https://doi.org/10.3390/coatings9100626
Chicago/Turabian StyleLu, Zhe, Guanlin Lyu, Abhilash Gulhane, Hyeon-Myeong Park, Jun Seong Kim, Yeon-Gil Jung, and Jing Zhang. 2019. "Experimental and Modeling Studies of Bond Coat Species Effect on Microstructure Evolution in EB-PVD Thermal Barrier Coatings in Cyclic Thermal Environments" Coatings 9, no. 10: 626. https://doi.org/10.3390/coatings9100626





