On the Activity Enhancing Role of Iron Oxide for Noble Metal Oxidation Catalysts: A CVD-Based Study with Differently Structured Combinations of Pt and FeOx Coatings on Al2O3
Abstract
:1. Introduction
2. Materials and Methods
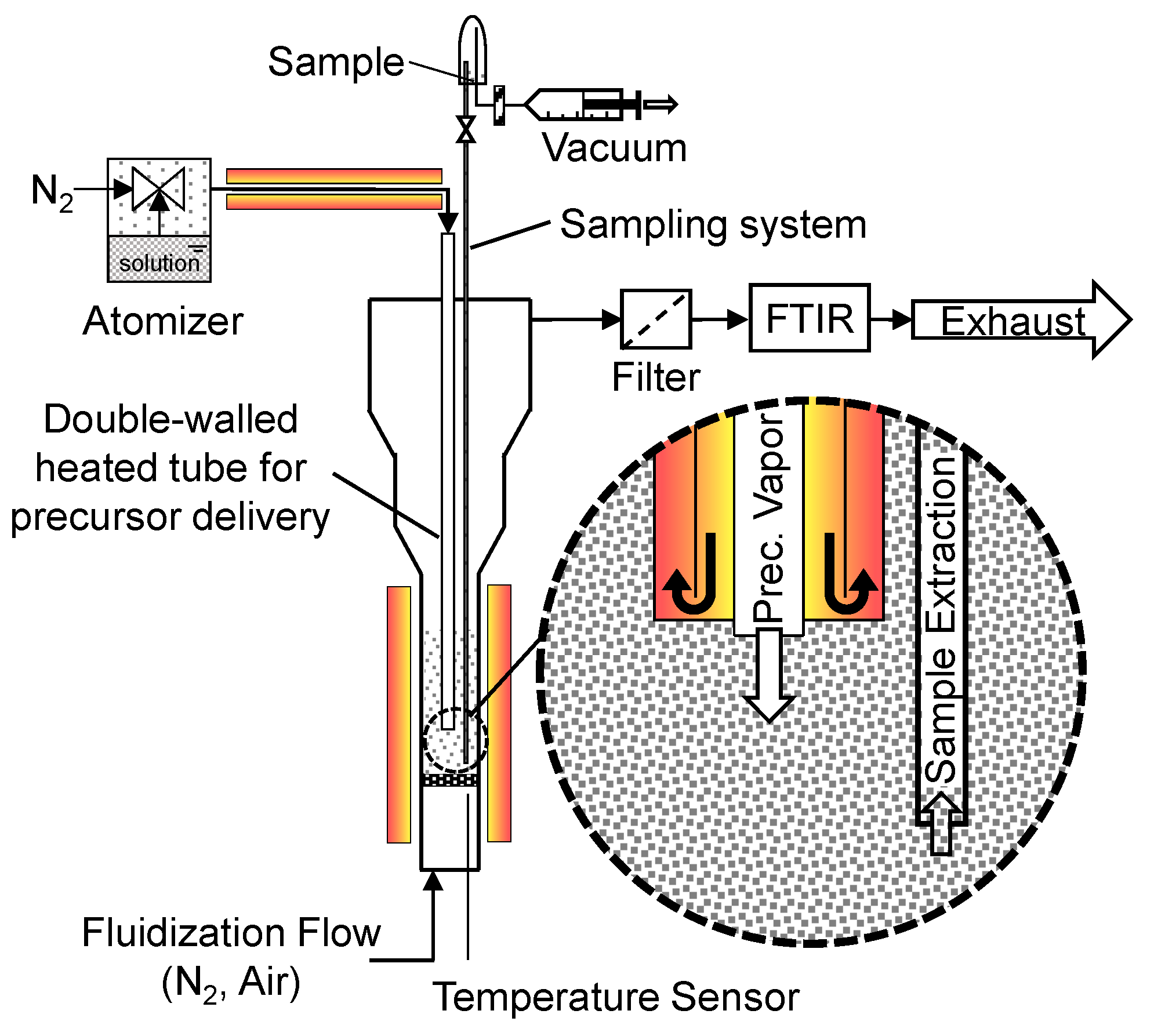
2.1. Apparatus for Production of Catalysts by Fluidized Bed Chemical Vapor Deposition (CVD)
2.2. CVD Precursors
2.3. Catalyst Characterization Methods
2.4. Al2O3 Substrate Material and its Characterization
3. Results and Discussion
3.1. CVD Coatings Routes
- Coating with FeOx by dosing EF at 300 °C in air
- Dosage of MeCpPtMe3 to the FeOx/Al2O3 particles in N2 at room temperature
- Decomposition of adsorbed MeCpPtMe3 at 300 °C in N2 for about 30 min, followed by exposure to air to ensure complete decomposition of potentially remaining precursor ligands
- 4.
- Dosage of MeCpPtMe3 dissolved in EF (no additional solvents) at 300 °C in N2, followed by air for complete decomposition of potentially remaining precursor ligands
- 5.
- Dosage of MeCpPtMe3 in N2 at room temperature
- 6.
- Dosage of EF in N2 at 300 °C, leading simultaneously also to decomposition of MeCpPtMe3 in N2, followed by air for complete decomposition of potentially remaining precursor ligands
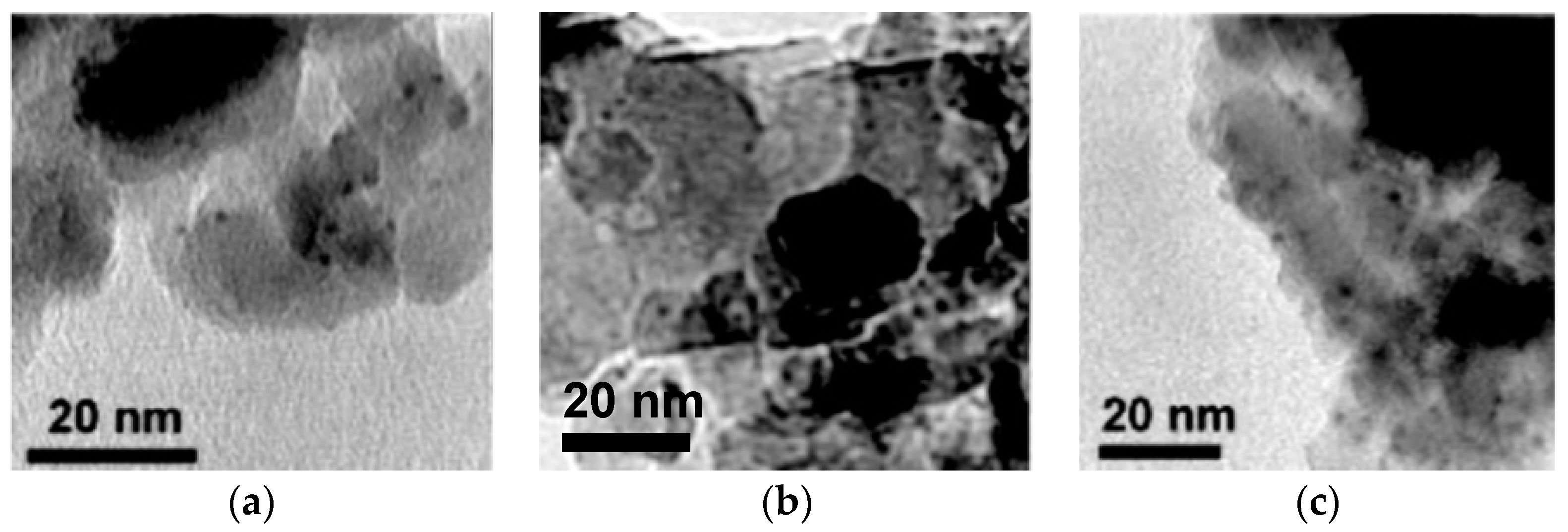
3.2. Comparison of Routes with Regard to Morphology, FeOx/Pt Interfacial Area and Catalytic Activity
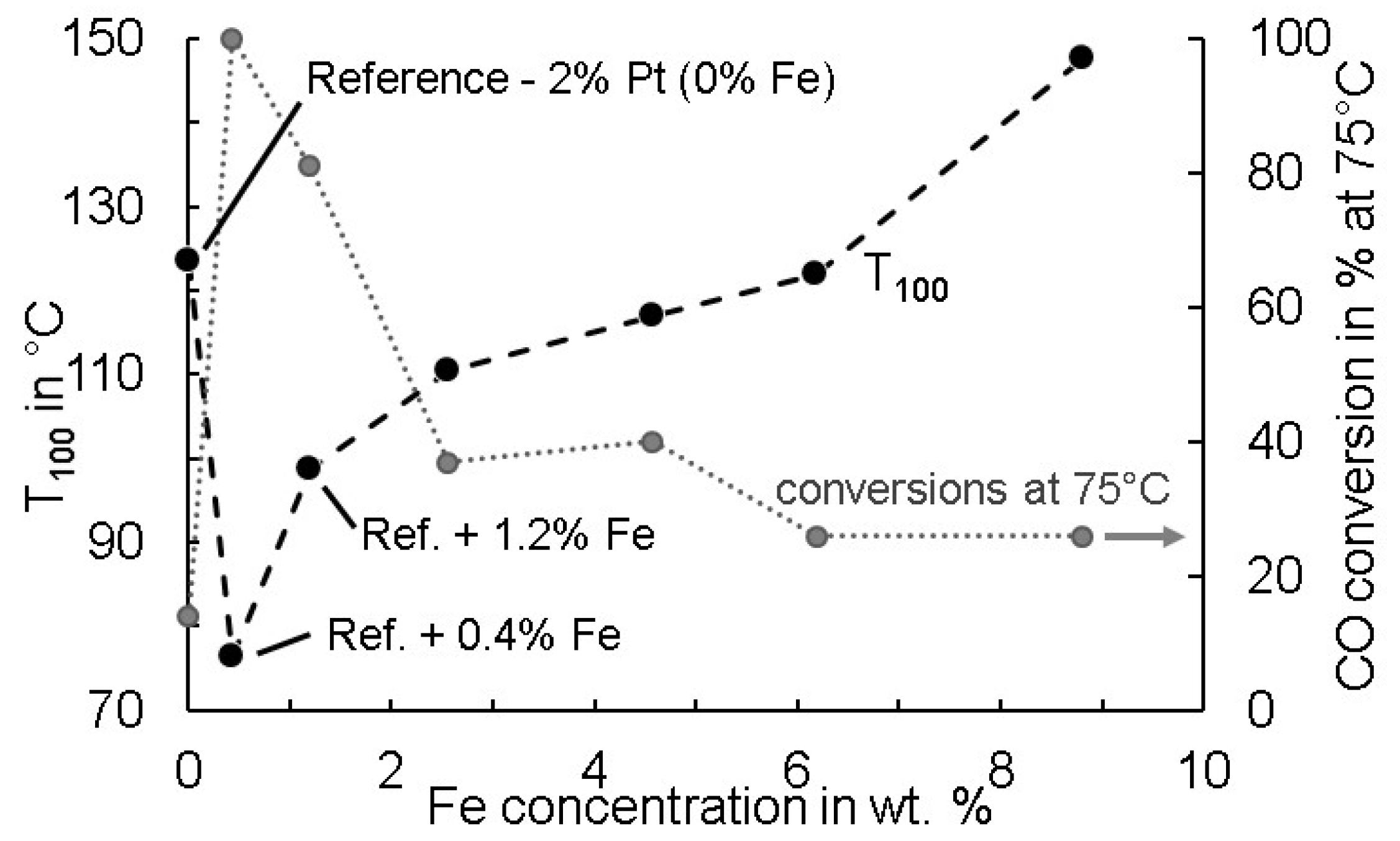
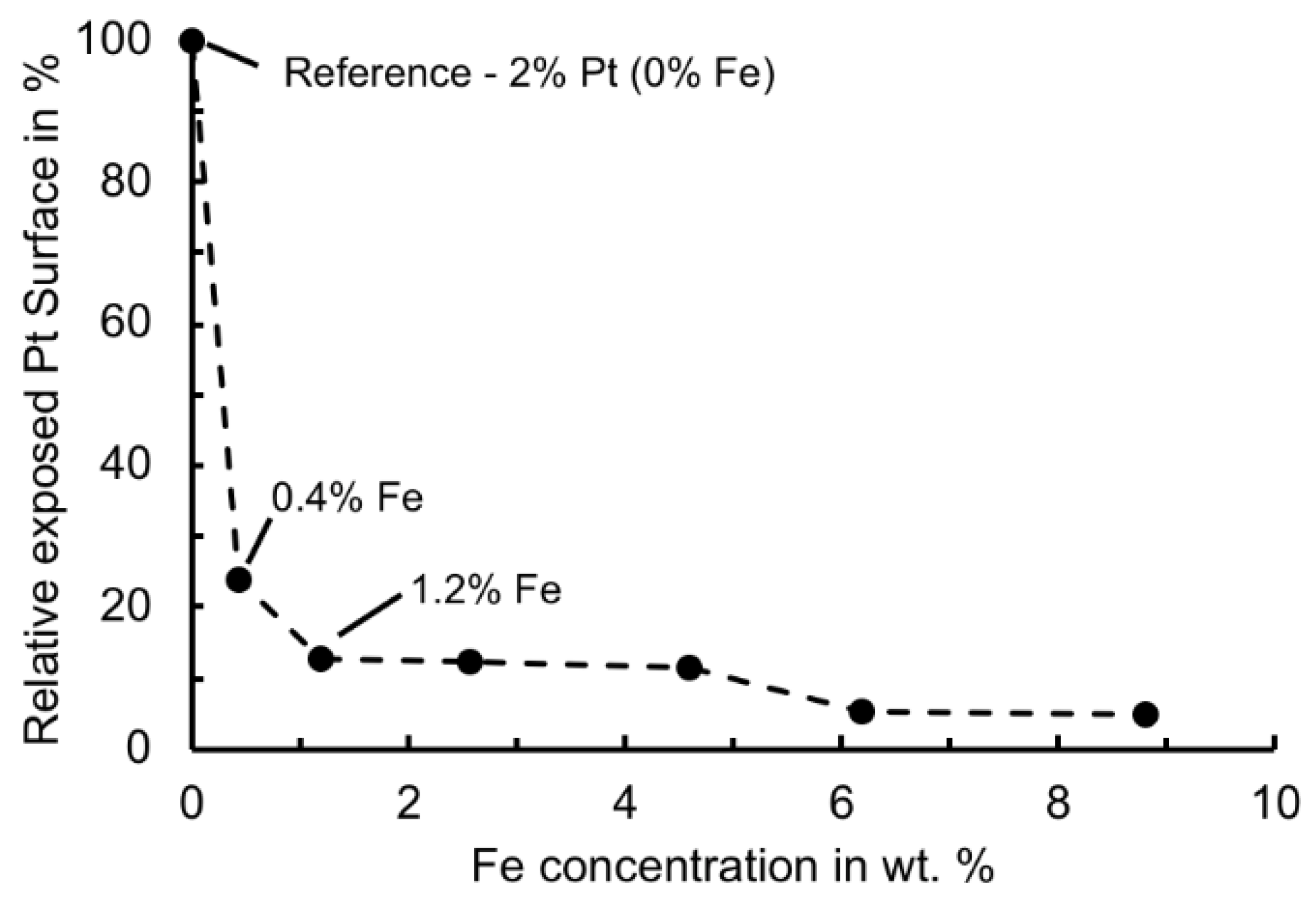
3.3. Identification of an Optimun FeOx–Pt Ratio with Regard to Catalyst Activity
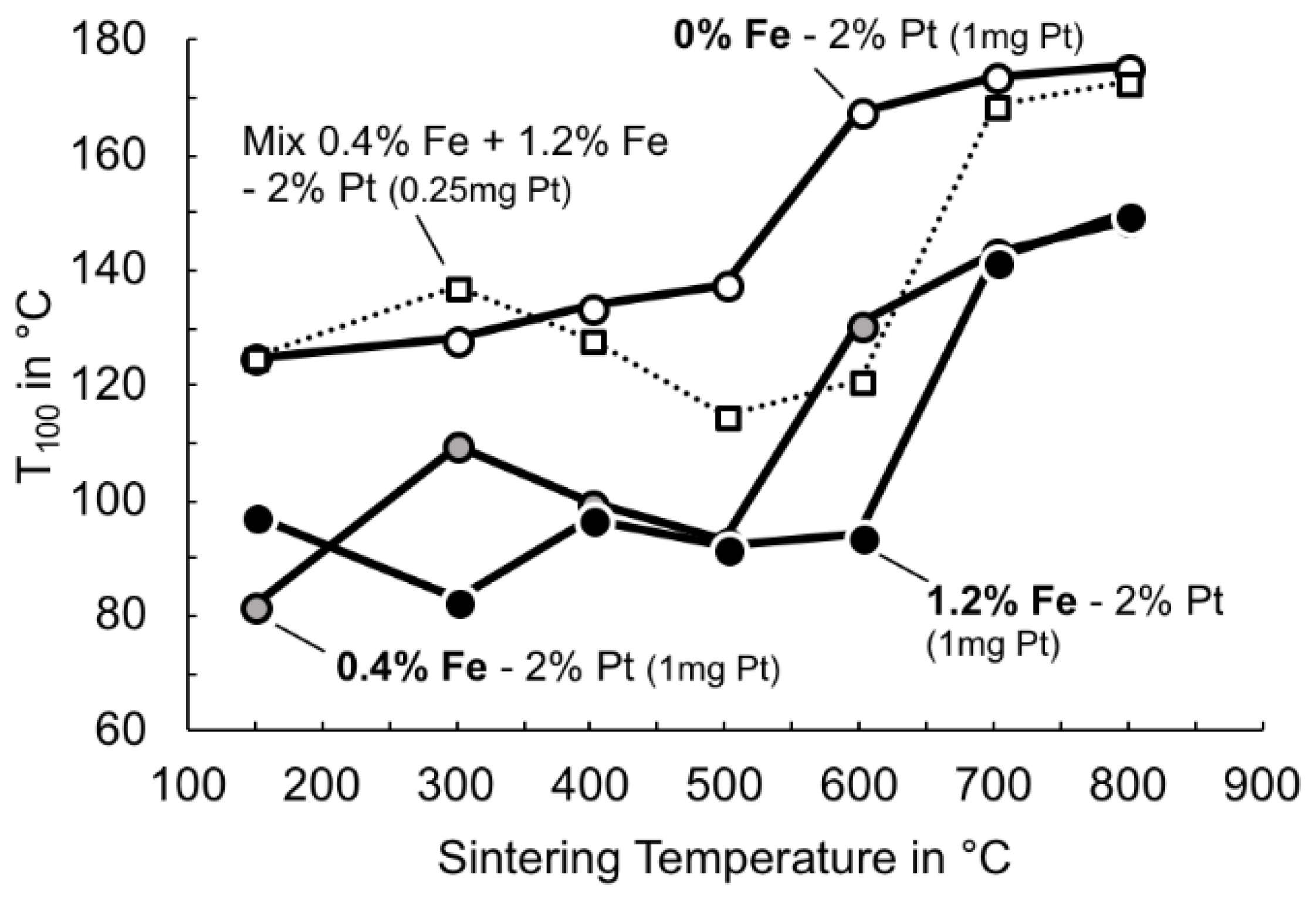
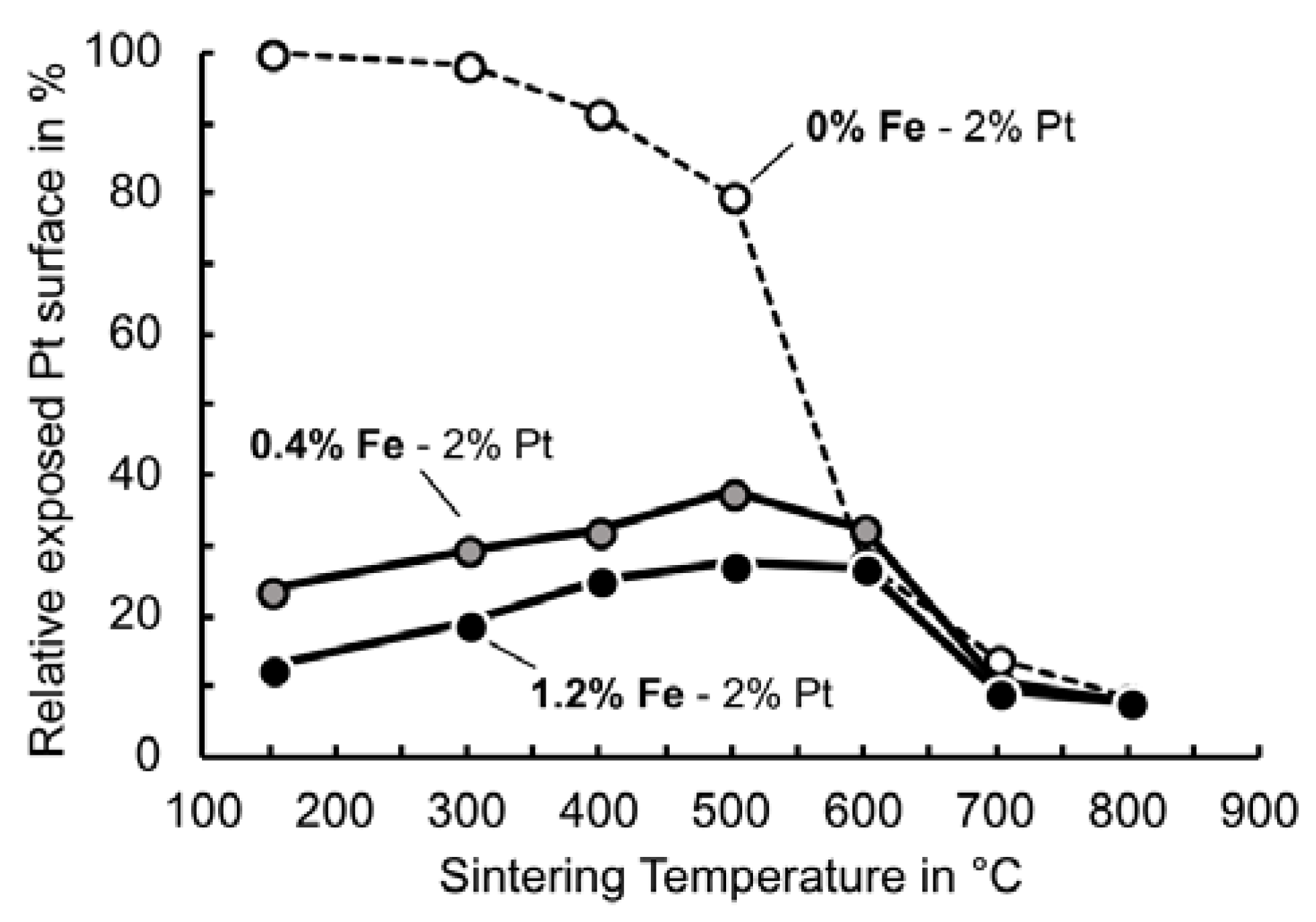
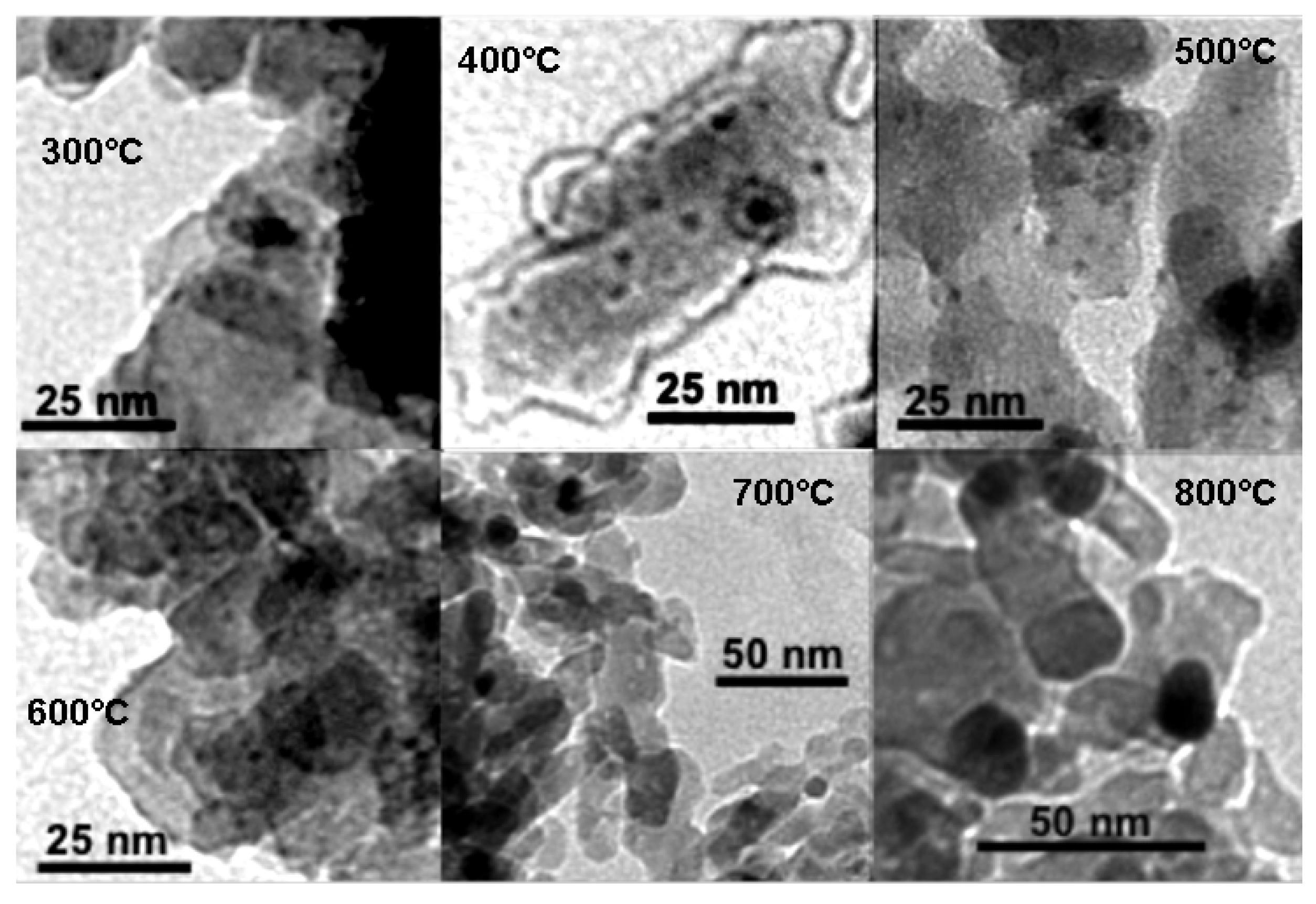
3.4. Effect of Sintering Temperature on Catalyst Behaviour
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Y.N.; Qin, Z.H.; Lewandowski, M.; Shaikhutdinov, S.; Freund, H.J. CO adsorption and dissociation on iron oxide supported Pt particles. Surf. Sci. 2009, 603, 3099–3103. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, M.; Sun, Y.N.; Qin, Z.H.; Shaikhutdinov, S.; Freund, H.J. Promotional effect of metal encapsulation on reactivity of iron oxide supported Pt catalysts. Appl. Catal. A Gen. 2011, 391, 407–410. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Qin, Z.; Lewandowski, M.; Kaya, S.; Shaikhutdinov, S. When an Encapsulating Oxide Layer Promotes Reaction on Noble Metals: Dewetting and in situ Formation of an “Inverted” FeOx/Pt Catalyst. Catal. Lett. 2008, 126, 31–35. [Google Scholar] [CrossRef]
- Xu, H.; Fu, Q.; Yao, Y.; Bao, X. Highly active Pt–Fe bicomponent catalysts for CO oxidation in the presence and absence of H2. Energy Environ. Sci. 2012, 5, 6313–6320. [Google Scholar] [CrossRef]
- Korotkikh, O.; Farrauto, R. Selective catalytic oxidation of CO in H2: Fuel cell applications. Catal. Today 2000, 62, 249–254. [Google Scholar] [CrossRef]
- Liu, X.; Korotkikh, O.; Farrauto, R. Selective catalytic oxidation of CO in H2: Structural study of Fe oxide-promoted Pt/alumina catalyst. Appl. Catal. A Gen. 2002, 226, 293–303. [Google Scholar]
- Pasin, L.; Meyer, J.; Eiche, E.; Kasper, G. Efficient aerosol assisted delivery of low volatile precursors for CVD applications. J. Aerosol Sci. 2018. submitted. [Google Scholar]
- Faust, M.; Seipenbusch, M. Highly controlled structuring of Pt nanoparticles on TiO2 and on ZrO2 by a modified MOCVD process. Surf. Coat. Technol. 2014, 259, 577–584. [Google Scholar] [CrossRef]
- Faust, M.; Enders, M.; Bruns, M.; Bräse, S.; Gao, K.; Seipenbusch, M. Synthesis of nanostructured Pt/oxide catalyst particles by MOCVD process at ambient pressure. Surf. Coat. Technol. 2013, 230, 284–289. [Google Scholar] [CrossRef]
- Heel, A.; Kasper, G. Catalyst Nanoparticles from a Continuous MOCVS/MOCVD Aerosol Process at Atmospheric Pressure. Aerosol Sci. Technol. 2005, 39, 1027–1037. [Google Scholar] [CrossRef]
- Binder, A.; Heel, A.; Kasper, G. Deposition of palladium nanodots of controlled size and density onto surface-modified SiO2 particles by an atmospheric pressure CVS/MOCVD process. Chem. Vap. Depos. 2007, 13, 48–54. [Google Scholar] [CrossRef]
- Maury, B.F. Trends in Precursor Selection for MOCVD. Chem. Vap. Depos. 1996, 2, 113–116. [Google Scholar] [CrossRef]
- Thurier, C.; Doppelt, P. Platinum OMCVD processes and precursor chemistry. Coord. Chem. Rev. 2008, 252, 155–169. [Google Scholar] [CrossRef]
- Li, J.; Liang, X.; King, D.M.; Jiang, Y.B.; Weimer, A.W. Highly dispersed Pt nanoparticle catalyst prepared by atomic layer deposition. Appl. Catal. B Environ. 2010, 97, 220–226. [Google Scholar] [CrossRef]
- Zhou, Y.; King, D.M.; Liang, X.; Li, J.; Weimer, A.W. Applied Catalysis B : Environmental Optimal preparation of Pt/TiO2 photocatalysts using atomic layer deposition. Appl. Catal. B Environ. 2010, 101, 54–60. [Google Scholar] [CrossRef]
- Xue, Z.; Thridandam, H. Organometallic chemical vapor deposition of platinum. Reaction kinetics and vapor pressures of precursors. Chem. Mater. 1992, 4, 162–166. [Google Scholar] [CrossRef]
- Charpak, F.S.G.; Perkov, V. Ethyl ferrocene in gas, condensed, or adsorbed phases: Three types of photosensitive elements for use in gaseous etectors. Nucl. Instrum. Methods Phys. Res. 1989, 277, 537–546. [Google Scholar] [CrossRef]
- Karlsruhe Institut for Technlogy. X-Ray fluorescence Spectrometers. In Encyclopedia of Spectroscopy and Spectrometry; Academic Press: Waltham, MA, USA, 1999; pp. 2467–2477. [Google Scholar]
- Karakaya, C.; Deutschmann, O. A simple method for CO chemisorption studies under continuous flow: Adsorption and desorption behavior of Pt/Al2O3 catalysts. Appl. Catal. A Gen. 2012, 445–446, 221–230. [Google Scholar] [CrossRef]
- Valverde, J.M.; Castellanos, A. Types of gas fluidization of cohesive granular materials. Phys. Rev. Lett. 2007, 75, 031306. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cai, Y.; Wachs, I.E. Reaction-Induced Spreading of Metal Oxides onto Surfaces of Oxide Supports during Alcohol Oxidation: Phenomenon, Nature, and Mechanisms. Langmuir 1999, 15, 1223–1235. [Google Scholar] [CrossRef]
- Weis, F.; Schneider, R.; Seipenbusch, M.; Kasper, G. Synthesis of Bi2O3/SiO2 core–shell nanoparticles by an atmospheric CVS/CVD process and their modification by hydrogen or electron-beam induced reduction. Surf. Coat. Technol. 2013, 230, 93–100. [Google Scholar] [CrossRef]
- Neubauer, N.; Palomaeki, J.; Karisola, P. Size-dependent ROS production by palladium and nickel nanoparticles in cellular and acellular environments—An indication for the catalytic nature of their interactions. Nanotoxicology 2015, 9, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Powell, Q.H. Gas Phase Production and Coating Titania in Tubular, Hot-Wall Reactors; University of New Mexico: Albuquerque, NM, USA, 1995. [Google Scholar]
- DIN 66136-1:2004-03 Bestimmung des Dispersionsgrades von Metallen durch Chemisorption—Teil 1: Grundlagen; DIN: Berlin, Germany, 2014; (InGerman).
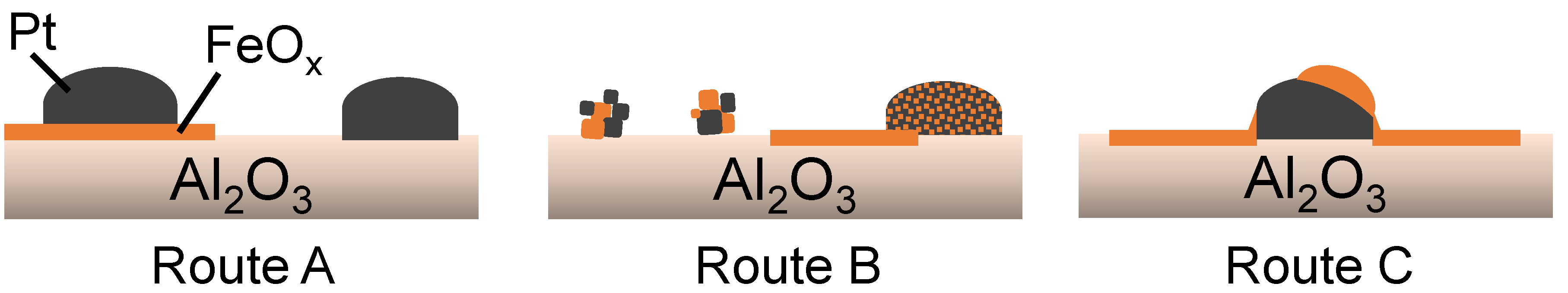
| Sample/Route | 0.3 wt % Pt and 3 wt % Fe | 0.8 wt % Pt and 3 wt % Fe | ||
|---|---|---|---|---|
| Pt surface Area [m2/g Sample] (±0.1 m2/g Sample) | Relative FeOx/Pt Interfacial Area SInterface (%) | Pt surface Area [m2/g Sample] (±0.1 m2/g Sample) | Relative FeOx/Pt Interfacial Area SInterface (%) | |
| Reference Pt (no Fe) | 0.3 | ~0 | 1.2 | ~0 * |
| Route A | 0.3 | – | ||
| Route B | – | – | 0.7 | 42 |
| Route C | 0.2 | 33 | 0.2 | 83 |
| Reference Fe (no Pt) | 0 | |||
| Sample/Route | T100 (°C) (±5 °C) | |
|---|---|---|
| 0.3 wt % Pt & 3 wt % Fe | 0.8 wt % Pt & 3 wt % Fe | |
| Reference Pt (no Fe) | 183 | 143 |
| Route A | 110 | – |
| Route B | – | 124 |
| Route C | 87 | 103 |
| Reference Fe (no Pt) | >300 | |
| Sinter Temp. (°C) | Reference Sample a (nm) | 0.4% Fe Sample b (nm) |
|---|---|---|
| 150 | 2.3 | 1.9 c |
| 300 | 2.3 | 1.9 |
| 400 | 2.7 | 2 |
| 500 | 3.2 | 2.3 |
| 600 | 9.1 | 5.1 |
| 700 | 18.1 | 13 |
| 800 | 29.5 | 20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasin, L.; Meyer, J.; Eiche, E.; Kasper, G. On the Activity Enhancing Role of Iron Oxide for Noble Metal Oxidation Catalysts: A CVD-Based Study with Differently Structured Combinations of Pt and FeOx Coatings on Al2O3. Coatings 2018, 8, 217. https://doi.org/10.3390/coatings8060217
Pasin L, Meyer J, Eiche E, Kasper G. On the Activity Enhancing Role of Iron Oxide for Noble Metal Oxidation Catalysts: A CVD-Based Study with Differently Structured Combinations of Pt and FeOx Coatings on Al2O3. Coatings. 2018; 8(6):217. https://doi.org/10.3390/coatings8060217
Chicago/Turabian StylePasin, Laila, Jörg Meyer, Elisabeth Eiche, and Gerhard Kasper. 2018. "On the Activity Enhancing Role of Iron Oxide for Noble Metal Oxidation Catalysts: A CVD-Based Study with Differently Structured Combinations of Pt and FeOx Coatings on Al2O3" Coatings 8, no. 6: 217. https://doi.org/10.3390/coatings8060217




