Study on Modified Water Glass Used in High Temperature Protective Glass Coating for Ti-6Al-4V Titanium Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Surface Morphologies of MWG
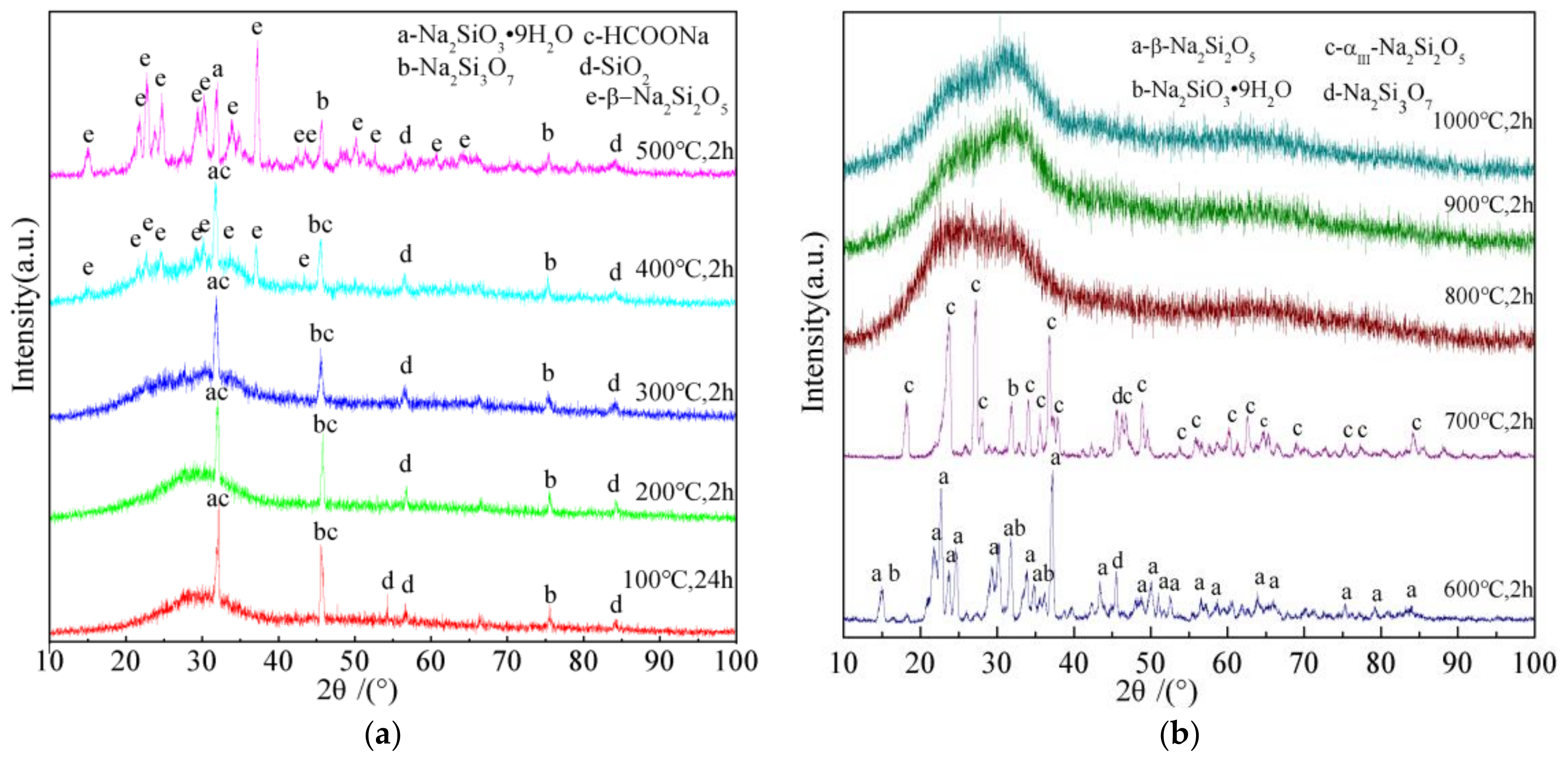
3.2. Phase Composition of MWG
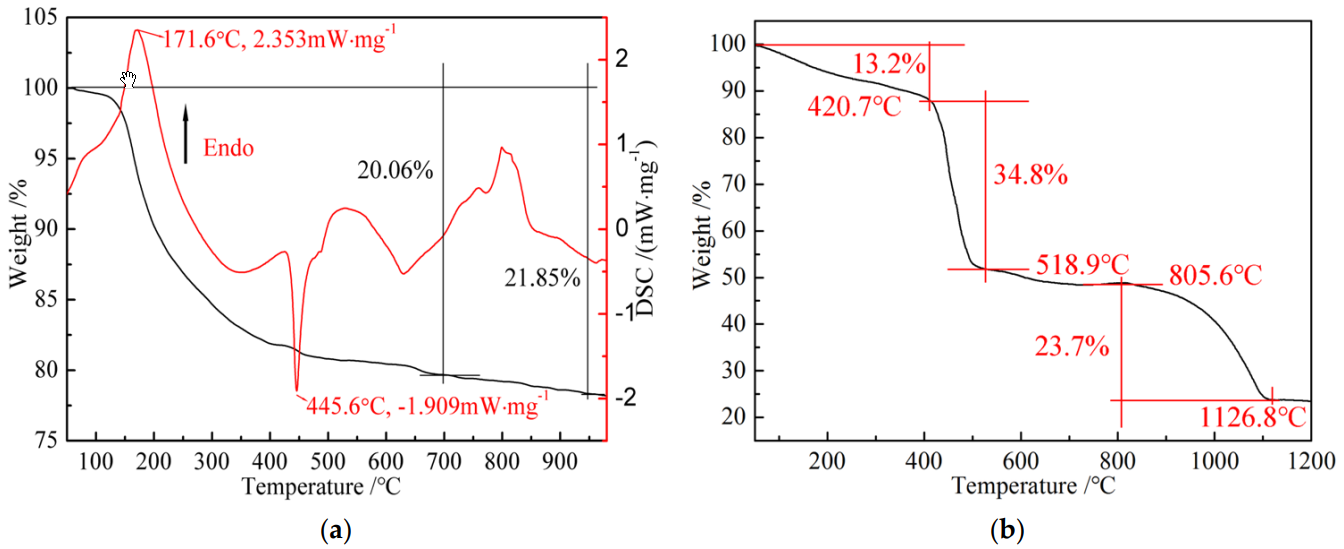
3.3. Thermal Behavior of MWG and PAANa
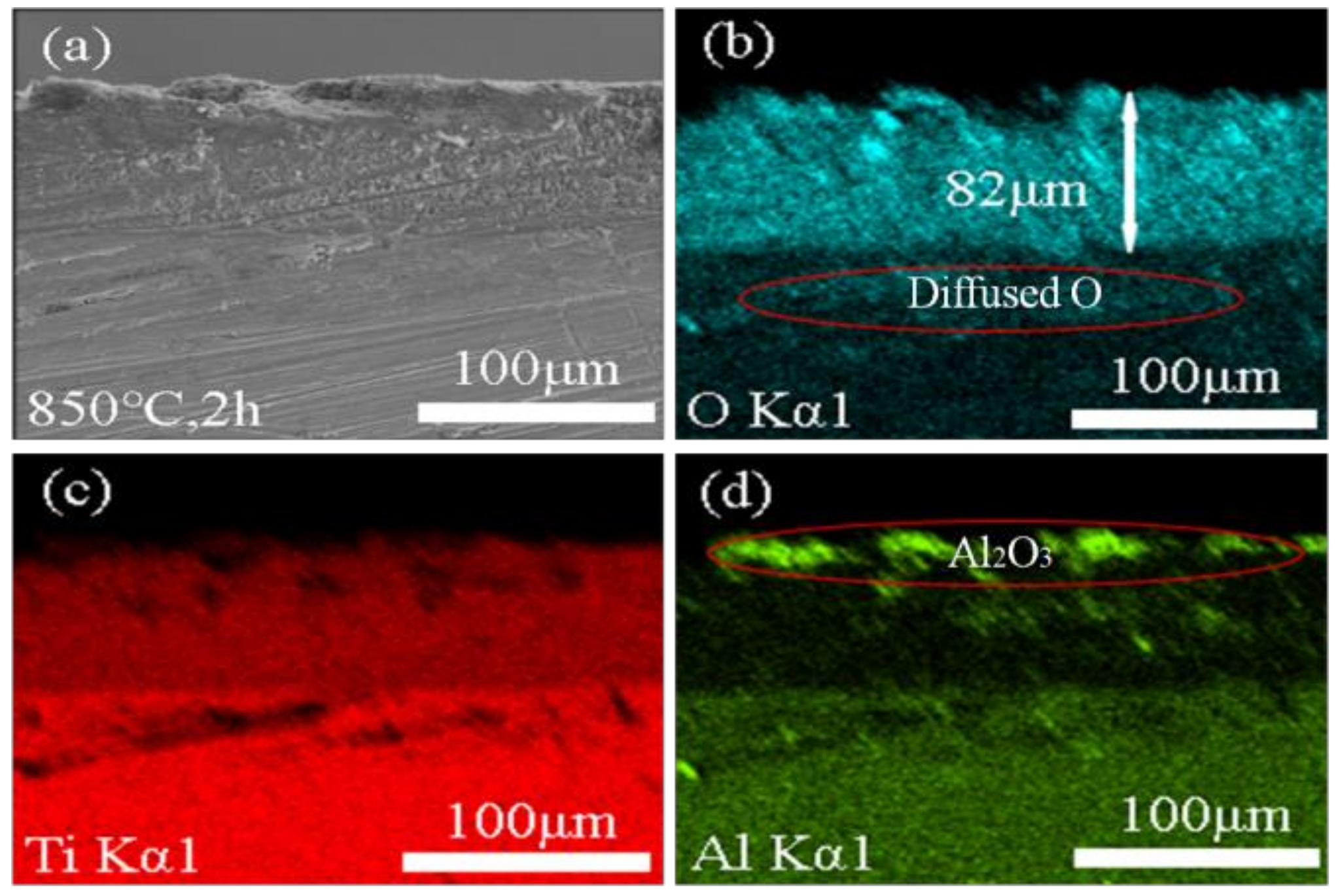
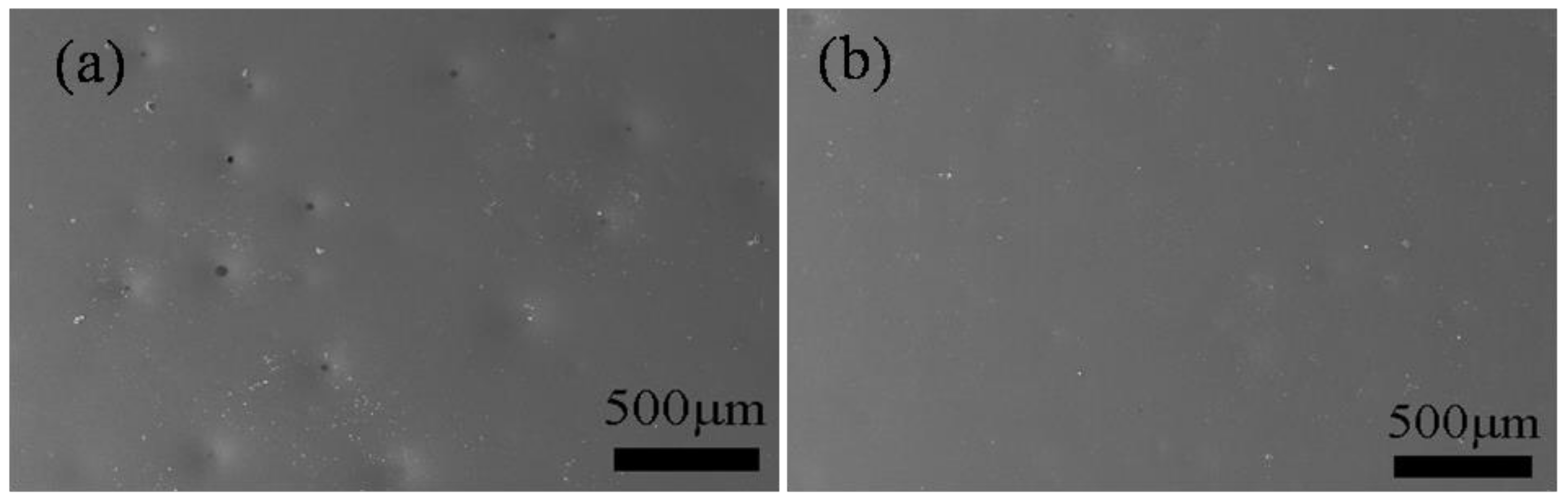
3.4. Oxidation Resistance of Ti-6Al-4V
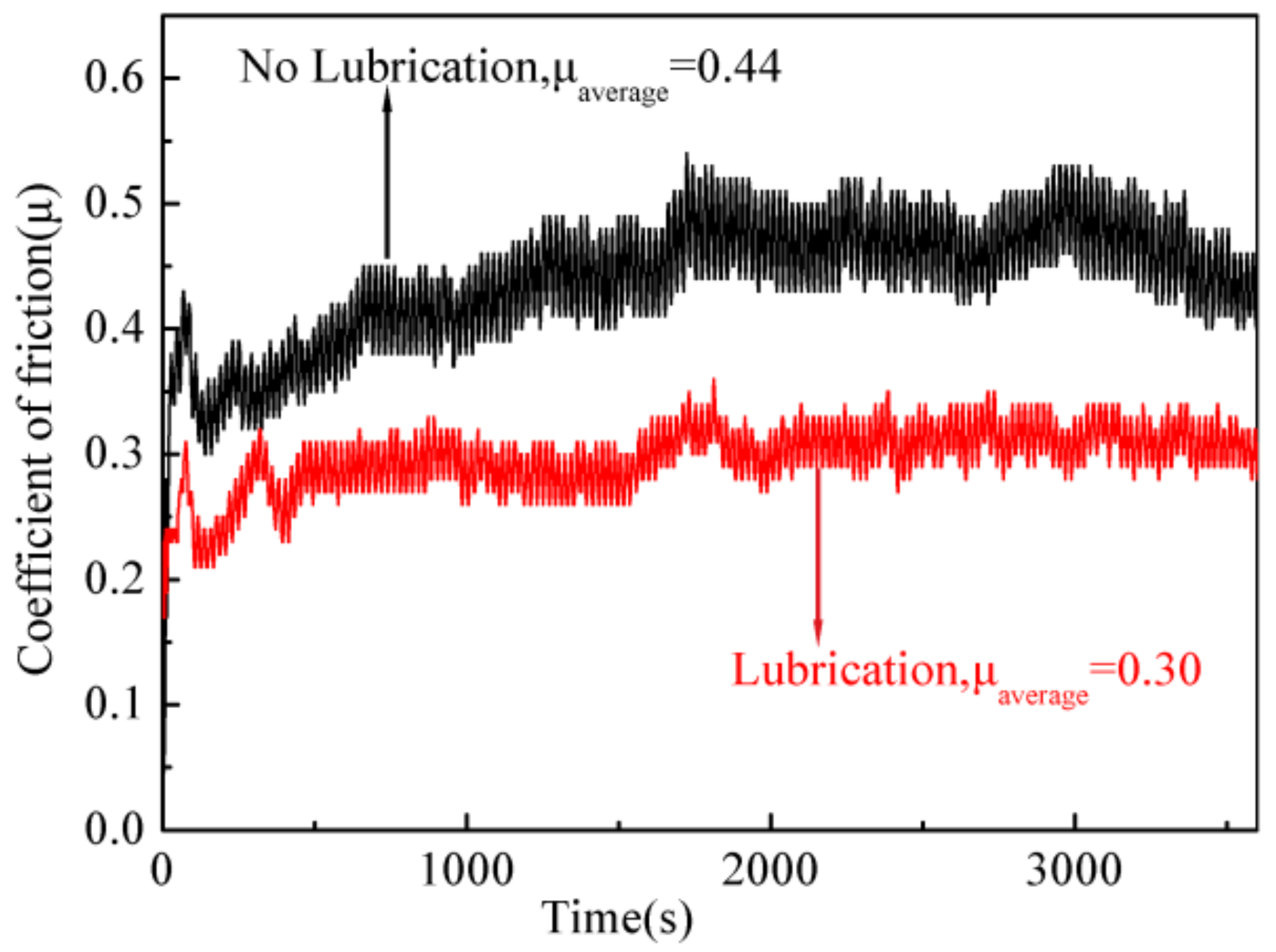
3.5. Tribology Study of the Lubricant
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Li, L.X.; Rao, K.P.; Lou, Y.; Peng, D.S. A study on hot extrusion of Ti–6Al–4V using simulations and experiments. Int. J. Mech. Sci. 2002, 44, 2415–2425. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Zeng, W.D.; Ma, X.; Tai, Q.G.; Li, Z.H.; Li, X.G. Determination of the friction factor of Ti–6Al–4V titanium alloy in hot forging by means of ring-compression test using fem. Tribol. Int. 2011, 44, 2074–2080. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Oxidation of Ti–6Al–4V alloy. J. Alloy Compd. 2009, 472, 241–246. [Google Scholar] [CrossRef]
- Dai, J.J.; Zhu, J.Y.; Chen, C.Z.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloy Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Li, L.X.; Peng, D.S.; Liu, J.A.; Liu, Z.Q.; Jiang, Y. An experimental study of the lubrication behavior of A5 glass lubricant by means of the ring compression test. J. Mater. Process. Technol. 2000, 102, 138–142. [Google Scholar] [CrossRef]
- Chen, M.H.; Li, W.B.; Shen, M.L.; Zhu, S.L.; Wang, F.H. Glass-ceramic coatings on titanium alloys for high temperature oxidation protection: Oxidation kinetics and microstructure. Corros. Sci. 2013, 74, 178–186. [Google Scholar] [CrossRef]
- Li, W.B.; Zhu, S.L.; Wang, C.; Chen, M.H.; Shen, M.L.; Wang, F.H. SiO2–Al2O3–glass composite coating on Ti–6Al–4V alloy: Oxidation and interfacial reaction behavior. Corros. Sci. 2013, 74, 367–378. [Google Scholar] [CrossRef]
- Xiao, Z.Q.; Tan, F.T.; Wang, W.; Lu, H.F.; Cai, Y.C.; Qiu, X.L.; Chen, J.G.; Qiao, X.L. Oxidation protection of commercial-purity titanium by Na2O–CaO–SiO2 and Na2O–CaO–Al2O3–SiO2 glass ceramic coatings. Ceram. Int. 2015, 41, 325–331. [Google Scholar] [CrossRef]
- Sarkar, S.; Datta, S.; Das, S.; Basu, D. Oxidation protection of gamma-titanium aluminide using glass-ceramic coatings. Surf. Coat. Technol. 2009, 203, 1797–1805. [Google Scholar] [CrossRef]
- Liu, P.; Wei, L.Q.; Ye, S.F.; Xu, H.W.; Chen, Y.F. Protecting stainless steel by glass coating during slab reheating. Surf. Coat. Technol. 2011, 205, 3582–3587. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Smeacetto, F.; Cempura, G.; Ajitdoss, L.C.; Salvo, M.; Czyrska-Filemonowicz, A. Microstructure and properties characterisation of the double layered glass–ceramic coating on near-α titanium alloy. Surf. Coat. Technol. 2010, 204, 3509–3516. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Smeacetto, F.; Czyrska-Filemonowicz, A. Microstructure, properties and oxidation behavior of the glass–ceramic based coating on near-α titanium alloy. Surf. Coat. Technol. 2009, 203, 2249–2253. [Google Scholar] [CrossRef]
- Liang, W.; Wu, J.P.; Zhang, L. Preparation and performance of high-temperature oxidation resistant of coatings for mild carbon alloyed steel. Geol. Sci. Technol. Inf. 2007, 26, 86–90. [Google Scholar]
- Zhang, X.J.; Gao, Y.H.; Ren, B.Y.; Tsubaki, N. Improvement of high-temperature oxidation resistance of titanium-based alloy by sol-gel method. J. Mater. Sci. 2010, 45, 1622–1628. [Google Scholar] [CrossRef]
- Matsumoto, K.; Izawa, M.; Nakanishi, T.; Tsubouchi, K. Tribological properties of water glass lubricant for hot metalworking. Tribol. Trans. 2009, 52, 553–559. [Google Scholar] [CrossRef]
- Schweins, R.; Huber, K. Collapse of sodium polyacrylate chains in calcium salt solutions. Eur. Phys. J. E 2001, 5, 117–126. [Google Scholar] [CrossRef]
- Chen, G.Q.; Li, N.N.; Fu, X.S.; Zhou, W.L. Preparation and characterization of a sodium polyacrylate/sodium silicate binder used in oxidation resistant coating for titanium alloy at high temperature. Powder Technol. 2012, 230, 134–138. [Google Scholar] [CrossRef]
- Kahlenberg, V.; Dorsam, G.; Wendschuh-Josties, M.; Fischer, R.X. The crystal structure of delta-Na2Si2O5. J. Solid State Chem. 1999, 146, 380–386. [Google Scholar] [CrossRef]
- Subasri, R.; Nafe, H. Phase evolution on heat treatment of sodium silicate water glass. J. Non-Cryst. Solids 2008, 354, 896–900. [Google Scholar] [CrossRef]
- Tieu, A.K.; Wan, S.H.; Kong, N.; Zhu, Q.; Zhu, H.T. Excellent melt lubrication of alkali metal polyphosphate glass for high temperature applications. RSC Adv. 2015, 5, 1796–1800. [Google Scholar] [CrossRef]


| SiO2 | B2O3 | Al2O3 | CaO | MgO | Na2O | BaO |
|---|---|---|---|---|---|---|
| 62 | 10 | 2 | 4 | 4 | 14 | 4 |
| Component | PAANa | Sodium Water Glass | H2O |
|---|---|---|---|
| WG | 0 | 30 | 70 |
| MWG | 0.5 | 30 | 69.5 |
| No. | A | B | C |
|---|---|---|---|
| Na | 27.79 | 17.88 | 21.35 |
| Si | 10.52 | 10.41 | 12.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhou, D.; Zhou, J.; Yang, L.; Fan, Q.; Yao, Q.; Yang, Y. Study on Modified Water Glass Used in High Temperature Protective Glass Coating for Ti-6Al-4V Titanium Alloy. Coatings 2018, 8, 158. https://doi.org/10.3390/coatings8050158
Yang S, Zhou D, Zhou J, Yang L, Fan Q, Yao Q, Yang Y. Study on Modified Water Glass Used in High Temperature Protective Glass Coating for Ti-6Al-4V Titanium Alloy. Coatings. 2018; 8(5):158. https://doi.org/10.3390/coatings8050158
Chicago/Turabian StyleYang, Shuang, Dali Zhou, Jiabei Zhou, Lei Yang, Qing Fan, Qianqian Yao, and Yongqiang Yang. 2018. "Study on Modified Water Glass Used in High Temperature Protective Glass Coating for Ti-6Al-4V Titanium Alloy" Coatings 8, no. 5: 158. https://doi.org/10.3390/coatings8050158





