1. Introduction
Material surfaces should have the optimal biocompatibility in vivo with the surrounding biological environment because the implant surface is in direct contact with biological tissue. In addition, the implant surface should allow for the physical, chemical, and electrical properties, as well as the increased biological activities induced by interactions between the material surface and the tissue. Titanium is used as a material possessing these properties and is best suited for applications in the orthopedic and dental fields, it is also widely used as a bone replacement material [
1,
2,
3]. A thin nanoscale TiO
2 film is formed on the titanium surface upon exposure to air by reacting it with oxygen while it is being made into an implant. However, naturally occurring thin TiO
2 films have a heterogeneous composition and a low density. Therefore, methods for achieving thin TiO
2 films with dense structures of homogeneous compositions have been studied to improve titanium’s biocompatibility. Moreover, because of the bio-inert character of natural thin TiO
2 films, it takes months until osseointegration sets in, thus prolonging the duration of the prosthetic treatment by up to several months. A number of studies have been conducted to overcome this problem by inducing bioactive property and enhancing the bone–implant adhesion through the surface modification of titanium implants [
4,
5]. Because such an implant surface accelerates osseointegration and enhances bone–implant adhesion, various surface-treatment methods have been studied, including surface coating, surface microstructure modification, and chemical property modification [
6,
7,
8].
Anodization is one of a variety of surface treatment methods; it is an electrochemical method in which the passive films formed by anodization and cathodic reduction reactions enhance the corrosion resistance of the surface. Furthermore, various surfaces can be obtained depending on voltage, current, time, temperature, and electrolyte type. The anodization of titanium forms nanotubes on the surface and increases its roughness and wettability [
8]. According to previous study results, anatase TiO
2 nanotubes, which can be obtained by heat-treating anodized TiO
2 nanotubes, increase the contact area with cells and thus promote interaction with osteoblasts [
9,
10].
In the alkali treatment method, which is a chemical surface treatment method, the titanium surface is soaked in a NaOH solution and the surface property is modified through ionic reaction. Bioactive sodium titanate gel layers with alkali ions are formed on the NaOH-treated titanium surface, and the surface layer composition and structure are modified through densification [
11,
12,
13,
14]. Na
2TiO
3 formed on the alkali-treated titanium surface increases the surface roughness and improves the contact property. Moreover, Na deposited on the surface forms sites within the body where the substitution of Ca and P ions is induced, thereby promoting the precipitation of hydroxyapatite (HAp) inducing osseointegration. This process can be accelerated by heat treatment [
11,
15]. However, it was reported that an alkali-treated surface releases Na ions into the body, resulting in a slowing down of cell proliferation [
16]. In another study related to Na ion extraction, biocompatibility could be improved by water treatment to accelerate HAp formation [
17].
Carbon nanotubes (CNTs) are tubular cylinders of carbon atoms forming hexagonal beehive-like structures, with each hexagon comprising six carbon atoms in which one atom is symmetrically bound to the other three atoms. They are representative new materials discovered by Iijima in 1991 [
18], and their extraordinary electrical, mechanical, and thermal properties based on their unique molecular structure have instigated research worldwide. They are promising candidates as new materials for applications in a wide range of industries such as information and communication technology, electronic devices, biosensors, transistors, hydrogen storage batteries, environmental fields, energy, and medicine. Not only do they have superior properties as industrial materials, but they also have unlimited potential as biomaterials. They have a wide spectrum of biological and medical applications. Specifically, CNT-based cell culture, drug delivery systems, implants, and bone formation are rapidly developing areas of research. They are also studied as a scaffold material in regenerative medicine [
19,
20]. Multi-walled CNT (MWCNT)-coated titanium has been reported to show stronger cell adhesion than untreated titanium [
3], and another study [
20] experimentally proved the feasibility of CNTs as biomaterials in in vitro and in vivo tests. On the basis of the results of these previous studies, it was hypothesized that a CNT coating on surface-treated titanium improves biocompatibility of the implant.
Therefore, in this study, the surface of pure titanium was modified by anodization, alkali treatment, and Multiwall Carbon Nanotubes (MWCNT) coating to form the biofunctional multilayer for dental and orthopedic applications. The modified surfaces of Ti were characterized by SEM, XRD, and AFM; the biocompatibility of the modified surfaces was evaluated with a cytotoxicity test using osteoblast.
3. Result
The surface morphologies of PT, PTN, PTNA, and PTNAC were observed with FE-SEM as shown
Figure 1. A smooth structure was observed on the surface of PT (
Figure 1A). An array of homogenous TiO
2 nanotubes was formed on the surface by anodization and the thickness of coating layer was about 600 nm (
Figure 1B). A nano-fibrous structure was formed on anodized Ti by alkali treatment in the 5 M NaOH solution, and an oxidized layer and an alkali layer was densely combined (
Figure 1C). The surface coated MWCNTs after anodization with Ti and alkali treatment showed a more complex network, more rigid than the PTN group (
Figure 1D).
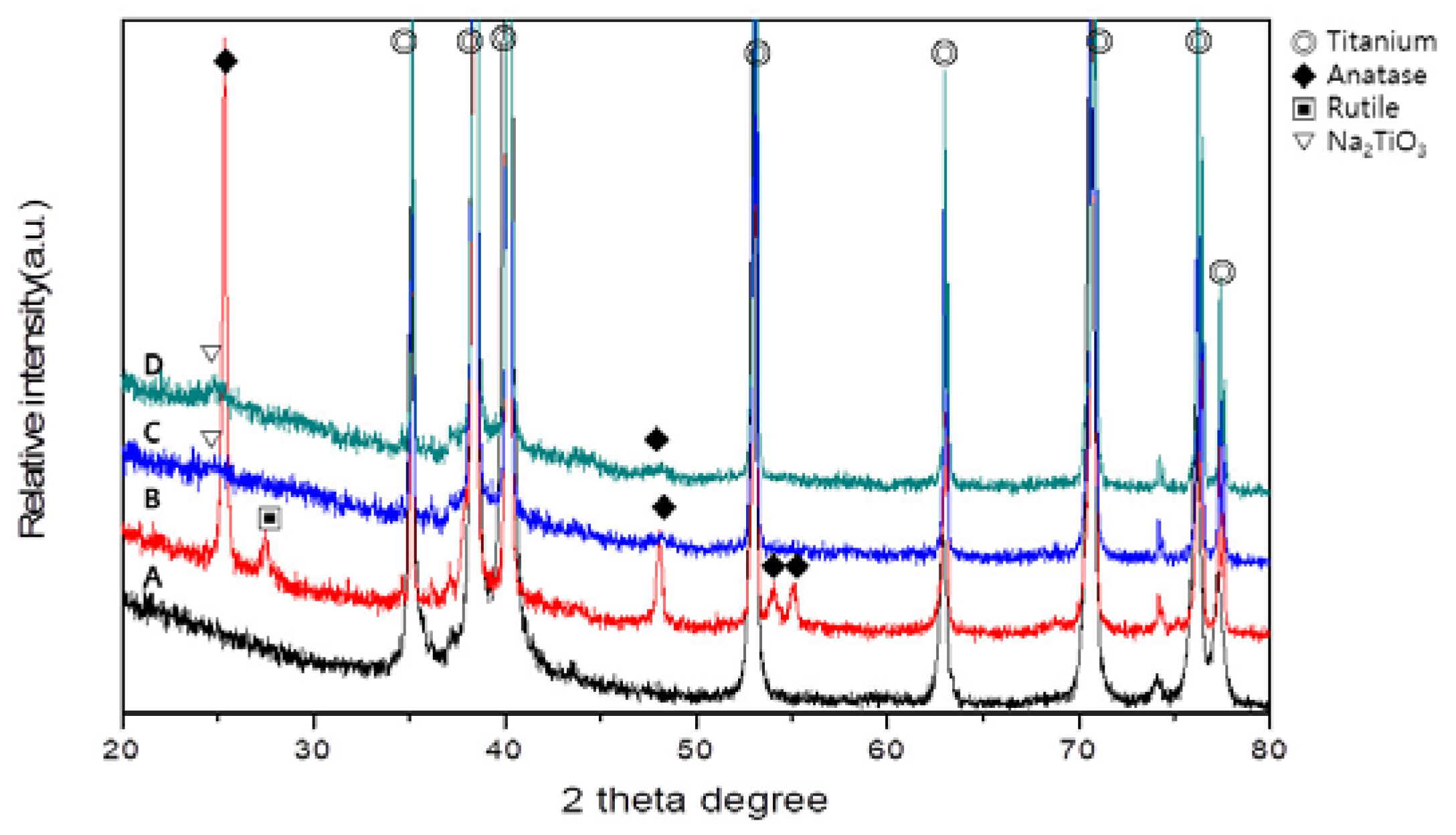
Figure 2 shows the XRD patterns of PT, PTN, PTNA, and PTNAC. Ti peaks were observed in all groups. Rutile and anatase phases were observed on the PTN surface (
Figure 2B). An anatase phase and Na
2TiO
3 phase were observed but rutile was not observed in PTNA and PTNAC (
Figure 2C,D).
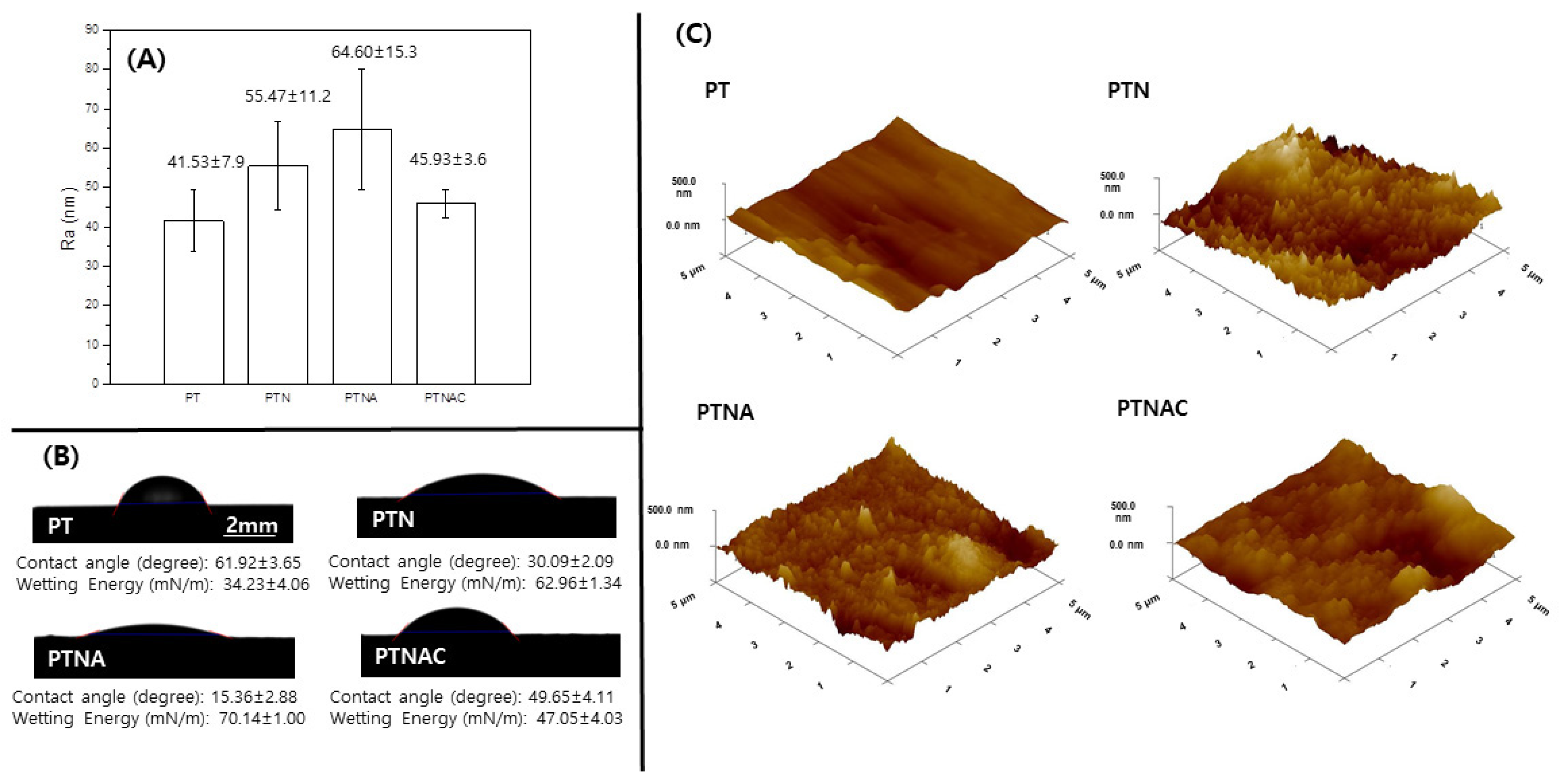
Figure 3 shows the value of the surface roughness, contact angle, and a 3D image of PT, PTN, PTNA, and PTNAC. The surface roughness values of PT, PTN, PTNA, and PTNAC are 41.53 ± 7.9, 55.47 ± 11.2, 64.60 ± 15.3, and 45.93 ± 3.6, respectively. The surface roughness was gradually increased by anodization and alkali treatment, but it was decreased by the MWCNT coating. Contact angle values of PT, PTN, PTNA, and PTNAC are 61.92 ± 3.65, 30.09 ± 2.09, 15.36 ± 2.88, and 49.65 ± 4.11; and the wetting energy values are 34.23 ± 4.06 mN/m, 62.96 ± 1.34 mN/m, 70.14 ± 1.00 mN/m, and 47.05 ± 4.03 mN/m, respectively. The smaller the contact angle of the surface, the higher the wetting energy value.
Figure 4 shows the FE-SEM images of the surface (A and E) of the PT, (B and F) of the PTN, (C and G) of the PTNA, and (D and H) of the PTNAC after immersion in the SBF solution for 7 and 10 days. A flat structure was observed on the surface of PT, but precipitates were confirmed on the surface of PTN, PTNA, and PTNAC after immersion for 7 days. Precipitates were observed on the surface of PT after immersion for 10 days (
Figure 4E), but precipitates on the surface of the other groups were confirmed after immersion for 7 days (
Figure 4B–D). Precipitates covered the entire surface of all the groups after immersion for 10 days.
Figure 5 shows the XRD pattern of the surfaces after immersion in SBF for 7 and 10 days. Peaks related to the hydroxyapatite phase and Tricalcium phosphate (TCP) phase were confirmed on PTN, PTNA, and PTNAC but were not on PT after 7 days. Also, peaks related to the hydroxyapatite phase and TCP phase were observed on PTN, PTNA, and PTNAC after 10 days. A peak related to the TCP phase were confirmed on PT but a hydroxyapatite phase was not confirmed on PT.
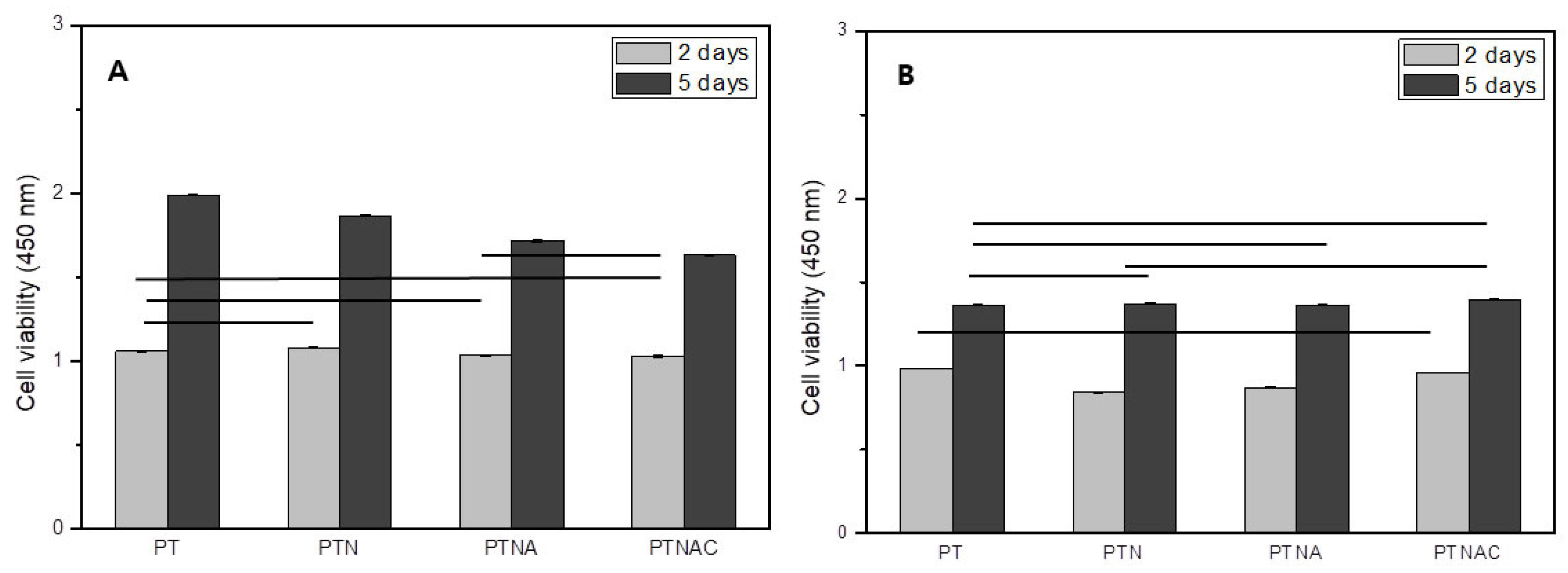
Figure 6 shows cell proliferation (
Figure 6A) and attachment (
Figure 6B) after cell culture for 2 and 5 days. Cell proliferation was not significantly different between all groups after the cells were cultured for 2 days (
Figure 6A). Cell proliferation was higher on PT than the other groups after the cells were cultured for 5 days (
Figure 6A).
Figure 6B shows the results of the WST assay performed on the specimens treated with 0.02% Trypsin-EDTA for 5 min after cell culture for 2 and 5 days. The cells attachment on the PT and PTNAC was higher than with PTN and PTNA after cell culture for 2 days. The cell adhesion was not significantly different between all groups after cell culture for 5 days. However, compared to the cell proliferation graph, it was confirmed that the cell adhesion rate of the carbon nanotube-coated specimen was improved.
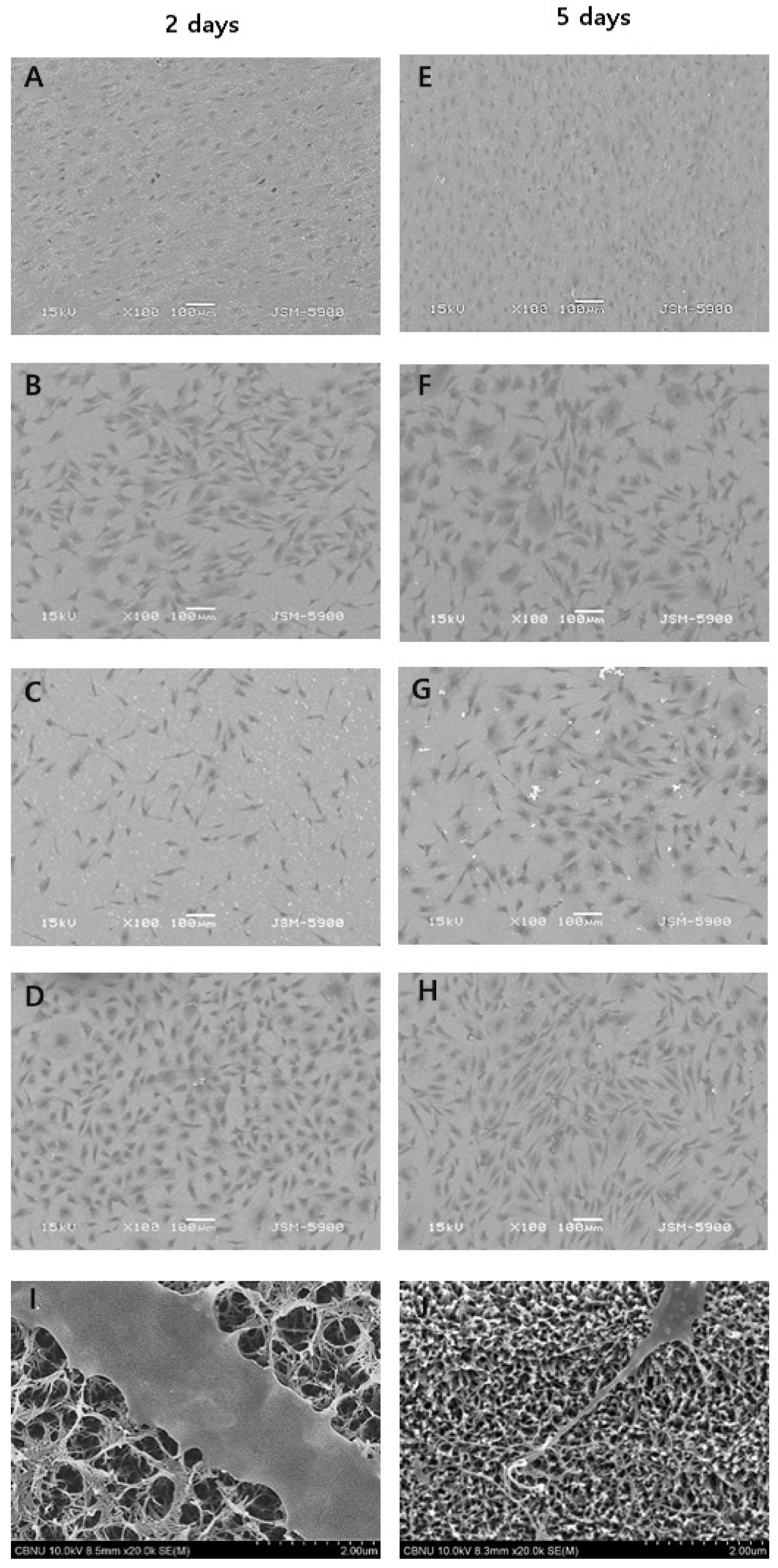
Figure 7 shows SEM images of cell morphologies after cells were cultured for 2 and 5 days. Cell morphology on PT showed a round shape and high cell density. The cell morphology on PTNA showed an elongated shape for 2 days but a round shape due to developed cytoplasm for 5 days. PTN and PTNAC showed more developed cytoplasm than PTNA.
4. Discussion
For successful osseointegration after implant surgery, cell compatibility is important. In other words, the incipient osteoblast adhesion, proliferation, and differentiation are crucial factors. To enhance the efficiency of osseointegration, a variety of surface treatment methods have recently been attempted. In particular, research into increasing the bioactivity of the titanium implant surface for early rapid bone formation have been continuously carried out [
21,
22]; incipient osteoblast formation is closely related to the implant surface properties. This study investigated the surface bioactivity and biocompatibility by changing the characteristics of the titanium surfaces. To enhance bioactivity of the titanium surface, radially elongated nanoscale hairy structures were formed by soaking the anodized titanium surface in an alkaline solution, followed by MWCNT coating. After that, the coating-dependent surface characteristics, HAp formation ability, and the interactions between osteoblasts and the surface-modified titanium were evaluated to determine its suitability as a biomaterial.
In this study, titanium was anodized in an electrolyte containing NHF
4. The anodized surface confirmed that nano-sized pores were formed in a homogeneous structure (
Figure 1B). At this time, the mechanism of the TiO
2 nanotube layer formation by anodic oxidation on the surface of titanium is seen to be the result of the growth of a dense oxide film layer which occurs electrochemically and because of the decomposition of oxide by fluorine ions. When the anodic oxidation process is performed under a condition of constant voltage, since an oxide film layer of a constant thickness is formed on the surface, the intensity of the electric field is radically reduced, so that the current shows an exponential decrease and reaches a state of equilibrium. However, because of the decomposition action of the fluorine ions liberated from the soluble fluorine compound contained in the electrolytic solution, the surface layer is activated again so that a large number of pores are generated, the reduced current slightly increases, and grows into dense nanotubes that form into a regular array [
23,
24]. The anodized titanium was immersed in a 5M NaOH solution for 24 h and heat-treated to form a dense network on the surface (
Figure 1C). The network structure of the alkali treatment was generated as follows. The alkali treatment with sodium hydroxide corrodes the titanium oxide film by the hydroxyl, and the reaction formula is as follows [
25]:
This reaction is assumed to occur concurrently with titanium hydration.
Negatively charged hydrates are formed on the matrix surface when the hydroxyl group is further added to the hydrated TiO
2.
These negatively charged hydrates bind to the alkali ions in the solution to form an alkali titanate hydroxide gel layer, resulting in a structurally heterogeneous porous net structure. The hydroxide gel layer is dehydrated under heat treatment and undergoes densification and forms stable amorphous crystalline sodium titanate, thus modifying the composition and structure of the surface layer. In addition, in this study, carbon nanotubes were coated on the titanium surface after the above surface treatment. The only alkali treated surface on the titanium and TiO
2 formed titanium surface (PTNA) are similar in appearance [
26]. However, it was identified that the heat-treated surface after the alkali treatment on the TiO
2 nanotube has a more uniform and dense surface than the surface only treated with alkali in the previous study [
4]. TiO
2 nanotube surfaces provide a favorable template for bone cell growth and differentiation [
27]. Also, the porous surface of TiO
2 increases the initial osseointegration [
28,
29]. Moreover, higher O ion concentration in the TiO
2 layer formed by anodization can make Na
+ react more easily during alkali treatment than the surface of untreated titanium, which results in the formation of amorphous sodium titanate [
30]. This amorphous sodium titanate in the TiO
2 layer can enhance initial bone formation more than when only a TiO
2 layer exists on the titanium surface.
CNT is a new material that is attracting attention in various research fields due to its excellent electrical conductivity and excellent physical and chemical properties. In addition, studies for applying CNTs as biomaterials have been increasing. After the carbon nanotube coating, the surface showed a more complicated network structure after the alkali treatment, and the presence of carbon nanotubes on the surface was also confirmed (
Figure 1D).
As a result of XRD, peaks of anatase and rutaile phases were found in the PTN group. The PTNA and PTNAC groups also showed weak an anatase phase and Na
2TiO
3 phase (
Figure 2). If TiO
2 formed on the titanium surface anodized in an electrolyte solution containing NH
4F and glycerol undergoes heat treatment, the anatase phase begins to form at 500 °C. In general, if a titanium specimen is heat-treated at around 600 °C after 24 h immersion in 5 M NaOH at 60 °C, crystalline a sodium titanate phase appears [
4].
Cells are generally adhered onto the rough surface of biomaterials. Factors that play an important role in the cell attachment process are surface roughness, wettability, and surface energy. In this study, the surface roughness of titanium increased through the treatment of the surface when compared with PT (
Figure 3). The surface roughness of PTNAC (among the surface-treated titanium in this study) was lowest since the CNTs were coated on the porous structure of TiO
2 by the alkali treatment. The surface with the high roughness generally shows high hydrophilicity. Highly hydrophilic surfaces makes organisms grow well, which is directly related to biocompatibility [
31]. The highly hydrophilic surface of titanium results in superior cell adhesion and cell proliferation [
31]. In this study, the surface of the titanium had a low contact angle when compared with the surface of PT. The PTNA group showed the lowest contact angle. The PTNAC group showed a higher contact angle than the PTN and PTNA groups since the CNTs coated on the surface decreased hydrophilic characteristics on the surfaces. However, the value of the contact angle for all groups was less than 90 degrees, which means the surfaces of all groups had a hydrophilic surface [
32].
The formation of apatite, a bone-like material, is of vital importance for biostability and osseointegration performance. In particular, if the titanium surface is treated with NaOH, a sodium titanate gel layer containing alkaline ions is formed. Such a titanium surface net structure is extremely dense and refines apatite, thus promoting the formation of amorphous sodium titanate [
11,
33,
34]. In this study, the apatite-forming ability was tested by immersing each surface-treated titanium specimen in a SBF solution for 7 and 10 days (
Figure 4 and
Figure 5); precipitates were confirmed on the surface of PTN, PTNA, and PTNAC after immersion for 7 days (
Figure 4). The XRD observations revealed the formation of HAp (
Figure 5). After the 10-day immersion, apatite was found to cover the surfaces of all specimens. The ionization of KH
2PO
4 and Na
2HPO
4 components in the SBF solution releases hydrogen ions, forming H
3O
+ ions by binding to water molecules. If an alkali-treated specimen is soaked in a SBF solution, Na
+ on the surface and H
3O
+ in the SBF solution are substituted to form the Ti–OH group, and if left to react further with the SBF solution, it binds with Ca
+ to form calcium titanate. Such a surface induces binding with PO
42−, thereby forming amorphous calcium phosphate, which then grows in the order of DCP-OCP-TCP-HAp [
14,
23,
35]. As such, the higher the hydrophilicity of the surface, the greater the HAp induction through the enhanced surface–SBF contact.
This study investigated the effect multiple-surface treated titanium had on cell growth. Cell proliferation was confirmed on each treated specimen after MC3T3-E1 cells were cultured for 2 days and 5 days (
Figure 6A). In this study, cell proliferation was not significantly different (
p > 0.05) after cells were cultured for 2 days when the specimens of treated surface (PTN, PTNA, and PTNAC) were compared to PT. In addition, cell proliferation decreased with added surface treatment after cells were cultured for 5 days. However, improved cell proliferation was confirmed in all groups after 5 days of cell culture. This may be explained by looking at a previous report that states that while the alkali-treated titanium surface shows a high apatite formation efficiency in the SBF solution, the amorphous sodium titanate layer formed on the surface releases sodium ions during implantation, thereby forming narrow-spaced pores and thus negatively affecting cell reactions [
16]. The alkaline treated PTNA and PTNAC groups have a favorable surface affinity for bonding with organisms. However, the surface obtained by the alkali treatment reacted with the culture medium and showed low cell proliferation because Na ions were eluted.
In this study, the cells attached to the surface of each group were examined by WST assay after 0.02% TryLE™ Express treatment for 5 min (
Figure 6B). After 2 days of cell culture, the attached cells were higher on the PT and PTNAC surfaces. In addition, there was no statistically significant difference in all specimens after cell culture for 5 days. However, compared to the cell proliferation graph, it was confirmed that the cell adhesion rate of the carbon nanotube-coated specimen (PTNAC) was improved. It is considered that the carbon nanotube affects cell adhesion. A previous study reported that CNTs exhibited a strong cell adhesion ability and attributed it to the mechanical bonding between the cell surface or filopodia and the protein adsorbed in CNTs [
36]. According to a study conducted by Terada et al. [
37], a collagen-coated dish showed higher cell proliferation and viability rates compared with a MWCNT-coated dish, but the latter showed stronger cell adhesion than the former. Aoki et al. [
38] and Zanello et al. [
39] reported that when osteoblasts were cultured in MWCNTs, the MWCNTs showed the same mechanical contact as the osteoblasts, and explained that this mechanical bonding is one of the reasons for the high bonding strength and that the large specific area of MWCNTs also contributes to the increased adhesion ability.
In order for cells to survive, adsorption of the cells into the surfaces should be stable, followed by proliferation. MT3T3-E1 osteoblasts extend filopodia to the surface for stable adsorption. As a result of observing the adsorption state of cells, including filopodia, during the adsorption process of the MC3T3-E1 osteoblast, more cells were adsorbed on the surface coated with carbon nanotubes (
Figure 7). This suggests that the surface coated with carbon nanotubes helps the cells to adsorb more strongly and that the strong adsorption of these cells is the result of the effect of MWCNT.