A Microstructural and Wear Resistance Study of Stainless Steel-Ag Coatings Produced through Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Antunes, R.A.; Rodas, A.C.D.; Lima, N.B.; Higa, O.Z.; Costa, I. Study of the corrosion resistance and in vitro biocompatibility of PVD TiCN-coated AISI 316 L austenitic stainless steel for orthopedic applications. Surf. Coat. Technol. 2010, 205, 2074–2081. [Google Scholar] [CrossRef]
- Parsapour, A.; Khorasani, S.N.; Fathi, M.H. Effect of surface treatment and metallic coating on corrosion behavior and biocompatibility of surgical 316L stainless steel implant. J. Mater. Sci. Technol. 2012, 28, 125–131. [Google Scholar] [CrossRef]
- Pareja López, A.N.D.R.É.S.; García García, C.P.; Abad Mejía, P.J.; Márquez Fernández, M.E. Citotoxic and genotoxic study of in vitro released products of stainless steel 316L with bioactive ceramic coatings. Iatreia 2007, 20, 12–20. (In Spanish) [Google Scholar]
- Gopi, D.; Ramya, S.; Rajeswari, D.; Kavitha, L. Corrosion protection performance of porous strontium hydroxyapatite coating on polypyrrole coated 316L stainless steel. Colloids Surf. B 2013, 107, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Anghelina, F.V.; Ungureanu, D.N.; Bratu, V.; Popescu, I.N.; Rusanescu, C.O. Fine structure analysis of biocompatible ceramic materials based hydroxyapatite and metallic biomaterials 316L. Appl. Surf. Sci. 2013, 285, 65–71. [Google Scholar] [CrossRef]
- Kannan, S.; Balamurugan, A.; Rajeswari, S. Hydroxyapatite coatings on sulfuric acid treated type 316L SS and its electrochemical behaviour in Ringer’s solution. Mater. Lett. 2003, 57, 2382–2389. [Google Scholar] [CrossRef]
- Kuroda, D.; Hiromoto, S.; Hanawa, T.; Katada, Y. Corrosion behavior of nickel-free high nitrogen austenitic stainless steel in simulated biological environments. Mater. Trans. 2002, 43, 3100–3104. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Ding, M.H.; Zheng, H.; Zhang, H.S.; Zhang, B.; Li, X.Q.; Wu, G.Y. Surface modification of biomedical AISI 316L stainless steel with zirconium carbonitride coatings. Appl. Surf. Sci. 2015, 340, 113–119. [Google Scholar] [CrossRef]
- Schneider, J.M.; Rebholz, C.; Voevodin, A.A.; Leyland, A.; Matthews, A. Deposition and characterization of nitrogen containing stainless steel coatings prepared by reactive magnetron sputtering. Vacuum 1996, 47, 1077–1080. [Google Scholar] [CrossRef]
- Kappaganthu, S.R.; Sun, Y. Formation of an MN-type cubic nitride phase in reactively sputtered stainless steel-nitrogen films. J. Cryst. Growth 2004, 267, 385–393. [Google Scholar] [CrossRef]
- Kappaganthu, S.R.; Sun, Y. Influence of sputter deposition conditions on phase evolution in nitrogen-doped stainless steel films. Surf. Coat. Technol. 2005, 198, 59–63. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Ávalos, A.; Haza, A.I.; Morales, P. Nanopartículas de plata: Aplicaciones y riesgos tóxicos para la salud humana y el medio ambiente. Rev. Complut. Cienc. Vet. 2013, 7, 1–23. [Google Scholar] [CrossRef]
- Taglietti, A.; Arciola, C.R.; D’Agostino, A.; Dacarro, G.; Montanaro, L.; Campoccia, D.; Cucca, L.; Vercellino, M.; Poggi, A.; Pallavicini, P.; et al. Antibiofilm activity of a monolayer of silver nanoparticles anchored to an amino-silanized glass surface. Biomaterials 2014, 35, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Velasco, S.C.; Cavaleiro, A.; Carvalho, S. Functional properties of ceramic-Ag nanocomposite coatings produced by magnetron sputtering. Prog. Mater. Sci. 2016, 84, 158–191. [Google Scholar] [CrossRef] [Green Version]
- Manninen, N.K.; Ribeiro, F.; Escudeiro, A.; Polcar, T.; Carvalho, S.; Cavaleiro, A. Influence of Ag content on mechanical and tribological behavior of DLC coatings. Surf. Coat. Technol. 2013, 232, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Dang, C.; Li, J.; Wang, Y.; Yang, Y.; Wang, Y.; Chen, J. Influence of Ag contents on structure and tribological properties of TiSiN-Ag nanocomposite coatings on Ti–6Al–4V. Appl. Surf. Sci. 2017, 394, 613–624. [Google Scholar] [CrossRef]
- Macías, H.A.; Yate, L.; Coy, L.E.; Olaya, J.; Aperador, W. Effect of nitrogen flow ratio on microstructure, mechanical and tribological properties of TiWSiNx thin film deposited by magnetron co-sputtering. Appl. Surf. Sci. 2018, 456, 445–456. [Google Scholar] [CrossRef]
- ASTM G99 Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus; ASTM International: West Conshohocken, PA, USA, 2006.
- Ju, H.; He, S.; Yu, L.; Asempah, I.; Xu, J. The improvement of oxidation resistance, mechanical and tribological properties of W2N films by doping silicon. Surf. Coat. Technol. 2017, 317, 158–165. [Google Scholar] [CrossRef]
- Sánchez-López, J.C.; Abad, M.D.; Carvalho, I.; Galindo, R.E.; Benito, N.; Ribeiro, S.; Henriquese, M.; Cavaleirof, A.; Carvalho, S. Influence of silver content on the tribomechanical behavior on Ag-TiCN bioactive coatings. Surf. Coat. Technol. 2012, 206, 2192–2198. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Hang, R.; Gao, A.; Zhang, X.; Huang, X.; Wang, Y.; Tang, B.; Zhao, L.; Chu, P.K. Nanostructured titanium–silver coatings with good antibacterial activity and cytocompatibility fabricated by one-step magnetron sputtering. Appl. Surf. Sci. 2015, 355, 32–44. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Debessai, M.; Filip, P. Zirconium nitride/silver nanocomposite structures for biomedical applications. J. Vac. Sci. Technol. B 2004, 22, 1134–1140. [Google Scholar] [CrossRef]
- Ferreri, I.; Lopes, V.; Tavares, C.J.; Cavaleiro, A.; Carvalho, S. Study of the effect of the silver content on the structural and mechanical behavior of Ag–ZrCN coatings for orthopedic prostheses. Mater. Sci. Eng. C 2014, 42, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Dahm, K.L.; Dearnley, P.A. On the nature, properties and wear response of s-phase (nitrogen-alloyed stainless steel) coatings on AISI 316L. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2000, 214, 181–198. [Google Scholar]
- Bourjot, A.; Foos, M.; Frantz, C. Basic properties of sputtered 310 stainless steel—Nitrogen coatings. In Metallurgical Coatings and Thin Films 1990; Sartwell, B.D., Zemel, J.N., MaGuire, G.E., Bresnock, F.N., Eds.; Elsevier: London, UK, 1990; pp. 533–542. [Google Scholar]
- Thornton, J.A. The microstructure of sputter-deposited coatings. J. Vac. Sci. Technol. A 1986, 4, 3059–3065. [Google Scholar] [CrossRef]
- Kelly, P.J.; Li, H.; Benson, P.S.; Whitehead, K.A.; Verran, J.; Arnell, R.D.; Iordanova, I. Comparison of the tribological and antimicrobial properties of CrN/Ag, ZrN/Ag, TiN/Ag, and TiN/Cu nanocomposite coatings. Surf. Coat. Technol. 2010, 205, 1606–1610. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Buršík, J.; Buršíková, V.; Souček, P.; Zábranský, L.; Vašina, P. Characterization of Ta-BC nanostructured hard coatings. IOP Conf. Ser. Mater. Sci. Eng. 2017, 175, 012020. [Google Scholar] [CrossRef]
- Pöhl, F.; Hardes, C.; Theisen, W. Scratch behavior of soft metallic materials. AIMS Mater. Sci. 2016, 3, 390–403. [Google Scholar] [CrossRef]
- Tschiptschin, A.P.; Garzon, C.M.; Lopez, D.M. Scratch resistance of high nitrogen austenitic stainless steels. In Tribology and Interface Engineering Series; Sinha, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 51, pp. 280–293. [Google Scholar]
- ASTM C1624-05 Standard Test Method for Adhesion Strength and Mechanical Failure Modes of Ceramic Coatings by Quantitative Single Point Scratch Testing; ASTM International: West Conshohocken, PA, USA, 2005.
- Mo, J.L.; Zhu, M.H. Tribological oxidation behaviour of PVD hard coatings. Tribol. Int. 2009, 42, 1758–1764. [Google Scholar] [CrossRef]
- Yu, X.; Qin, Y.; Wang, C.B.; Yang, Y.Q.; Ma, X.C. Effects of nanocrystalline silver incorporation on sliding tribological properties of Ag-containing diamond-like carbon films in multi-ion beam assisted deposition. Vacuum 2013, 89, 82–85. [Google Scholar] [CrossRef]
- Ju, H.; Yu, D.; Yu, L.; Ding, N.; Xu, J.; Zhang, X.; Zheng, Y.; Yang, L.; He, X. The influence of Ag contents on the microstructure, mechanical and tribological properties of ZrN-Ag films. Vacuum 2018, 148, 54–61. [Google Scholar] [CrossRef]


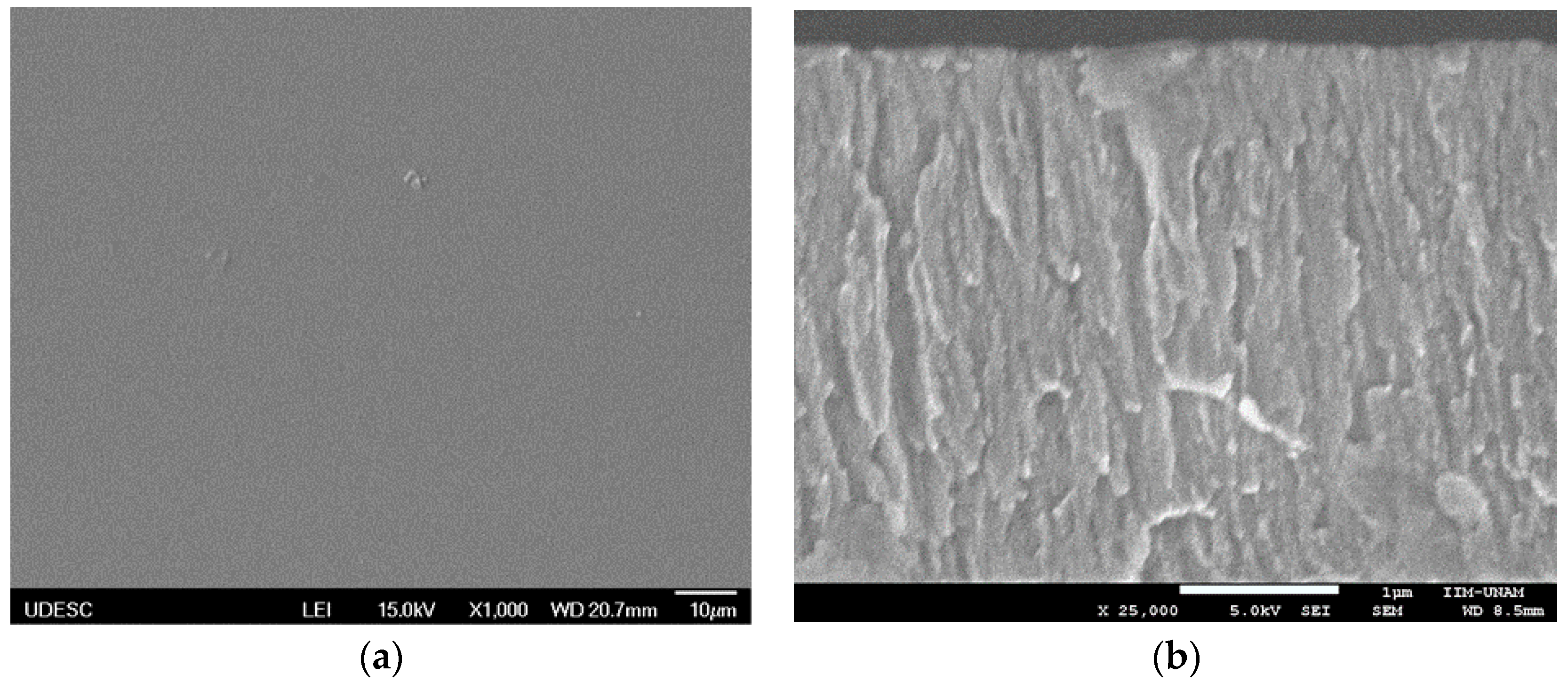
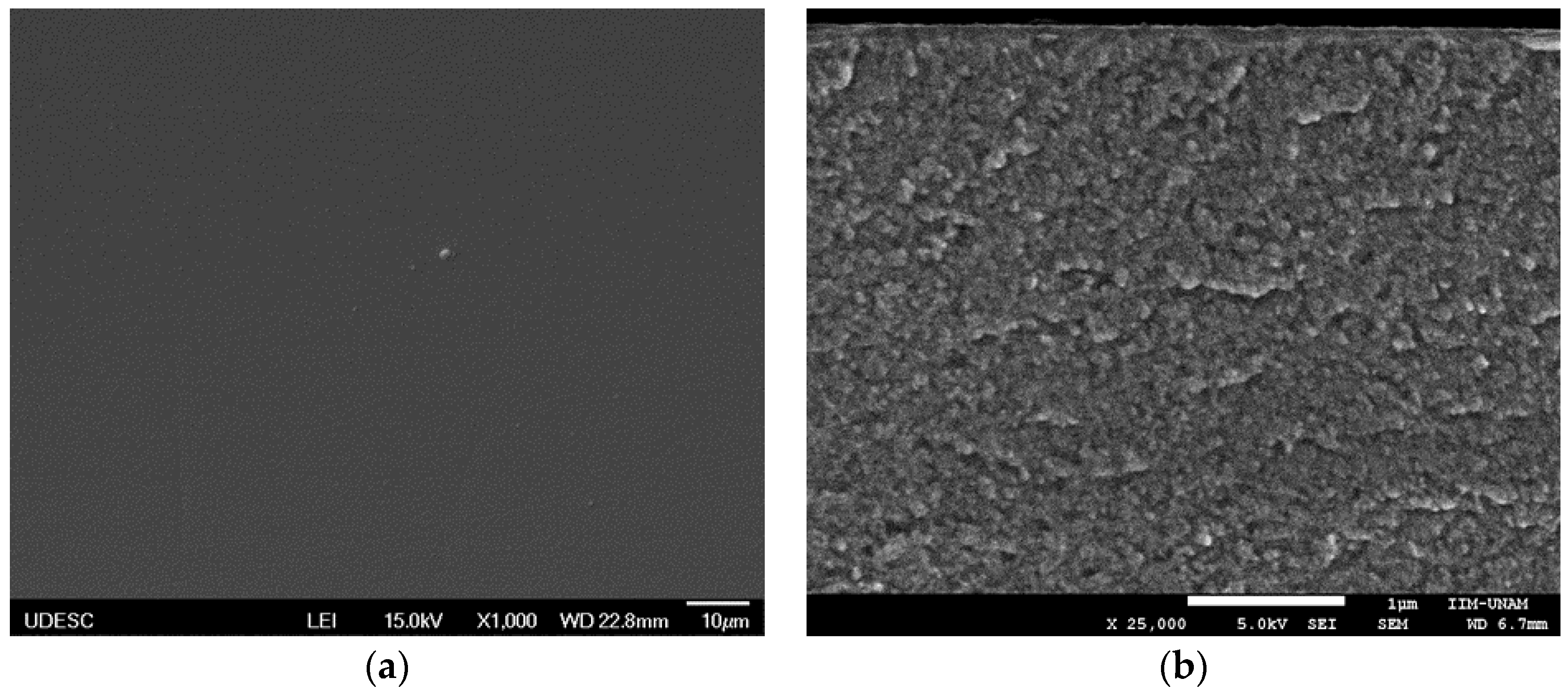
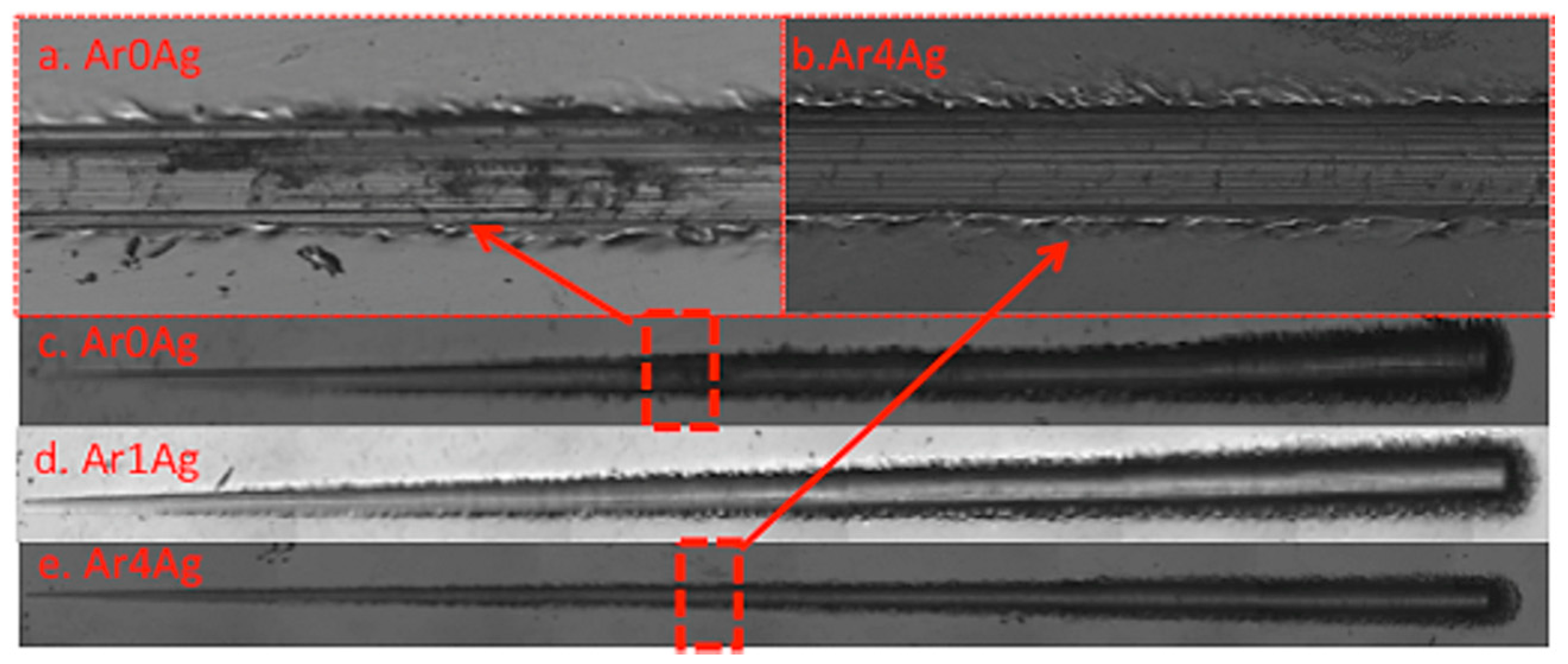

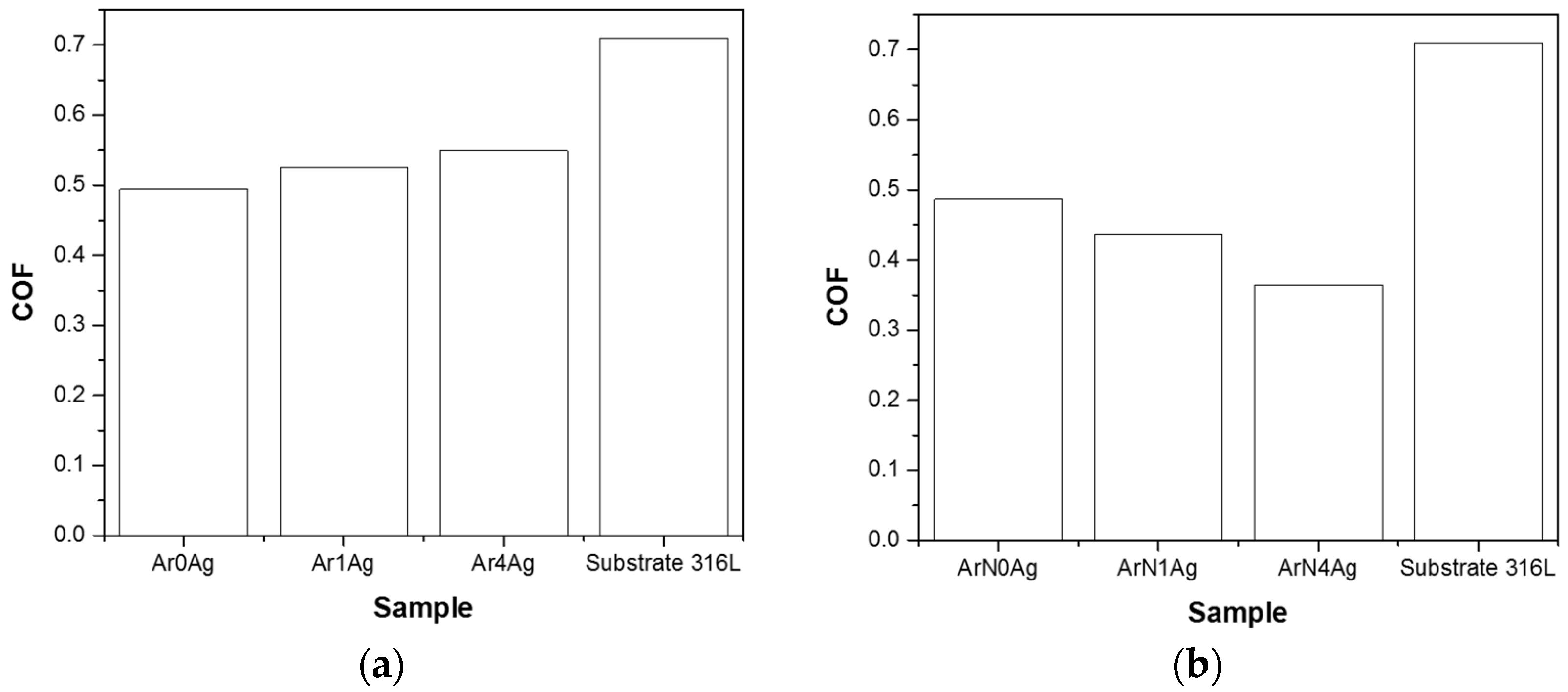
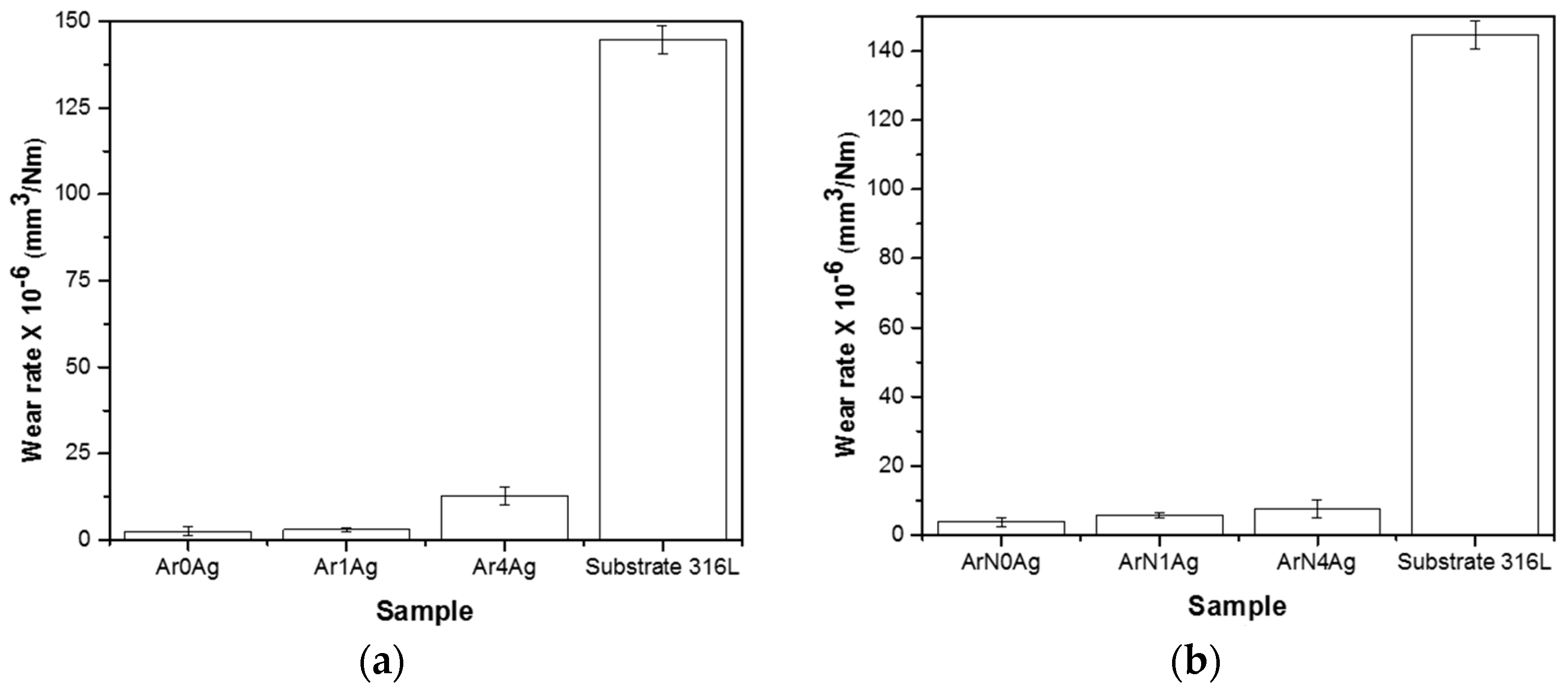
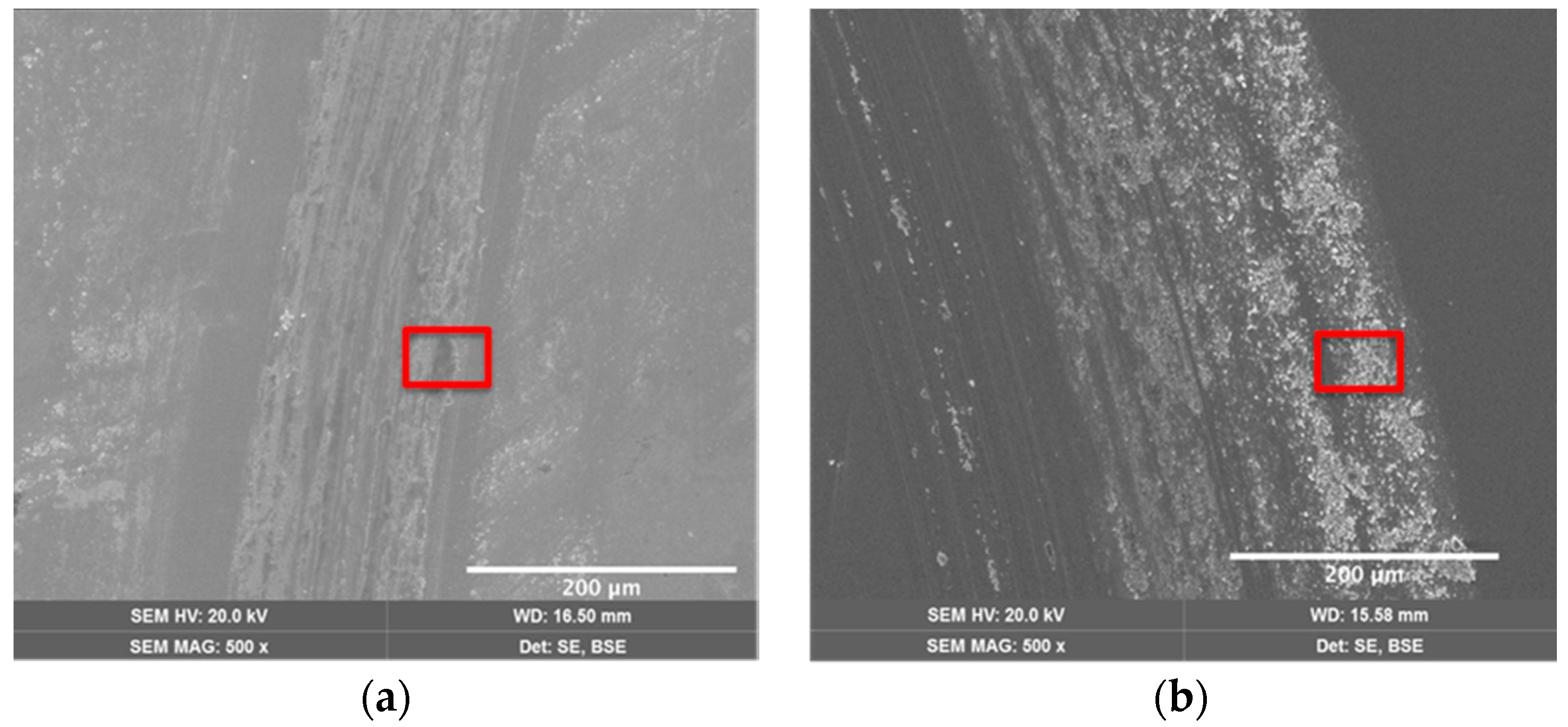
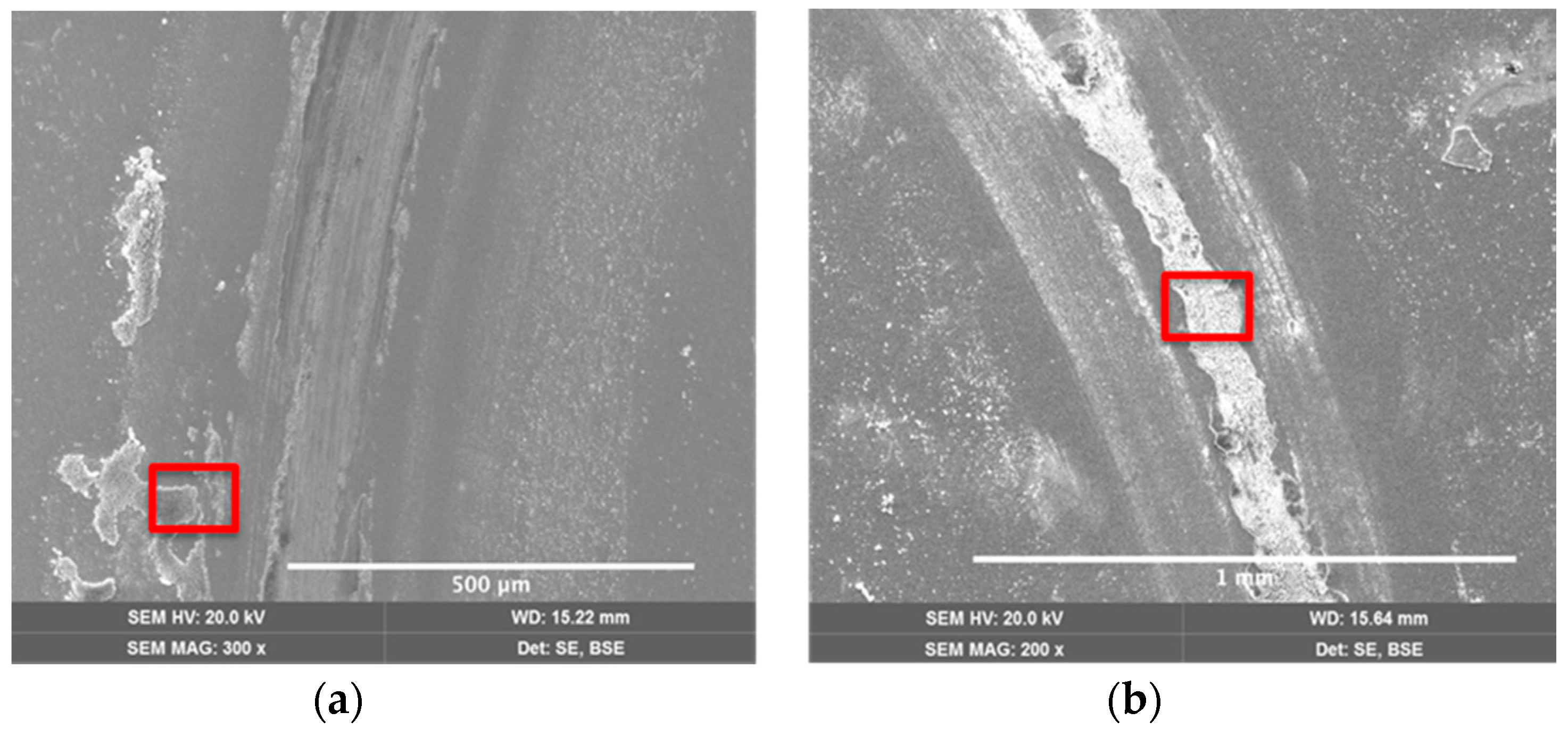
| Coating | Silver Pieces | Atmosphere |
|---|---|---|
| Ar0Ag | 0 | Ar |
| Ar1Ag | 1 | Ar |
| Ar4Ag | 4 | Ar |
| ArN0Ag | 0 | Ar + N2 |
| ArN1Ag | 1 | Ar + N2 |
| ArN4Ag | 4 | Ar + N2 |
| Coating | Phase | Reflection (h k l) | D (nm) | ε | a (nm) |
|---|---|---|---|---|---|
| Ar0Ag | BCC | 110 | 15.45 | 0.10 | 0.288 |
| Ar1Ag | BCC | 200 110 | 8.28 19.24 | 0.31 0.08 | 0.289 0.288 |
| Ar4Ag | BCC | 110 | 16.46 | 0.09 | 0.287 |
| ArN0Ag | FCC | 200 | 12.93 | 0.15 | 0.363 |
| ArN1Ag | FCC | 200 | 9.69 | 0.19 | 0.363 |
| AgN4Ag | FCC | 111 | 4.24 | 0.38 | 0.353 |
| Coating | Hardness (GPa) | Elastic Module (GPa) | H/E | H3/E2 (GPa) | Chemical Composition (at.%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Fe | Ni | Mn | Mo | Ag | |||||
| Ar0Ag | 8.1 ± 0.8 | 211 ± 14 | 0.039 ± 6.4 × 10−5 | 0.012 ± 5.1 × 10−5 | 16.3 | 69.1 | 9.6 | 2.8 | 2.3 | 0.0 |
| Ar1Ag | 8.6 ± 0.4 | 210 ± 12 | 0.041 ± 4.2 × 10−5 | 0.014 ± 3.6 × 10−5 | 16.3 | 68.1 | 9.8 | 2.6 | 2.2 | 1.0 |
| Ar4Ag | 10 ± 0.5 | 242 ± 15 | 0.042 ± 4.7 × 10−5 | 0.018 ± 4.9 × 10−5 | 15.7 | 64.8 | 9.5 | 2.6 | 2.2 | 5.2 |
| ArN0Ag | 8.2 ± 0.3 | 187 ± 15 | 0.044 ± 5.1 × 10−5 | 0.016 ± 4.3 × 10−5 | 16.4 | 68.6 | 9.8 | 3.1 | 2.2 | 0.0 |
| ArN1Ag | 9.9 ± 0.3 | 196 ± 8 | 0.051 ± 3.6 × 10−5 | 0.025 ± 4.3 × 10−5 | 17.2 | 67.7 | 9.5 | 3.0 | 2.2 | 0.5 |
| ArN4Ag | 13.2 ± 0.5 | 214 ± 7 | 0.062 ± 4.4 × 10−5 | 0.050 ± 8.9 × 10−5 | 14.4 | 59.5 | 8.7 | 2.4 | 2.0 | 13.1 |
| Target 316L | – | – | – | – | 17.4 | 65.6 | 8.9 | 3.8 | 4.2 | 0.0 |
| Substrate 316L | 2.8 ± 0.3 | 189 ± 15 | 0.014 ± 2.7 × 10−5 | 0.001 ± 2.9 × 10−6 | 16.4 | 69.9 | 10.1 | 1.5 | 2.1 | 0.0 |
| Coating | Chemical Composition (at.%) | ||||||
|---|---|---|---|---|---|---|---|
| Fe | Cr | Ni | O | Mn | Al | Ag | |
| Ar0Ag | 55.0 | 13.3 | 6.3 | 24.1 | 0.4 | 1.0 | – |
| Ar4Ag | 40.3 | 10.1 | 5.0 | 42.6 | 0.3 | – | 0.3 |
| ArN0Ag | 48.2 | 12.4 | 6.1 | 32.8 | 0.5 | – | – |
| ArN4Ag | 32.8 | 8.5 | 3.9 | 52.6 | 0.3 | – | 1.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
España P., C.L.; Recco, A.A.C.; Olaya, J.J. A Microstructural and Wear Resistance Study of Stainless Steel-Ag Coatings Produced through Magnetron Sputtering. Coatings 2018, 8, 381. https://doi.org/10.3390/coatings8110381
España P. CL, Recco AAC, Olaya JJ. A Microstructural and Wear Resistance Study of Stainless Steel-Ag Coatings Produced through Magnetron Sputtering. Coatings. 2018; 8(11):381. https://doi.org/10.3390/coatings8110381
Chicago/Turabian StyleEspaña P., Claudia L., Abel A. C. Recco, and Jhon J. Olaya. 2018. "A Microstructural and Wear Resistance Study of Stainless Steel-Ag Coatings Produced through Magnetron Sputtering" Coatings 8, no. 11: 381. https://doi.org/10.3390/coatings8110381




