Hardening of HVOF-Sprayed Austenitic Stainless-Steel Coatings by Gas Nitriding
Abstract
:1. Introduction
2. Materials and Methods
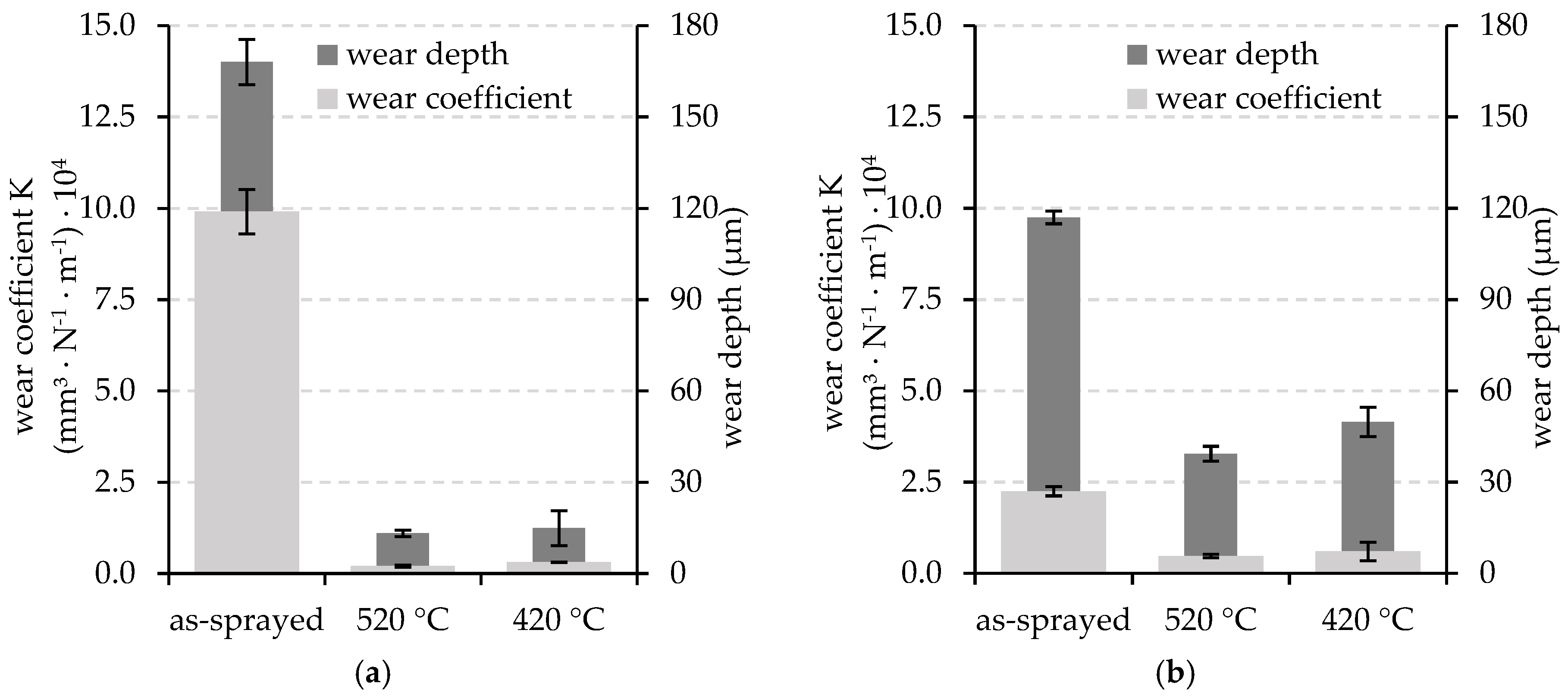
3. Results
3.1. Chemical Composition
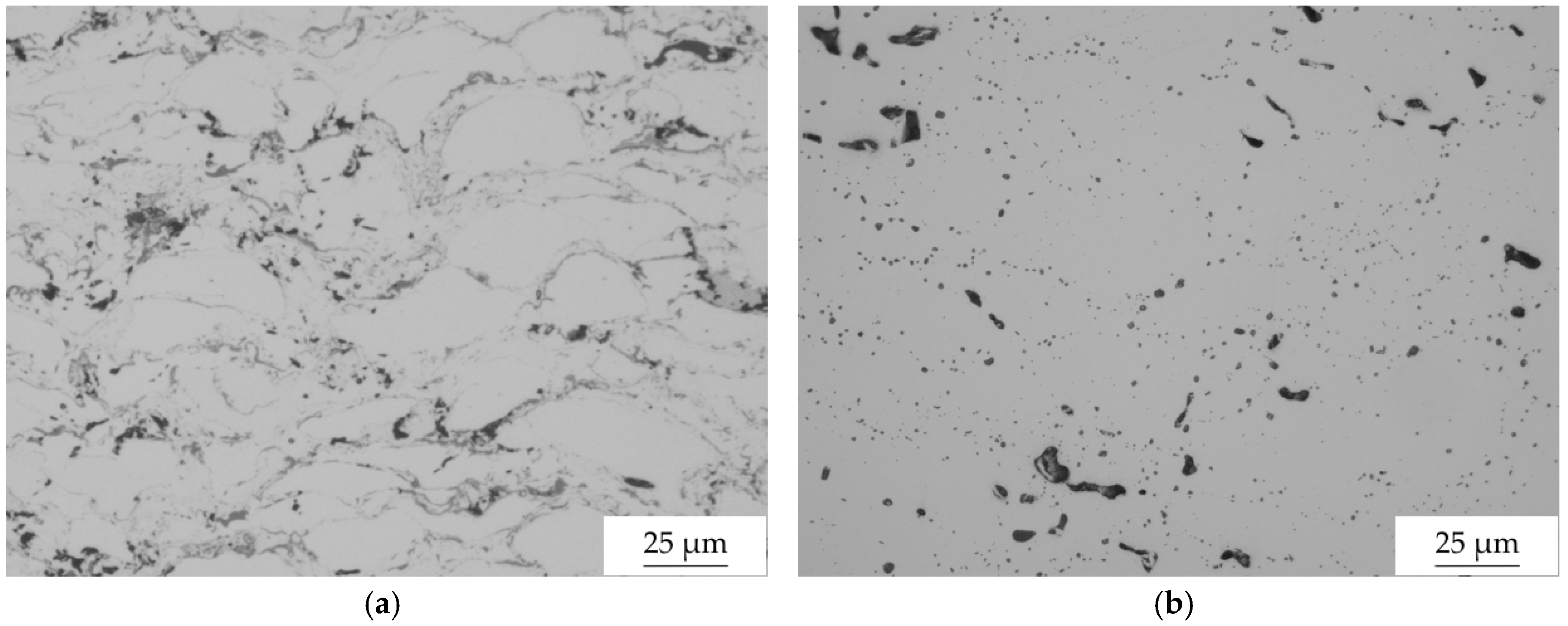
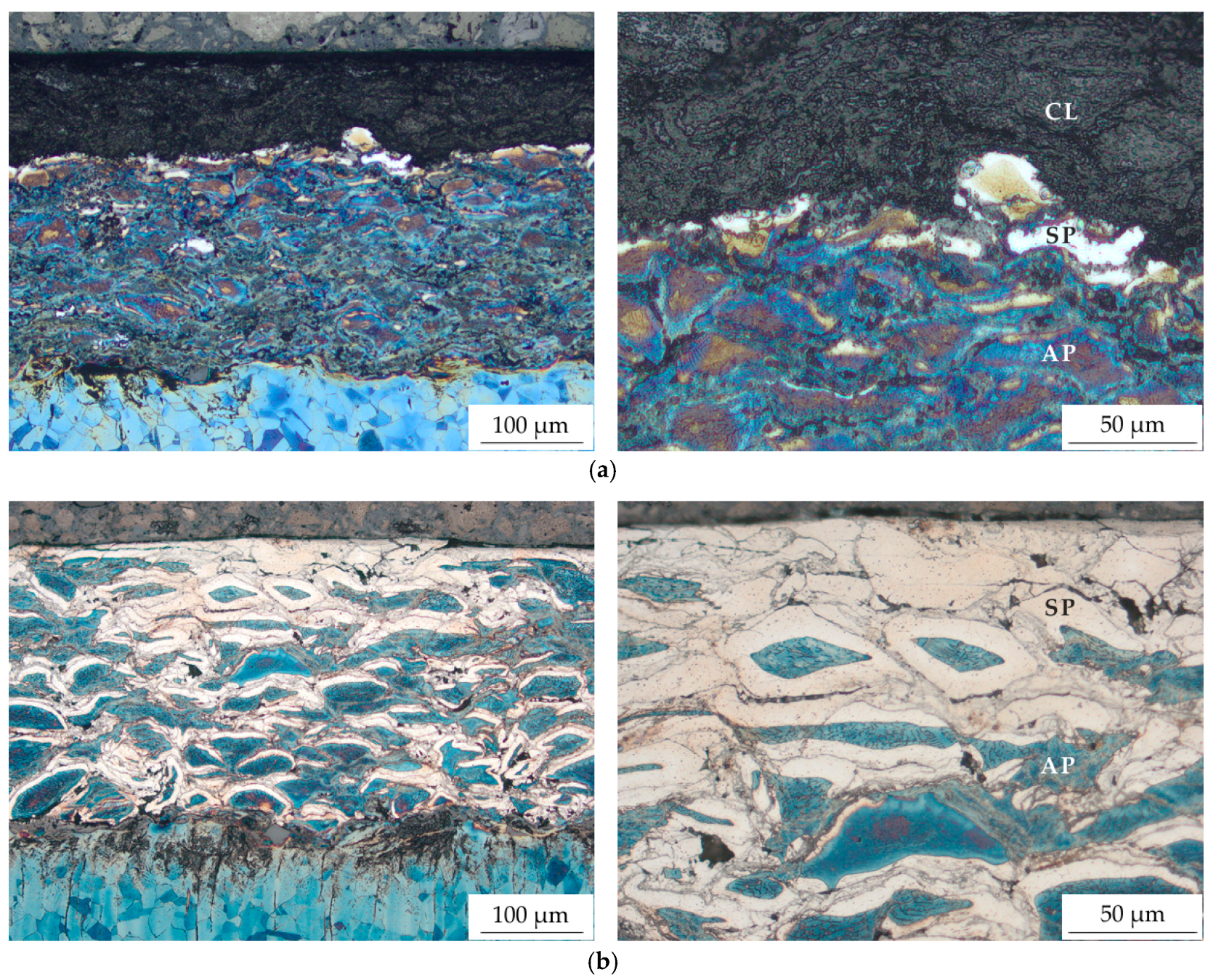
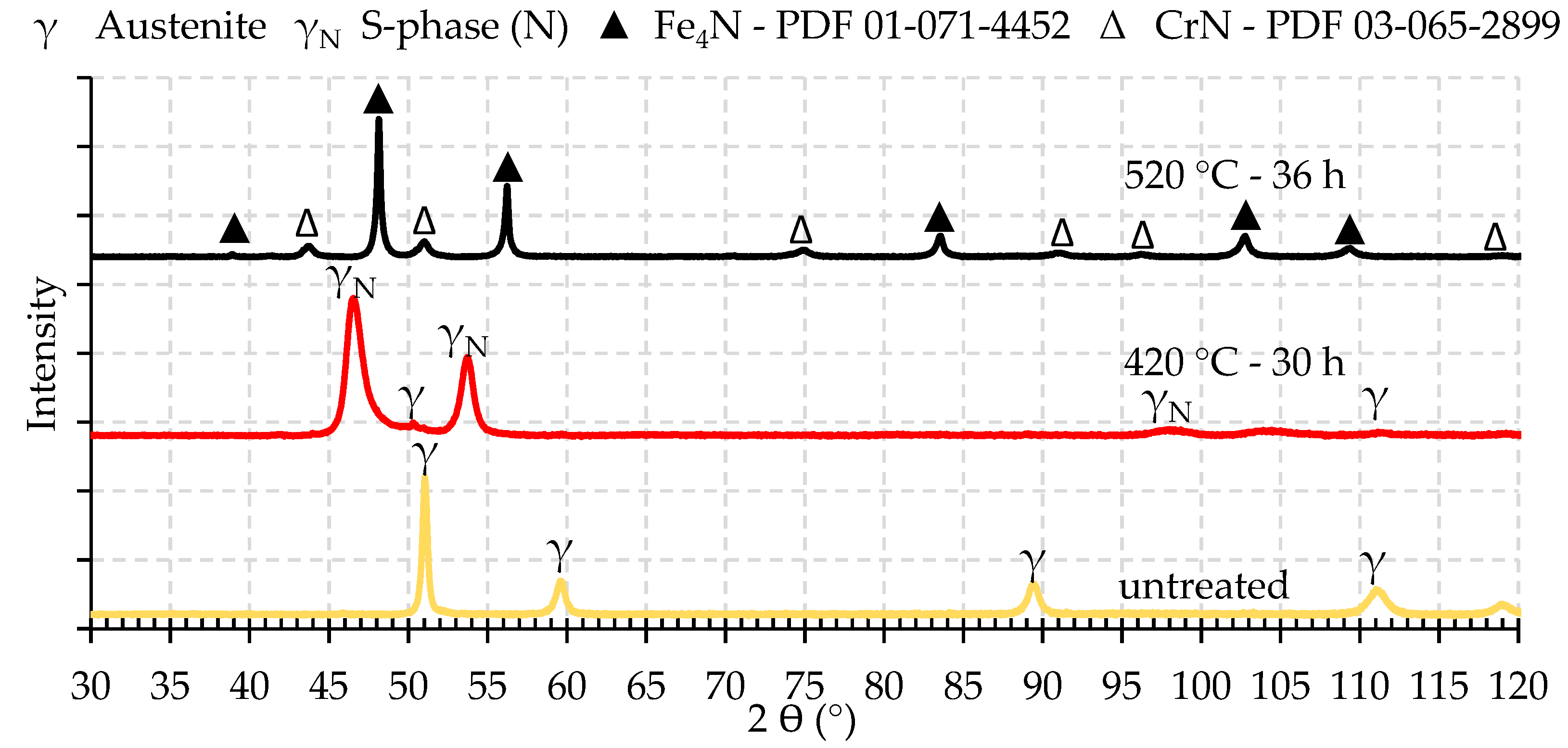
3.2. Microstructure Analyses
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Somers, M.A.J.; Christiansen, T.L. Low temperature surface hardening of stainless steel. In Thermochemical Surface Engineering of Steels; Mittemeijer, E.J., Somers, M.A.J., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 557–579. [Google Scholar]
- Zhao, C.; Li, C.X.; Dong, H.; Bell, T. Low temperature plasma nitrocarburising of AISI 316 austenitic stainless steel. Surf. Coat. Technol. 2005, 191, 195–200. [Google Scholar]
- Bell, T. Current status of supersaturated surface engineered S-phase materials. Key Eng. Mater. 2008, 373, 289–295. [Google Scholar] [CrossRef]
- Adachi, S.; Ueda, N. Combined plasma carburizing and nitriding of sprayed AISI 316L coating for improved wear resistance. Surf. Coat. Technol. 2014, 259, 44–49. [Google Scholar] [CrossRef]
- Nestler, M.C.; Spies, H.; Hermann, K. Production of duplex coatings by thermal spraying and nitriding. Surf. Eng. 1996, 12, 299–302. [Google Scholar] [CrossRef]
- Park, G.; Bae, G.; Moon, K.; Lee, C. Effect of plasma nitriding and nitrocarburinzing on HVOF-sprayed stainless steel coatings. J. Therm. Spray Technol. 2013, 22, 1366–1373. [Google Scholar] [CrossRef]
- Wielage, B.; Rupprecht, C.; Lindner, T.; Hunger, R. Surface modification of austenitic thermal spray coatings by low-temperature carburization. In Proceedings of the International Thermal Spray Conference & Exposition, Long Beach, CA, USA, 11–14 May 2015. DVS 276. [Google Scholar]
- Adachi, S.; Ueda, N. Formation of S-phase layer on plasma sprayed AISI 316L stainless steel coating by plasma nitriding at low temperature. Thin Solid Films 2012, 523, 11–14. [Google Scholar] [CrossRef]
- Adachi, S.; Ueda, N. Formation of expanded austenite on a cold-sprayed AISI 316L coating by low-temperature plasma nitriding. J. Therm. Spray Technol. 2015, 24, 1399–1407. [Google Scholar] [CrossRef]
- Adachi, S.; Ueda, N. Surface hardness improvement of plasma-sprayed AISI 316L stainless steel coating by low-temperature plasma carburizing. Adv. Powder Technol. 2013, 24, 818–823. [Google Scholar] [CrossRef]
- Lindner, T.; Mehner, T.; Lampke, T. Surface modification of austenitic thermal-spray coatings by low-temperature nitrocarburizing. IOP Conf. Ser. Mater. Sci. Eng. 2016, 118, 012008. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Hummelshøj, T.S.; Somers, M.A.J. Expanded austenite, crystallography and residual stress. Surf. Eng. 2010, 26, 242–247. [Google Scholar] [CrossRef]
- Brink, B.K.; Ståhl, K.; Christiansen, T.L.; Oddershede, J. On the elusive crystal structure of expanded austenite. Scr. Mater. 2017, 131, 59–62. [Google Scholar] [CrossRef]
- ASTM G 133 Standard Test Method for Linearly Reciprocating Ball-on-Flat Sliding Wear; ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM G 99 Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus; ASTM International: West Conshohocken, PA, USA, 2016.
- Rajendrana, P.; Devaraju, A. Experimental evaluation of mechanical and tribological behaviours of gas nitride treated AISI 316LN austenitic stainless steel. Mater. Today Proc. 2018, 5, 14333–14338. [Google Scholar] [CrossRef]
- Subbiah, R.; Rajavel, R. Dry sliding wear behaviour analysis of nitrided 316LN grade austenitic stainless steels using gas nitriding process. J. Theor. Appl. Inf. Technol. 2010, 19, 98–101. [Google Scholar]

| Kerosene Flow Rate (L/h) | Oxygen Flow Rate (L/min) | λ | Combustion Chamber Pressure (bar) | Spraying Distance (mm) | Nozzle (mm) | Transverse Speed (m/s) | Offset (mm) | Powder Feed Rate (g/min) | Gas Feed (Argon) (L/min) | Wiper |
|---|---|---|---|---|---|---|---|---|---|---|
| 24 | 900 | 1.1 | 7.1 | 350 | 150/14 | 1 | 5 | 70 | 2 × 8 | NL |
| Process Gas | Volume Flow (L/h) | Temperature (°C) | Duration (h) |
|---|---|---|---|
| NH3 | 1000 | 520; 420 | 36; 30 |
| Reciprocating Ball-on-Plane Test | Ball-on-Disk Test | ||
|---|---|---|---|
| Normal load | 26 N | Normal load | 20 N |
| Frequency | 40 Hz | Radius | 5 mm |
| Time | 900 s | Speed | 96 RPM |
| Amplitude | 0.5 mm | Cycles | 15916 |
| Ø Al2O3 | 10 mm | Ø Al2O3 | 6 mm |
| Sample | Feedstock | HVOF Coating | Substrate |
|---|---|---|---|
| Fe | 66.2 | 67 | 70 |
| Cr | 16.7 | 16.5 | 16.8 |
| Ni | 12.8 | 12.7 | 10.1 |
| Mo | 2.8 | 2.3 | 1.9 |
| Mn | 1.5 | 1.5 | 1.2 |
| O2 | 0.1 | 0.65 | – |
| Phase | Austenite | S-phase | Compound Layer |
|---|---|---|---|
| HV 0.01 | 334 ± 15 | 958 ± 86 | 861 ± 102 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindner, T.; Kutschmann, P.; Löbel, M.; Lampke, T. Hardening of HVOF-Sprayed Austenitic Stainless-Steel Coatings by Gas Nitriding. Coatings 2018, 8, 348. https://doi.org/10.3390/coatings8100348
Lindner T, Kutschmann P, Löbel M, Lampke T. Hardening of HVOF-Sprayed Austenitic Stainless-Steel Coatings by Gas Nitriding. Coatings. 2018; 8(10):348. https://doi.org/10.3390/coatings8100348
Chicago/Turabian StyleLindner, Thomas, Pia Kutschmann, Martin Löbel, and Thomas Lampke. 2018. "Hardening of HVOF-Sprayed Austenitic Stainless-Steel Coatings by Gas Nitriding" Coatings 8, no. 10: 348. https://doi.org/10.3390/coatings8100348
APA StyleLindner, T., Kutschmann, P., Löbel, M., & Lampke, T. (2018). Hardening of HVOF-Sprayed Austenitic Stainless-Steel Coatings by Gas Nitriding. Coatings, 8(10), 348. https://doi.org/10.3390/coatings8100348







