Polymeric Antimicrobial Coatings Based on Quaternary Ammonium Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Precursors
2.3. Introduction of Antimicrobial Species
2.3.1. Covalently Bound Antimicrobial Groups
2.3.2. Electrostatically Attached Antimicrobial Groups
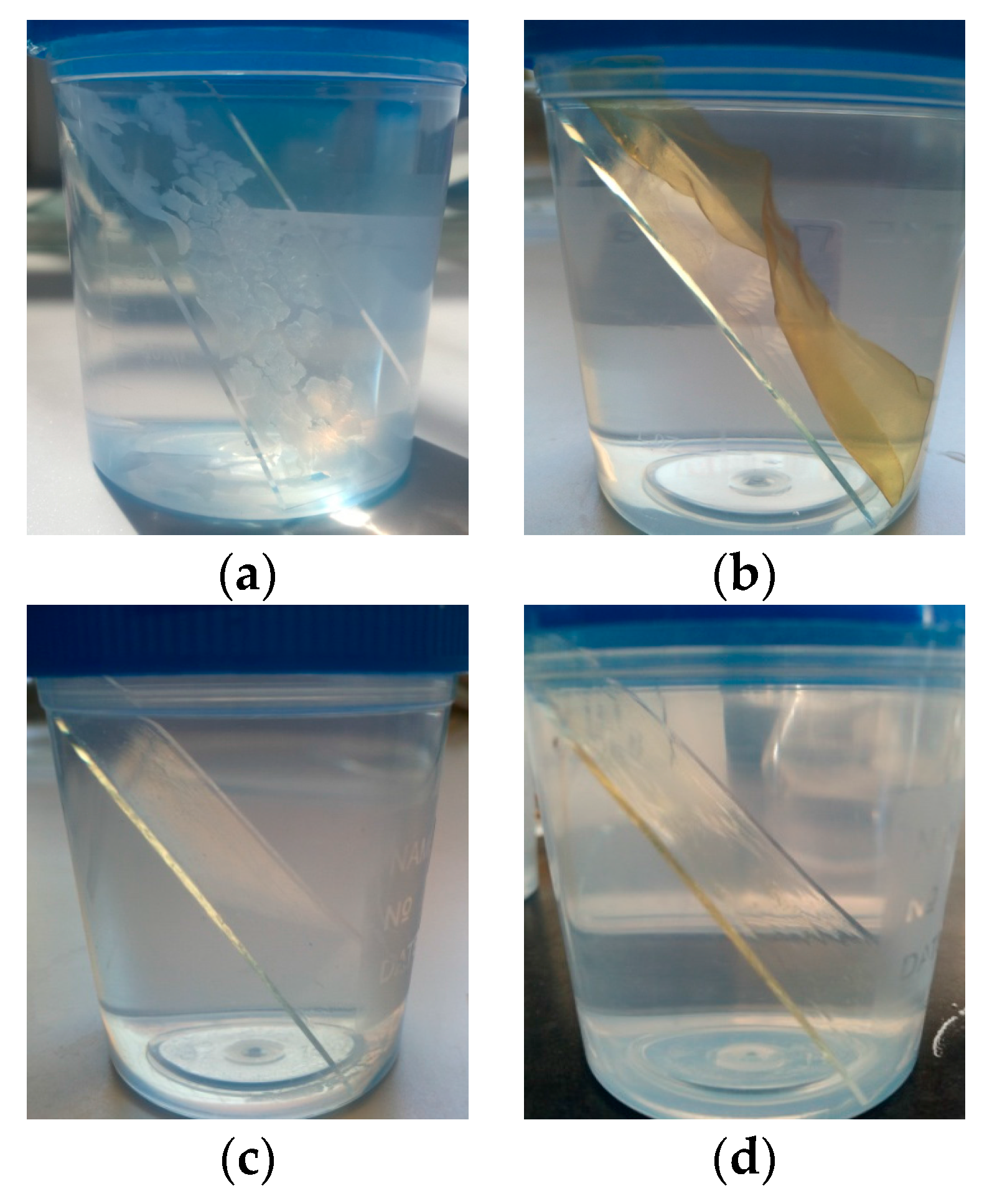
2.4. Preparation of Antimicrobial Coatings
2.5. Immersion of Coatings in Ultra-Pure Water and Aqueous NaCl Solutions
2.6. Characterization Techniques
2.6.1. Scanning Electron Microscopy (SEM) Examination
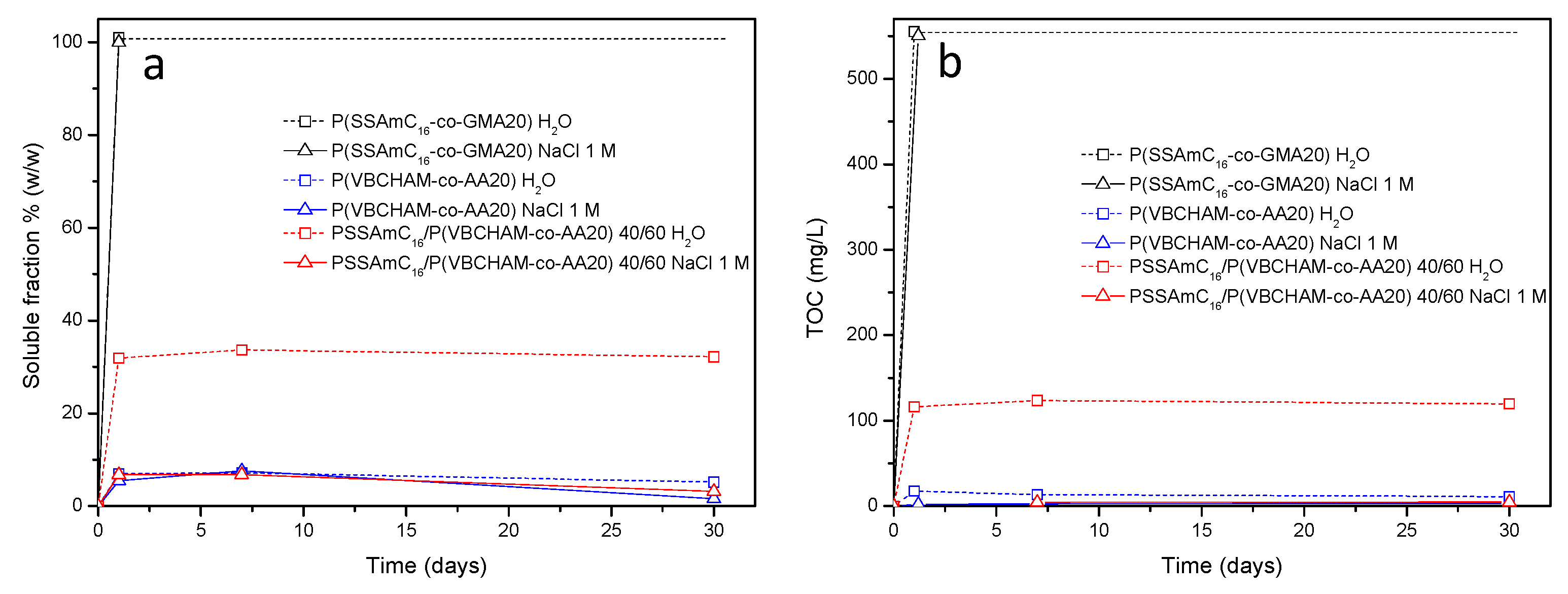
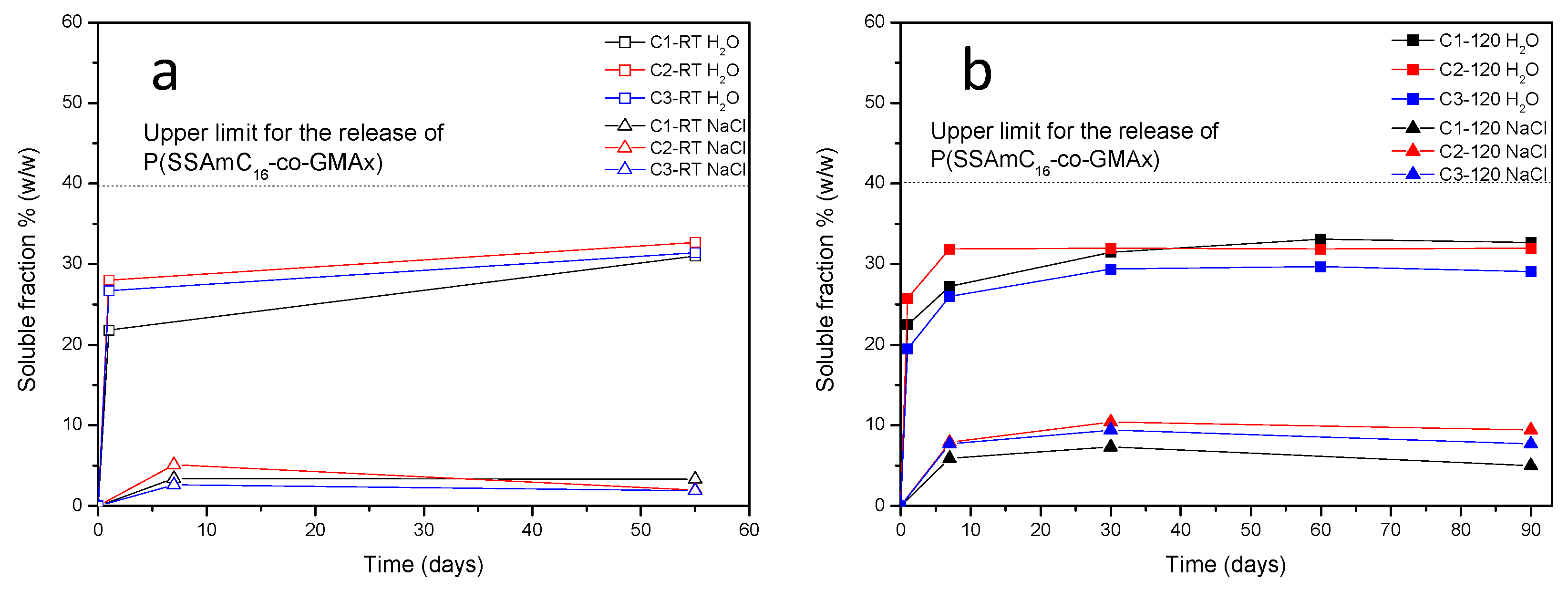
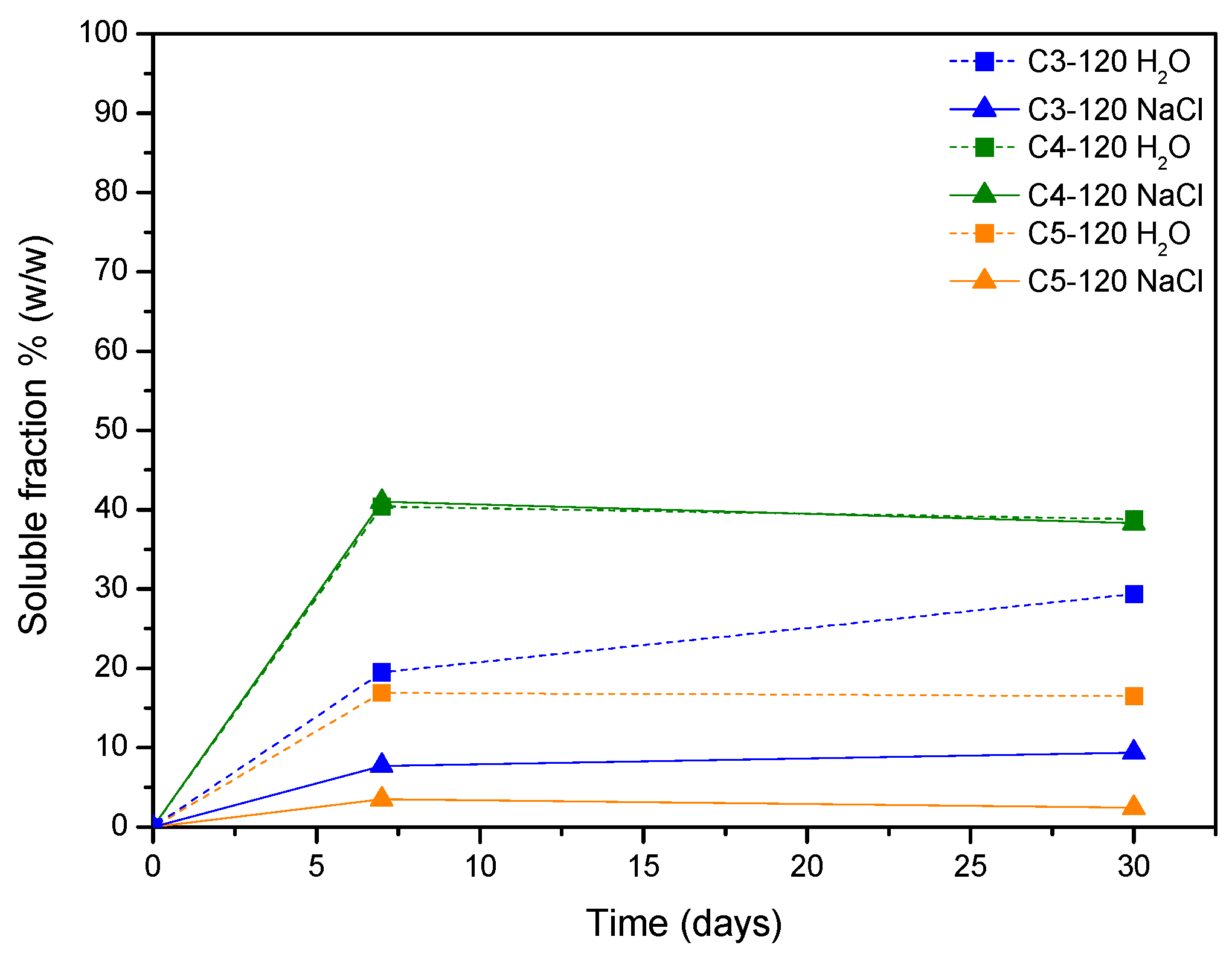
2.6.2. Release Studies
2.6.3. Total Organic Carbon (TOC) and Total Nitrogen (TN) Measurements
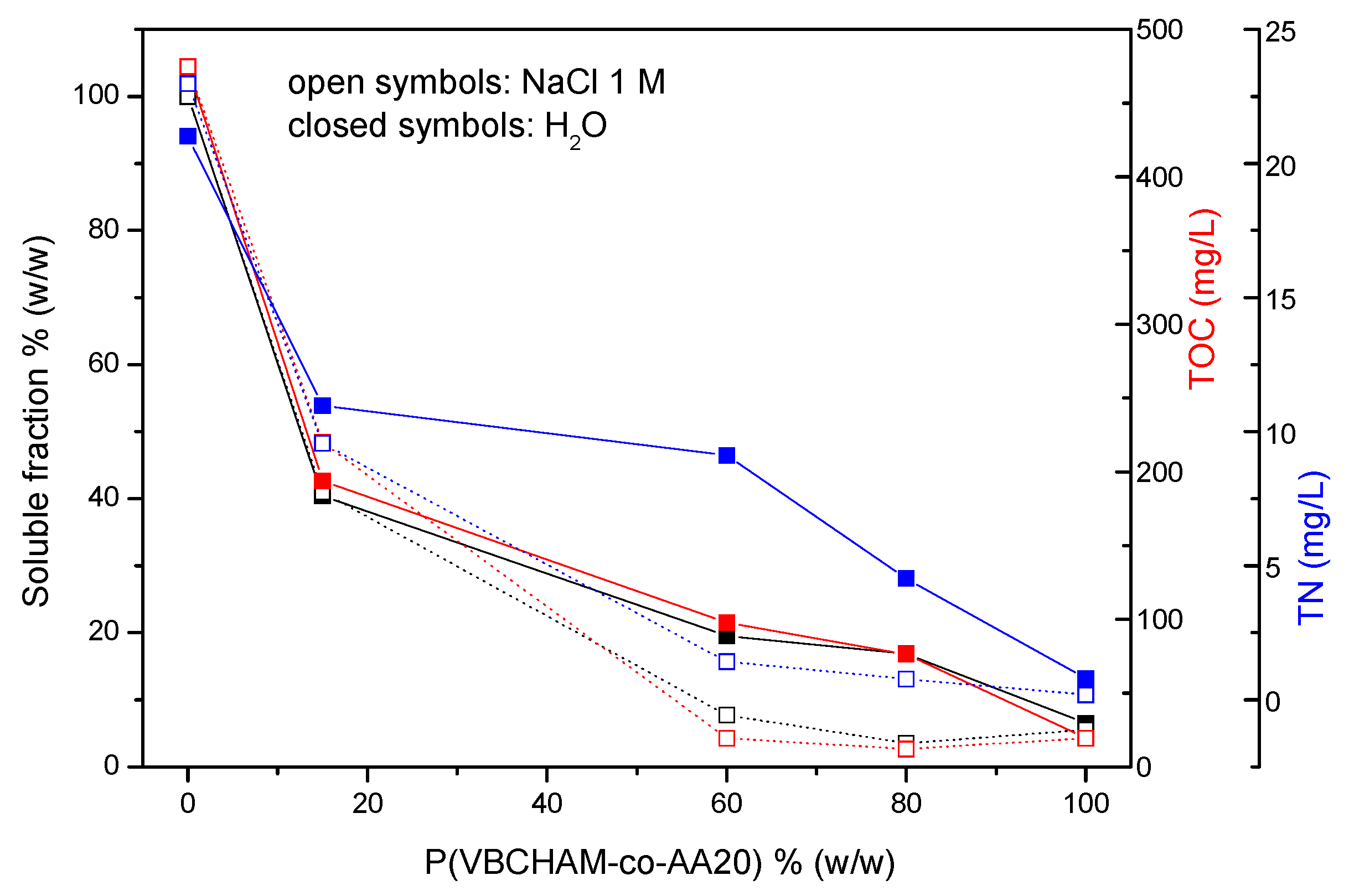
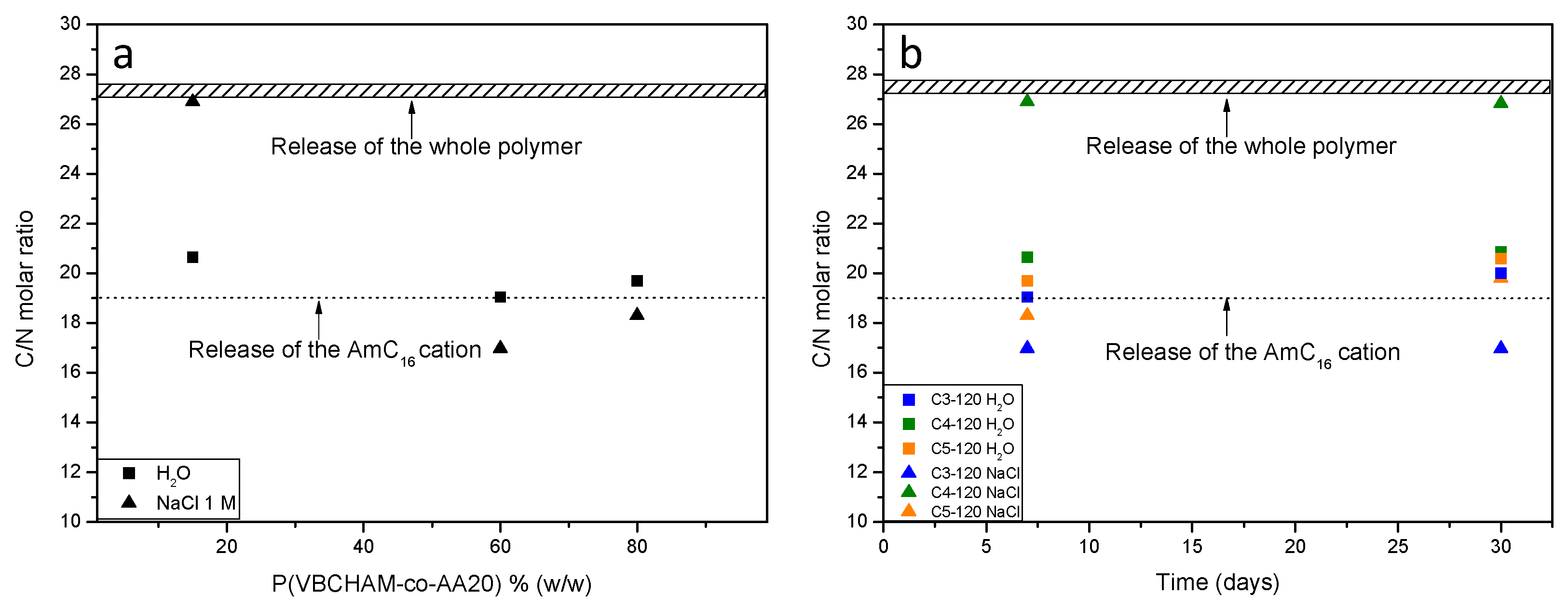
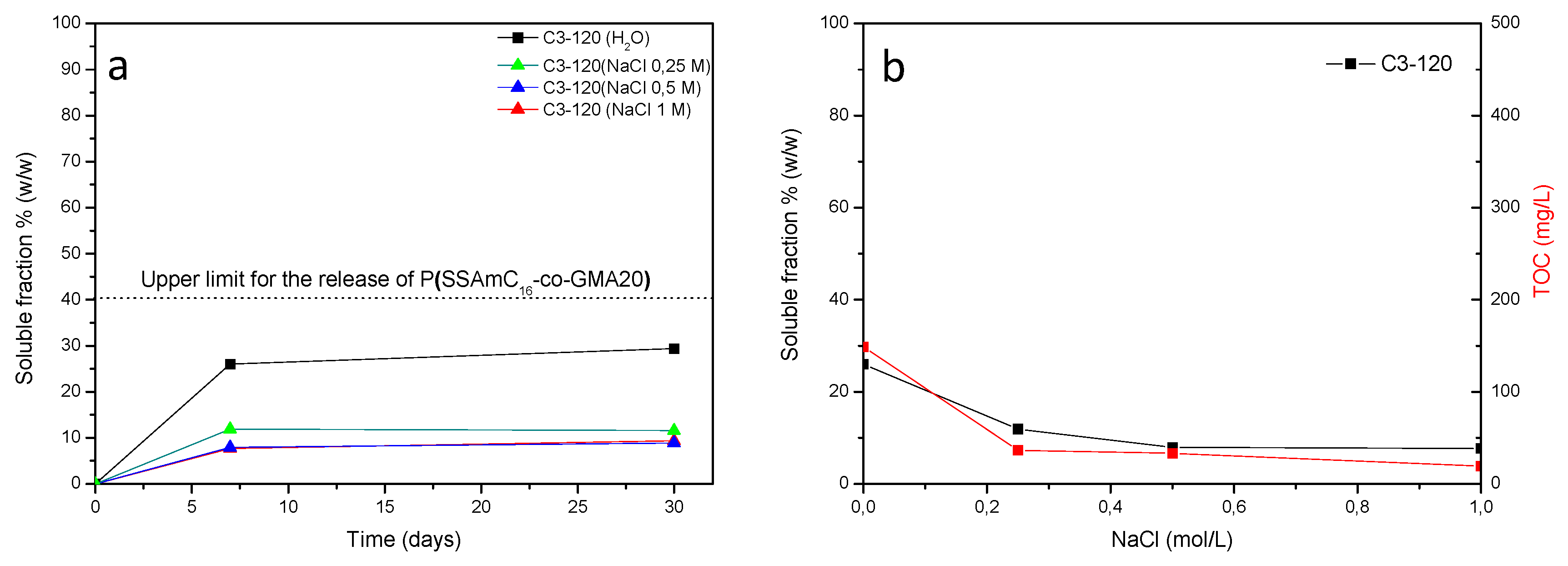
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krumm, C.; Tiller, J.C. Antimicrobial Polymers and Surfaces–Natural Mimics or Surpassing Nature? In Bio-Inspired Polymers, 1st ed.; Bruns, N., Kilbinger, A.F.M., Eds.; RSC Polymer Chemistry Series: Cambridge, UK, 2017; pp. 490–522. [Google Scholar]
- Yang, Y.; Cai, Z.; Huang, Z.; Tan, X.; Zhan, X. Antimicrobial cationic polymers: From structural design to functional control. Polym. J. 2017, 1–12. [Google Scholar] [CrossRef]
- Schnaider, L.; Brahmachari, S.; Schmidt, N.W.; Mensa, B.; Shaham-Niv, S.; Bychenko, D.; Adler-Abramovich, L.; Shimon, L.J.W.; Kolusheva, S.; DeGrado, W.F.; et al. Self-assembling dipeptide antibacterial nanostructures with membrane disrupting activity. Nat. Commun. 2017, 8, 1365. [Google Scholar] [CrossRef] [PubMed]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernandez-Garcia, M. The roadmap of antimicrobial polymeric materials in macromolecular nanotechnology. Eur. Polym. J. 2015, 65, 46–62. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernandez-Garcia, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Álvarez-Paino, M.; Muñoz-Bonilla, A.; Fernández-García, M. Antimicrobial polymers in the nano-world. Nanomaterials 2017, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Niu, L.; Ma, S.; Li, J.; Tay, F.R.; Chen, J. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Simoncic, B.; Tomsic, B. Structures of novel antimicrobial agents for textiles—A review. Text. Res. J. 2010, 80, 1721–1737. [Google Scholar] [CrossRef]
- Ren, W.; Cheng, W.; Wang, G.; Liu, Y. Developments in antimicrobial polymers. J. Appl. Polym. Sci. 2017, 55, 632–639. [Google Scholar] [CrossRef]
- Huang, K.-S.; Yang, C.-H.; Huang, S.-L.; Chen, C.-Y.; Lu, Y.-Y.; Lin, Y.-S. Recent advances in antimicrobial polymers: A mini-review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Peng, H.; Blakey, I.; Whittaker, A.K. Biocidal polymers: A mechanistic overview. Polym. Rev. 2017, 57, 276–310. [Google Scholar] [CrossRef]
- Zubris, D.L.; Minbiole, K.P.; Wuest, W.M. Polymeric quaternary ammonium compounds: Versatile antimicrobial materials. Curr. Top. Med. Chem. 2017, 17, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Koepsel, R.R.; Murata, H.; Zadan, S.; Campbell, A.S.; Russell, A.J. Bactericidal specificity and resistance profile of poly(quaternary ammonium) polymers and protein–poly(quaternary ammonium) conjugates. Biomacromolecules 2017, 18, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Abdel-Hay, F.I.; El-Shanshoury, A.E.-R.R.; El-Newehy, M.H. Biologically active polymers. V. Synthesis and antimicrobial activity of modified poly(glycidyl methacrylate-co-2-hydroxyethyl methacrylate) derivatives with quaternary ammonium and phosphonium salts. J. Polym. Sci. A Polym. Chem. 2002, 40, 2384–2393. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Tang, C. Controlling macromolecular structures towards effective antimicrobial polymers. Polymer 2015, 63, A1–A29. [Google Scholar] [CrossRef]
- Tiwari, A. Handbook of Antimicrobial Coatings, 1st ed.; Elsevier Science & Technology Books: New York, NY, USA, 2017; pp. 1–596. ISBN 0128119829. [Google Scholar]
- Li, H.; Bao, H.; Bok, K.X.; Lee, C.Y.; Li, B.; Zin, M.T.; Kang, L. High durability and low toxicity antimicrobial coatings fabricated by quaternary ammonium silane copolymers. Biomater. Sci. 2016, 4, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Sisti, L.; Cruciani, L.; Totaro, G.; Vannini, M.; Berti, C.; Aloisio, I.; Di Gioia, D. Antibacterial coatings on poly(fluoroethylenepropylene) films via grafting of 3-hexadecyl-1-vinylimidazolium bromide. Prog. Org. Coat. 2012, 73, 257–263. [Google Scholar] [CrossRef]
- Wei, T.; Tang, Z.; Yu, Q.; Chen, H. Smart antibacterial surfaces with switchable bacteria-killing and bacteria-releasing capabilities. ACS Appl. Mater. Interfaces 2017, 9, 37511–37523. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wu, Z.; Chen, H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomater. 2015, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Voo, Z.X.; Khan, M.; Xu, Q.; Narayanan, K.; Ng, B.W.J.; Ahmad, R.B.; Hedrick, J.L.; Yang, Y.Y. Antimicrobial coatings against biofilm formation: The unexpected balance between antifouling and bactericidal behavior. Polym. Chem. 2016, 7, 656–668. [Google Scholar] [CrossRef]
- Voo, Z.X.; Khan, M.; Narayanan, K.; Seah, D.; Hedrick, J.L.; Yang, Y.Y. Antimicrobial/antifouling polycarbonate coatings: Role of block copolymer architecture. Macromolecules 2015, 48, 1055–1064. [Google Scholar] [CrossRef]
- Kaura, R.; Liu, S. Antibacterial surface design—Contact kill. Prog. Surf. Sci. 2016, 91, 136–153. [Google Scholar] [CrossRef]
- Alarfaj, A.A.; Lee, H.H.; Munusamy, M.A.; Ling, Q.-D.; Kumar, S.; Chang, Y.; Chen, Y.-M.; Lin, H.-R.; Lu, Y.-T.; Wu, G.-J.; et al. Development of biomaterial surfaces with and without microbial nanosegments. J. Polym. Eng. 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Gao, J.; White, E.M.; Liu, Q.; Locklin, J. Evidence for the phospholipid sponge effect as the biocidal mechanism in surface-bound polyquaternary ammonium coatings with variable cross-linking density. ACS Appl. Mater. Interfaces 2017, 9, 7745–7751. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.E.; Fonseca, A.C.; Mendonça, P.V.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Recent developments in antimicrobial polymers: a review. Materials 2016, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Kougia, E.; Tselepi, M.; Vasilopoulos, G.; Lainioti, G.Ch.; Koromilas, N.D.; Druvari, D.; Bokias, G.; Vantarakis, A.; Kallitsis, J.K. Evaluation of antimicrobial efficiency of new polymers comprised by covalently attached and/or electrostatically bound bacteriostatic species, based on quaternary ammonium compounds. Molecules 2015, 20, 21313–21327. [Google Scholar] [CrossRef] [PubMed]
- Koromilas, N.D.; Lainioti, G.Ch.; Vasilopoulos, G.; Vandarakis, A.; Kallitsis, J.K. Synthesis of antimicrobial block copolymers bearing immobilized bacteriostatic groups. Polym. Chem. 2016, 7, 3562–3575. [Google Scholar] [CrossRef]
- Druvari, D.; Koromilas, N.D.; Lainioti, G.Ch.; Bokias, G.; Vasilopoulos, G.; Vandarakis, A.; Baras, I.; Dourala, N.; Kallitsis, J.K. Polymeric quaternary ammonium-containing coatings with potential dual contact-based and release-based antimicrobial activity. ACS Appl. Mater. Interfaces 2016, 8, 35593–35605. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Iatridi, Z.; Moschakou, M.; Damigos, P.; Bokias, G.; Kallitsis, J.K. Development of Cu2+-and/or phosphonium-based polymeric biocidal materials and their potential application in antifouling paints. Prog. Org. Coat. 2012, 75, 190–199. [Google Scholar] [CrossRef]
- Thalberg, K.; Lindman, B.; Bergfeldt, K. Phase behavior of systems of polyacrylate and cationic surfactants. Langmuir 1991, 7, 2893–2898. [Google Scholar] [CrossRef]
- Oikonomou, E.; Bokias, G.; Kallitsis, J.K.; Iliopoulos, I. Formation of hybrid wormlike micelles upon mixing cetyl trimethylammonium bromide with poly(methyl methacrylate-co-sodium styrene sulfonate) copolymers in aqueous solution. Langmuir 2011, 27, 5054–5061. [Google Scholar] [CrossRef] [PubMed]
- Mathioudakis, G.N.; Soto Beobide, A.; Koromilas, N.D.; Kallitsis, J.K.; Bokias, G.; Voyiatzis, G.A. Evaluation of the release characteristics of covalently attached or electrostatically bound biocidal polymers utilizing SERS and UV-Vis absorption. eXPRESS Polym. Lett. 2016, 10, 750–761. [Google Scholar] [CrossRef]



| Precursors | Feed Composition % (mol AA or GMA) | 1H NMR Composition % (mol AA or GMA) | Mw | PDI | Antimicrobial Copolymers |
|---|---|---|---|---|---|
| P(VBC-co-AA7) | 10 | 7 | 22,000 | 1.7 | P(VBCHAM-co-AA7) |
| P(VBC-co-AA20) | 20 | 20 | 28,200 | 2.7 | P(VBCHAM-co-AA20) |
| P(SSΝa-co-GMA6) | 5 | 6 | 27,800 | 1.9 | P(SSAmC16-co-GMA6) |
| P(SSΝa-co-GMA20) | 15 | 20 | 12,200 | 1.8 | P(SSAmC16-co-GMA20) |
| Complementary Copolymers | Composition, % w/w | Curing Temperature and Time of Curing | Polymeric Coating | |
|---|---|---|---|---|
| P(SSAmC16-co-GMA20) | P(VBCHAM-co-AA7) | 40/60 | RT (1 day) | C1-RT |
| 120 °C (1 day) | C1-120 | |||
| P(SSAmC16-co-GMA6) | P(VBCHAM-co-AA20) | 40/60 | RT (1 day) | C2-RT |
| 120 °C (1 day) | C2-120 | |||
| P(SSAmC16-co-GMA20) | P(VBCHAM-co-AA20) | 40/60 | RT (1 day) | C3-RT |
| 120 °C (1 day) | C3-120 | |||
| 85/15 | 120 °C (1 day) | C4-120 | ||
| 20/80 | 120 °C (1 day) | C5-120 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Druvari, D.; Koromilas, N.D.; Bekiari, V.; Bokias, G.; Kallitsis, J.K. Polymeric Antimicrobial Coatings Based on Quaternary Ammonium Compounds. Coatings 2018, 8, 8. https://doi.org/10.3390/coatings8010008
Druvari D, Koromilas ND, Bekiari V, Bokias G, Kallitsis JK. Polymeric Antimicrobial Coatings Based on Quaternary Ammonium Compounds. Coatings. 2018; 8(1):8. https://doi.org/10.3390/coatings8010008
Chicago/Turabian StyleDruvari, Denisa, Nikos D. Koromilas, Vlasoula Bekiari, Georgios Bokias, and Joannis K. Kallitsis. 2018. "Polymeric Antimicrobial Coatings Based on Quaternary Ammonium Compounds" Coatings 8, no. 1: 8. https://doi.org/10.3390/coatings8010008






