Influence of Oxygen Contents on the Microstructure, High Temperature Oxidation and Corrosion Resistance Properties of Cr–Si–O–N Coatings
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
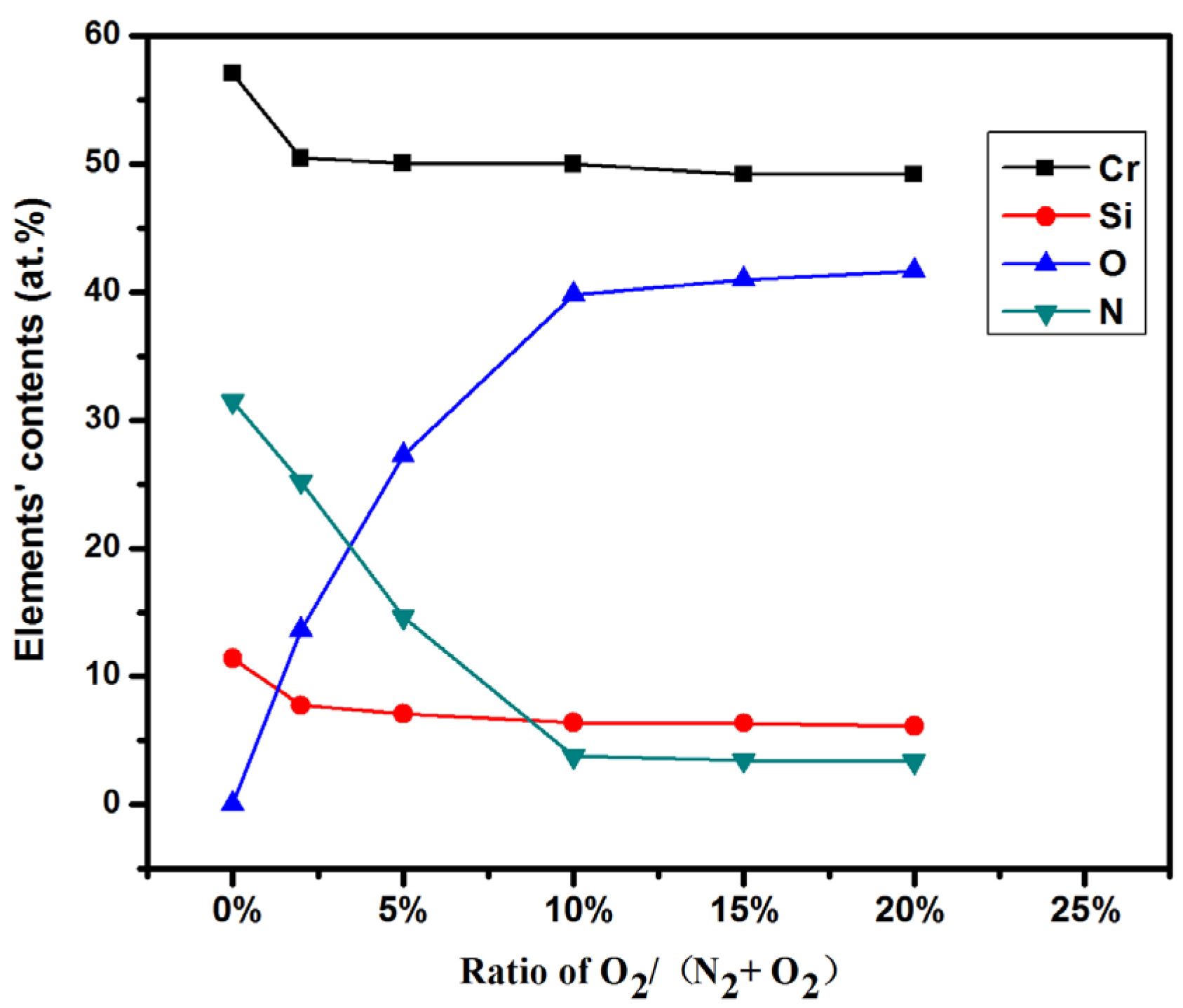
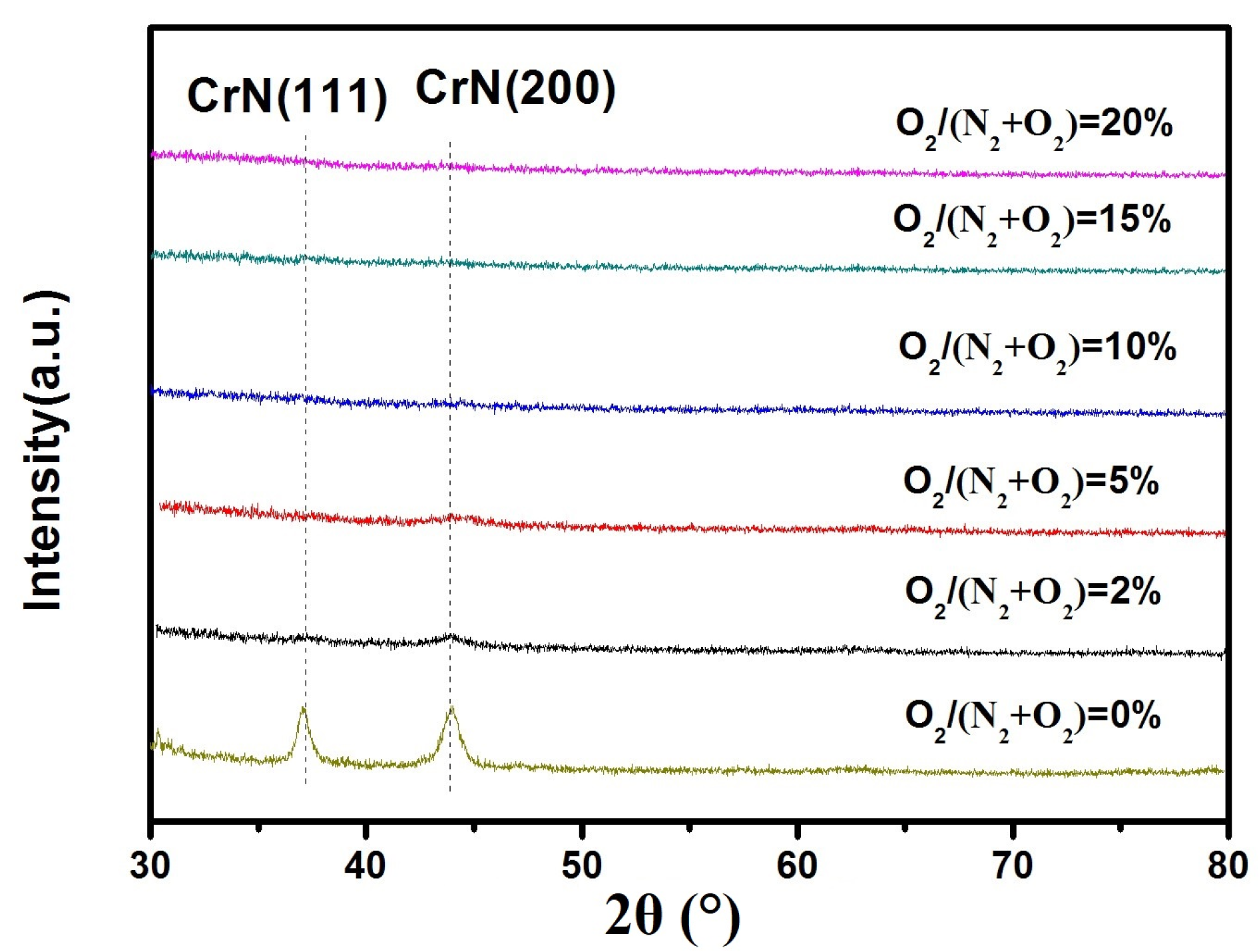
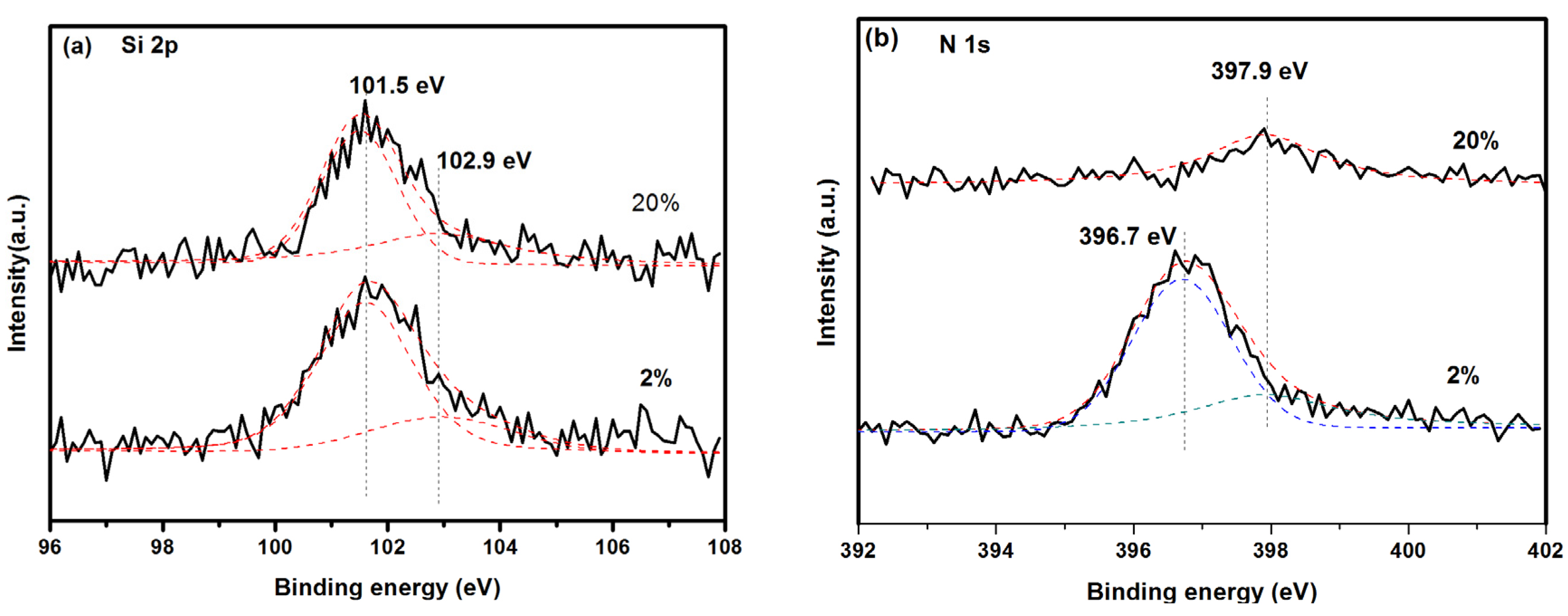
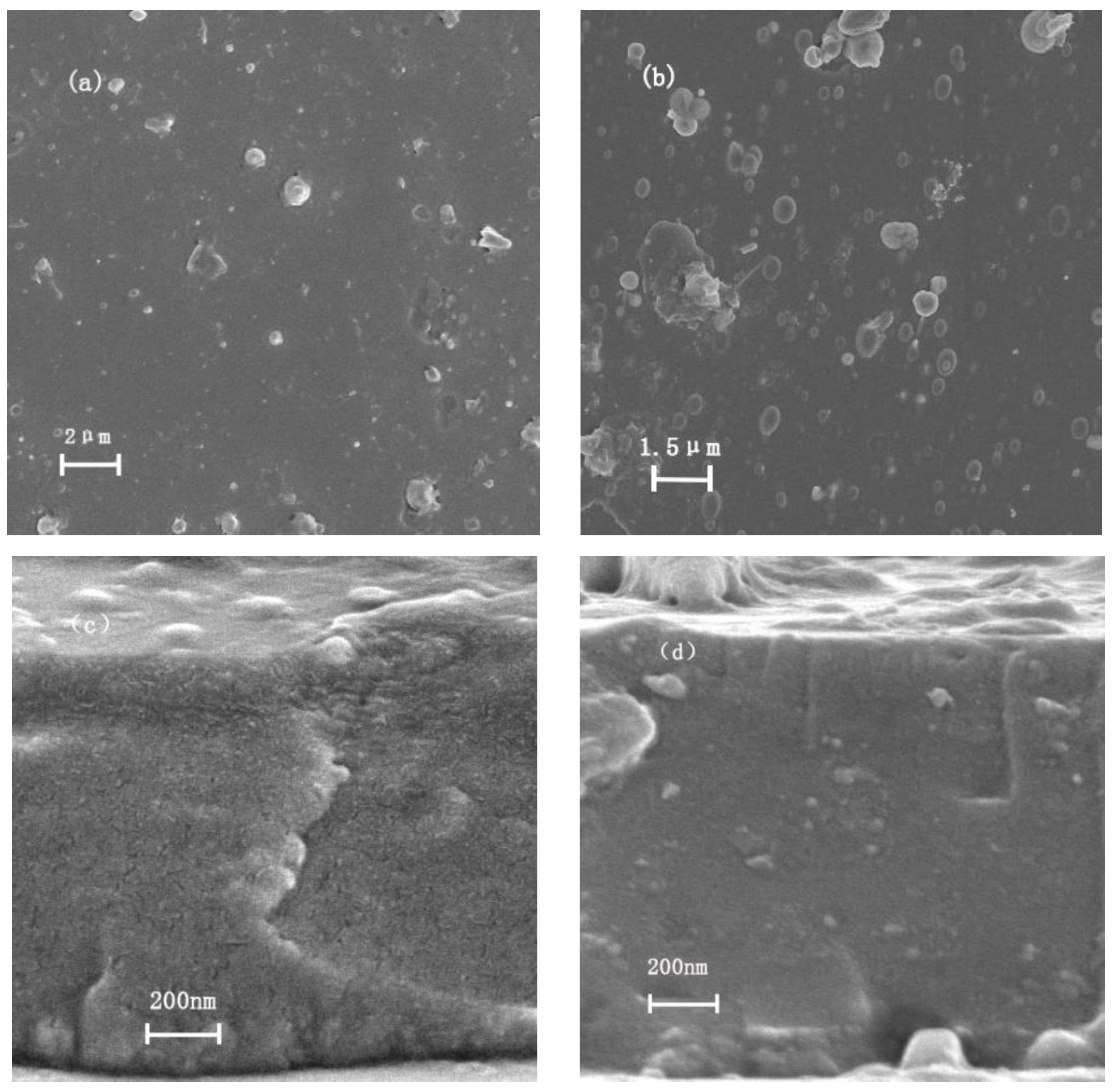
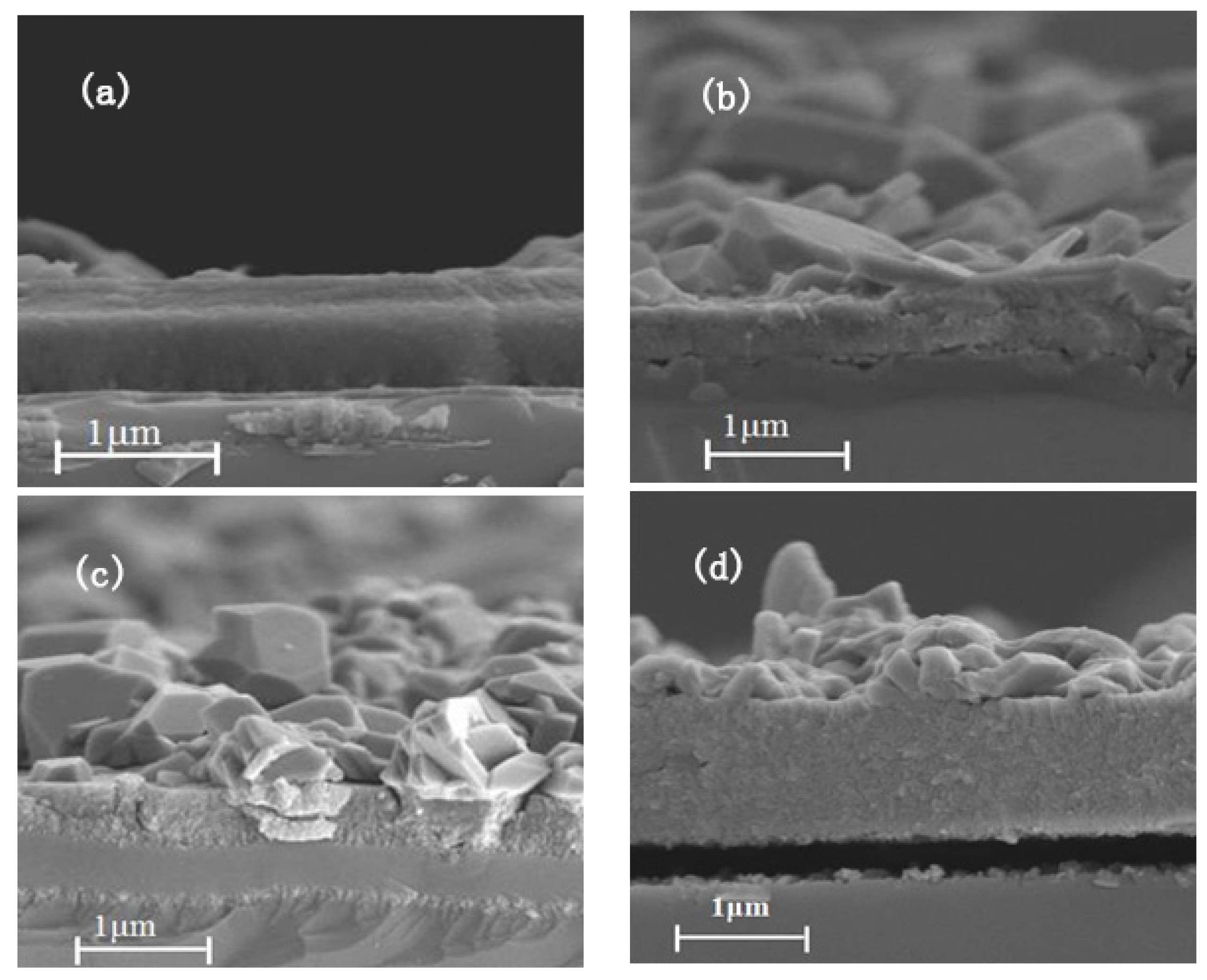
3.1. Composition and Microstructure
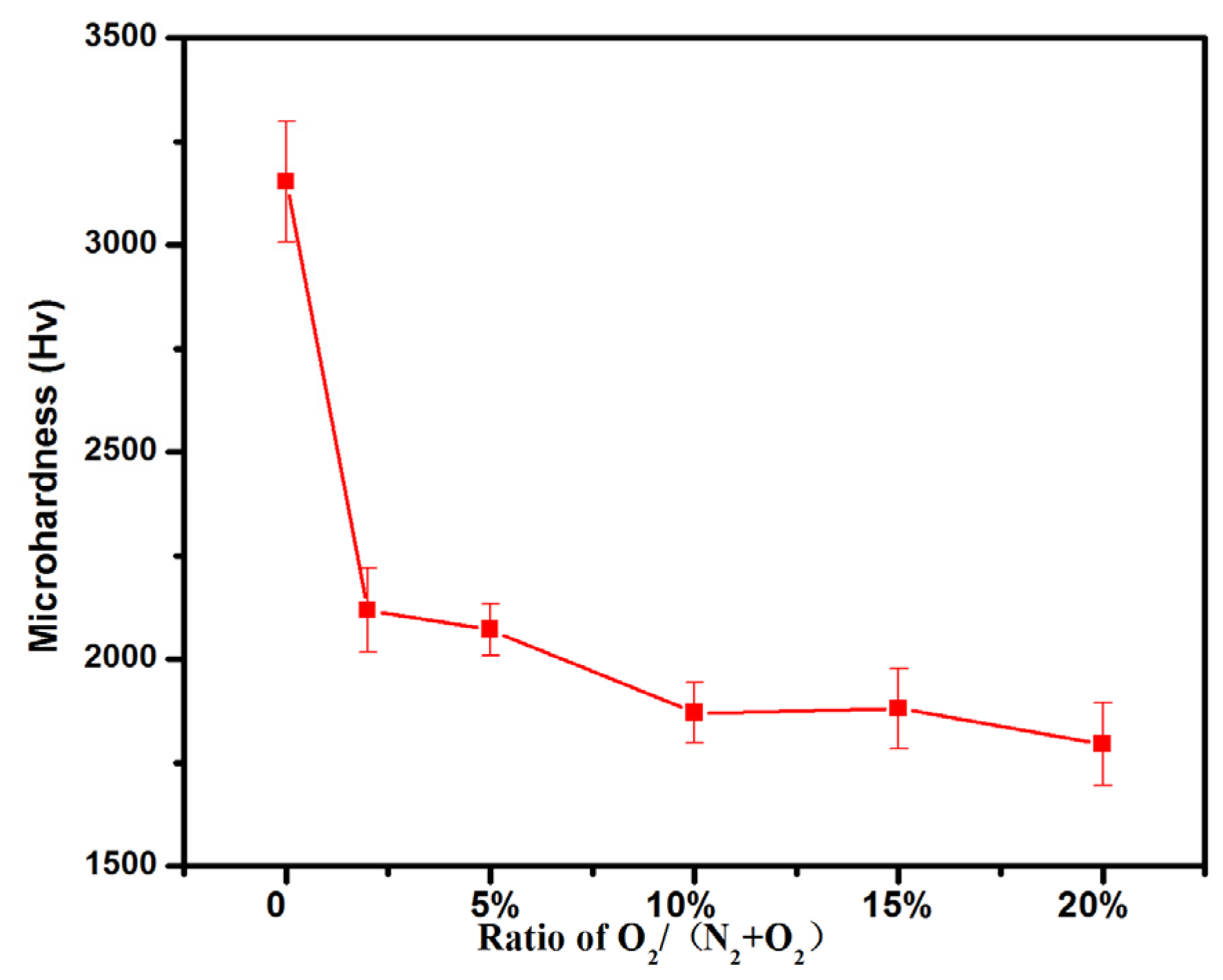
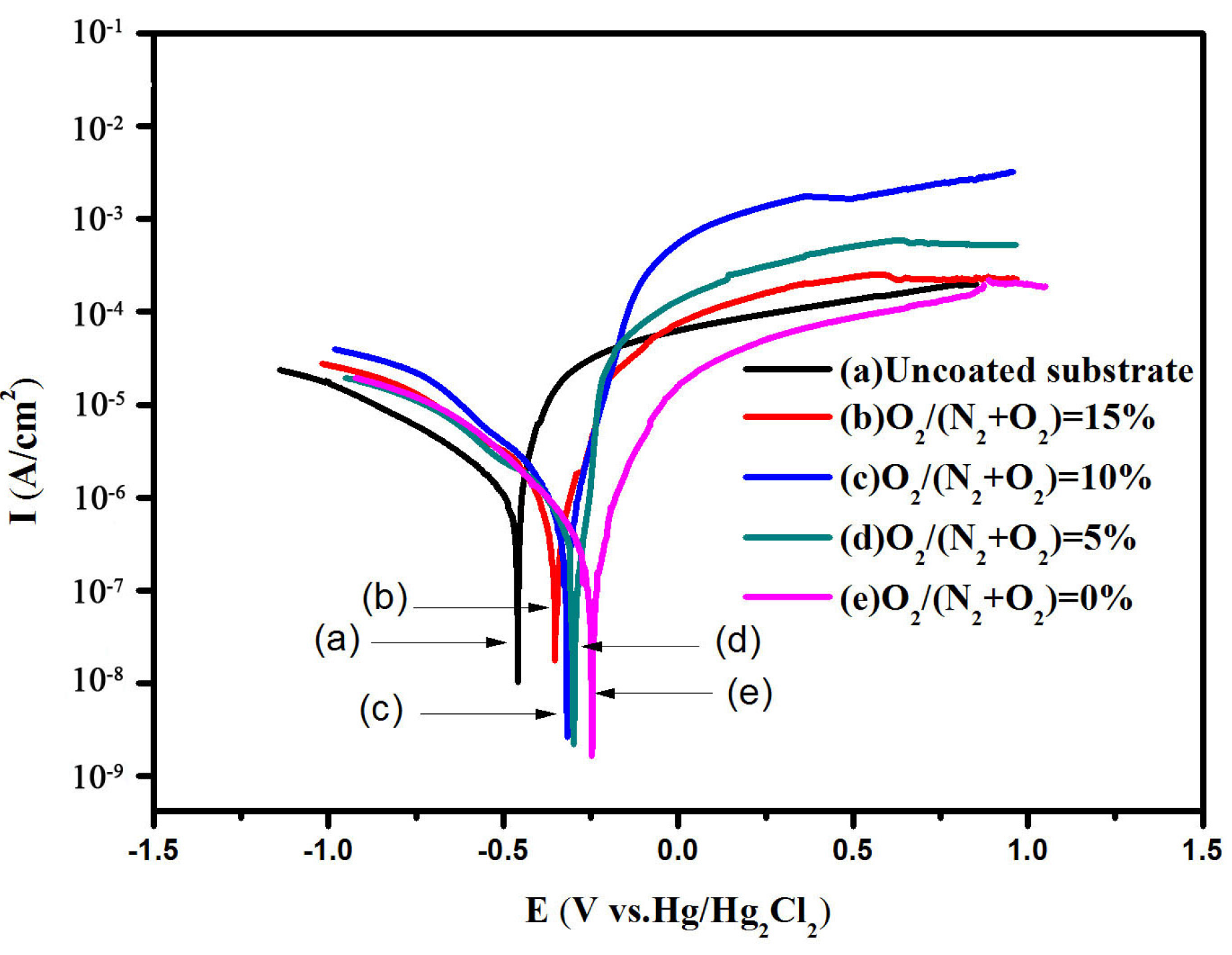
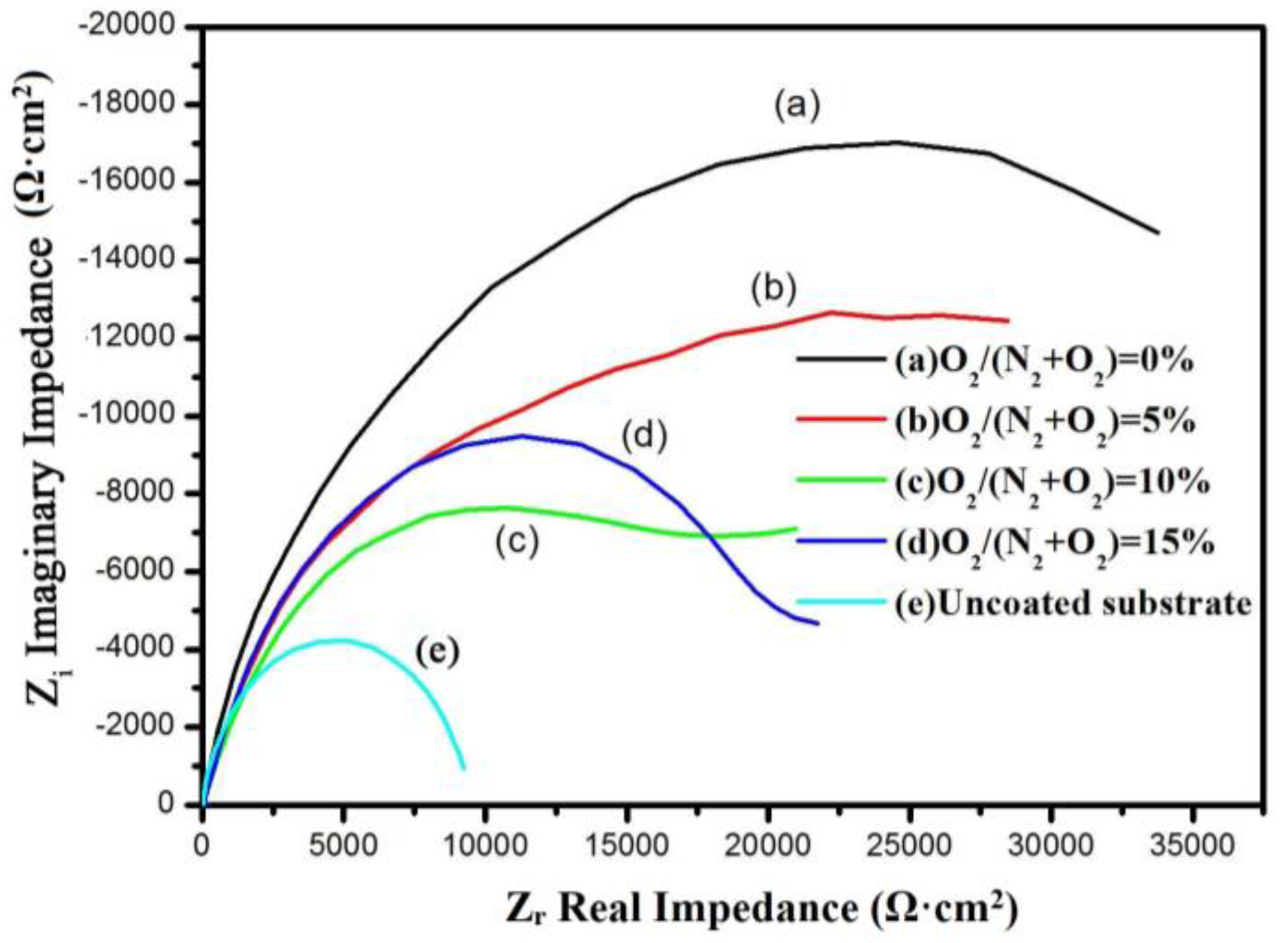
3.2. Microhardness, High Temperature Test and Corrosion Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Creus, J.; Idrissi, H.; Mazille, H.; Sanchette, F.; Jacquot, P. Improvement of the corrosion resistance of CrN coated steel by an interlayer. Surf. Coat. Technol. 1998, 107, 183–190. [Google Scholar] [CrossRef]
- Mercs, D.; Bonasso, N.; Naamane, S.; Bordes, J.-M.; Coddet, C. Mechanical and tribological properties of Cr-N and Cr–Si–N coatings reactively sputter deposited. Surf. Coat. Technol. 2005, 200, 403–407. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lu, F.-H. Oxidation behavior of chromium nitride films. Thin Solid Films 2006, 515, 2179–2184. [Google Scholar] [CrossRef]
- Navinšek, B.; Panjan, P.; Milošev, I. Industrial applications of CrN (PVD) coatings, deposited at high and low temperatures. Surf. Coat. Technol. 1997, 97, 182–191. [Google Scholar] [CrossRef]
- Veprek, S.; Veprek-Heijman, M.G.J.; Karvankova, P.; Prochazka, J. Different approaches to superhard coatings and nanocomposites. Thin Solid Films 2005, 476, 1–29. [Google Scholar] [CrossRef]
- Veprek, S.; Mannling, H.D.; Niederhofer, A.; Ma, D.; Mukherjee, S. Degradation of superhard nanocomposites by built-in impurities. J. Vac. Sci. Technol. 2004, 22, L5–L9. [Google Scholar] [CrossRef]
- Veprek, S.; Veprek-Heijman, M.G.J. Limits to the preparation of superhard nanocomposites: Impurities, deposition and annealing temperature. Thin Solid Films 2012, 522, 274–282. [Google Scholar] [CrossRef]
- Veprek, S.; Mannling, H.D.; Karvankova, P.; Prochazka, J. The issue of the reproducibility of deposition of superhard nanocomposites with hardness of ≥50 GPa. Surf. Coat. Technol. 2004, 200, 3876–3885. [Google Scholar] [CrossRef]
- Camps, I.; Muhl, S.; Camps, E.; Quinones-Galvan, J.G.; Flores, M. Tribological properties of TiSiN thin films deposited by laser ablation. Surf. Coat. Technol. 2014, 255, 74–78. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, L.; Liu, Z.; Pei, F.; Du, Y. Improving thermal stability of TiSiN nanocomposite coatings by multilayered epitaxial growth. Surf. Coat. Technol. 2017, 321, 180–185. [Google Scholar] [CrossRef]
- Yao, Y.; Li, J.; Wang, Y.; Ye, Y.; Zhu, L. Influence of the negative bias in ion plating on the microstructural and tribological performances of Ti-Si-N coatings in seawater. Surf. Coat. Technol. 2015, 280, 154–162. [Google Scholar] [CrossRef]
- Ma, D.; Ma, S.; Xu, K.; Veprek, S. Effecting of oxygen and chlorine on nano-structured TiN/Si3N4 films hardness. Mater. Lett. 2005, 59, 838–841. [Google Scholar]
- Lee, J.D.; Wang, Q.M.; Kim, S.-H.; Wang, T.-G.; Shin, D.-W.; Kim, K.H. Microstructure and mechanical properties of quaternary Cr–Si–O–N films by a hybrid coating system. Surf. Coat. Technol. 2012, 206, 3721–3727. [Google Scholar] [CrossRef]
- Wang, Q.; Kim, K.H. Microstructural control of Cr–Si–N films by a hybrid arc ion plating and magnetron sputtering process. Acta Mater. 2009, 57, 4974–4987. [Google Scholar] [CrossRef]
- Khatibi, A.; Sjolen, J.; Greczynski, G.; Jensen, J.; Eklund, P.; Hultman, L. Structural and mechanical properties of Cr-Al-O-N thin films grown by cathodic arc deposition. Acta Mater. 2012, 60, 6494–6507. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jung, W.S.; Han, J.G.; Seo, S.M.; Kim, J.H.; Bae, Y.H. The synthesis of CrSiN film deposited using magnetron sputtering system. Surf. Coat. Technol. 2005, 200, 1026–1030. [Google Scholar] [CrossRef]
- Ingo, G.M.; Zacchetti, N. XPS investigation on the growth model of a-SiNx and silicon and nitrogen chemical bondings. High Temp. Sci. 1988, 28, 137–151. [Google Scholar]
- Wang, L.; Zhang, S.; Chen, Z.; Li, J.; Li, M. Influence of deposition parameters on hard Cr–Al–N coatings deposited by multi-arc ion plating. Appl. Surf. Sci. 2012, 258, 3629–3636. [Google Scholar] [CrossRef]
- Vepřek, S. Conventional and new approaches towards the design of novel superhard materials. Surf. Coat. Technol. 1997, 97, 15–22. [Google Scholar] [CrossRef]
- Dong, B.L.; Nguyen, T.D.; Sun, K.K. Air-oxidation of nano-multilayered CrAlSiN thin films between 800 and 1000 °C. J. Nanosci. Nanothchno. 2009, 203, 1199–1204. [Google Scholar]
- Kim, J.W.; Kim, K.H.; Lee, D.B.; Moore, J.J. Study on high-temperature oxidation behaviors of Cr–Si–N films. Surf. Coat. Technol. 2006, 200, 6702–6705. [Google Scholar] [CrossRef]
- Pshyk, A.V.; Coy, L.E.; Nowaczyk, G.; Kempiński, M.; Peplińska, B.; Pogrebnjak, A.D.; Beresnev, V.M.; Jurga, S. High temperature behavior of functional TiAlBSiN nanocomposite coatings. Surf. Coat. Technol. 2016, 305, 49–60. [Google Scholar] [CrossRef]
- Saladukhin, I.A.; Abadias, G.; Uglov, V.V.; Zlotski, S.V.; Michel, A.; van Vuuren, A.J. Thermal stability and oxidation resistance of ZrSiN nanocomposite and ZrN/SiNx multilayered coatings: A comparative study. Surf. Coat. Technol. 2017, 332, 428–439. [Google Scholar] [CrossRef]
- Grips, V.K.W.; Barshilia, H.C.; Ezhil Selvi, V.; Kalavati; Rajam, K.S. Electrochemical behavior of single layer CrN, TiN, TiAlN coatings and nanolayered TiAlN/CrN multilayer coatings prepared by reactive direct current magnetron sputtering. Thin Solid Films 2006, 514, 204–211. [Google Scholar]
- Zhang, L.J.; Mohammed, E.A.A.; Adriaens, A. Synthesis and electrochemical behavior of a magnesium fluoride-polydopamine-stearic acid composite coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2016, 307, 56–64. [Google Scholar] [CrossRef]



| Deposition Parameters | Cr Adhesive Layer | CrN Inter Layer | Cr–Si–O–N |
|---|---|---|---|
| Ar flow rates (sccm) | 9 | – | – |
| N2 flow rates (sccm) | – | 60 | 45 |
| O2/(N2 + O2) ratio | – | – | 0–20% |
| Working pressure (Pa) | 1.0 | 1.0 | 1.0 |
| Deposition time (s) | 300 | 300 | 3000 |
| Cr cathode arc current (A) | 80 | 80 | – |
| CrSi cathode arc current (A) | – | – | 70 |
| Negative bias voltage (V) | −160 | −140 | −100 |
| Samples | Cr–Si–O–N (2%) | Cr–Si–O–N (10%) | ||||
|---|---|---|---|---|---|---|
| 800 °C | 1100 °C | 1200 °C | 800 °C | 1100 °C | 1200 °C | |
| Cr | 39.05% | 29.70% | 25.92% | 34.45% | 25.01% | 22.47% |
| Si | 9.31% | 14.05% | 18.81% | 13.49% | 17.23% | 19.10% |
| O | 43.10% | 54.3% | 53.84% | 47.08% | 56.52% | 57.33% |
| N | 8.54% | 1.86% | 1.43% | 4.98% | 1.24% | 1.10% |
| Samples | Ecorr (V) | Icorr (μA/cm2) | Rp (kΩ·cm2) | Corr rate (mm/A) |
|---|---|---|---|---|
| Substrate | −0.4650 | 2.65 | 14.95 | 13.46 × 10−3 |
| Cr–Si–N | −0.2541 | 0.35 | 123.39 | 4.02 × 10−3 |
| Cr–Si–O–N (5%) | −0.2645 | 0.41 | 98.63 | 5.94 × 10−3 |
| Cr–Si–O–N (10%) | −0.3099 | 0.72 | 49.92 | 6.25 × 10−3 |
| Cr–Si–O–N (15%) | −0.3489 | 0.96 | 49.69 | 7.01 × 10−3 |
| Samples | Rs (Ω·cm2) | CPEc-Y0 (μF·cm2) | Rc (Ω·cm2) | CPEdl-Y0 (μF·cm2) | Rct (kΩ·cm2) |
|---|---|---|---|---|---|
| Substrate | 19.11 | – | – | 95.55 | 9.46 |
| Cr–Si–N | 19.91 | 25.60 | 2573 | 52.34 | 50.15 |
| Cr–Si–O–N (5%) | 20.23 | 85.61 | 1970 | 46.39 | 30.15 |
| Cr–Si–O–N (10%) | 22.60 | 89.83 | 1898 | 62.33 | 22.57 |
| Cr–Si–O–N (15%) | 20.82 | 87.62 | 1851 | 53.01 | 23.48 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Chen, S.; Zou, C.; Tian, C.; Wang, Z.; Liao, S. Influence of Oxygen Contents on the Microstructure, High Temperature Oxidation and Corrosion Resistance Properties of Cr–Si–O–N Coatings. Coatings 2018, 8, 19. https://doi.org/10.3390/coatings8010019
Liang J, Chen S, Zou C, Tian C, Wang Z, Liao S. Influence of Oxygen Contents on the Microstructure, High Temperature Oxidation and Corrosion Resistance Properties of Cr–Si–O–N Coatings. Coatings. 2018; 8(1):19. https://doi.org/10.3390/coatings8010019
Chicago/Turabian StyleLiang, Jinjue, Shimin Chen, Changwei Zou, Canxin Tian, Zesong Wang, and Shijun Liao. 2018. "Influence of Oxygen Contents on the Microstructure, High Temperature Oxidation and Corrosion Resistance Properties of Cr–Si–O–N Coatings" Coatings 8, no. 1: 19. https://doi.org/10.3390/coatings8010019




