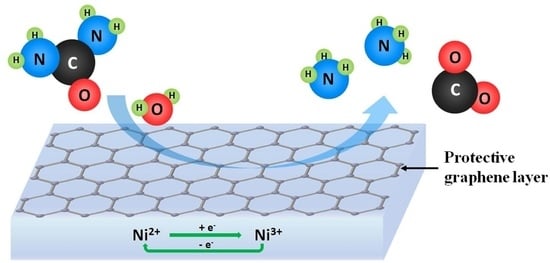
Ammonia Generation via a Graphene-Coated Nickel Catalyst
Abstract
:1. Introduction
2. Experimental Methods
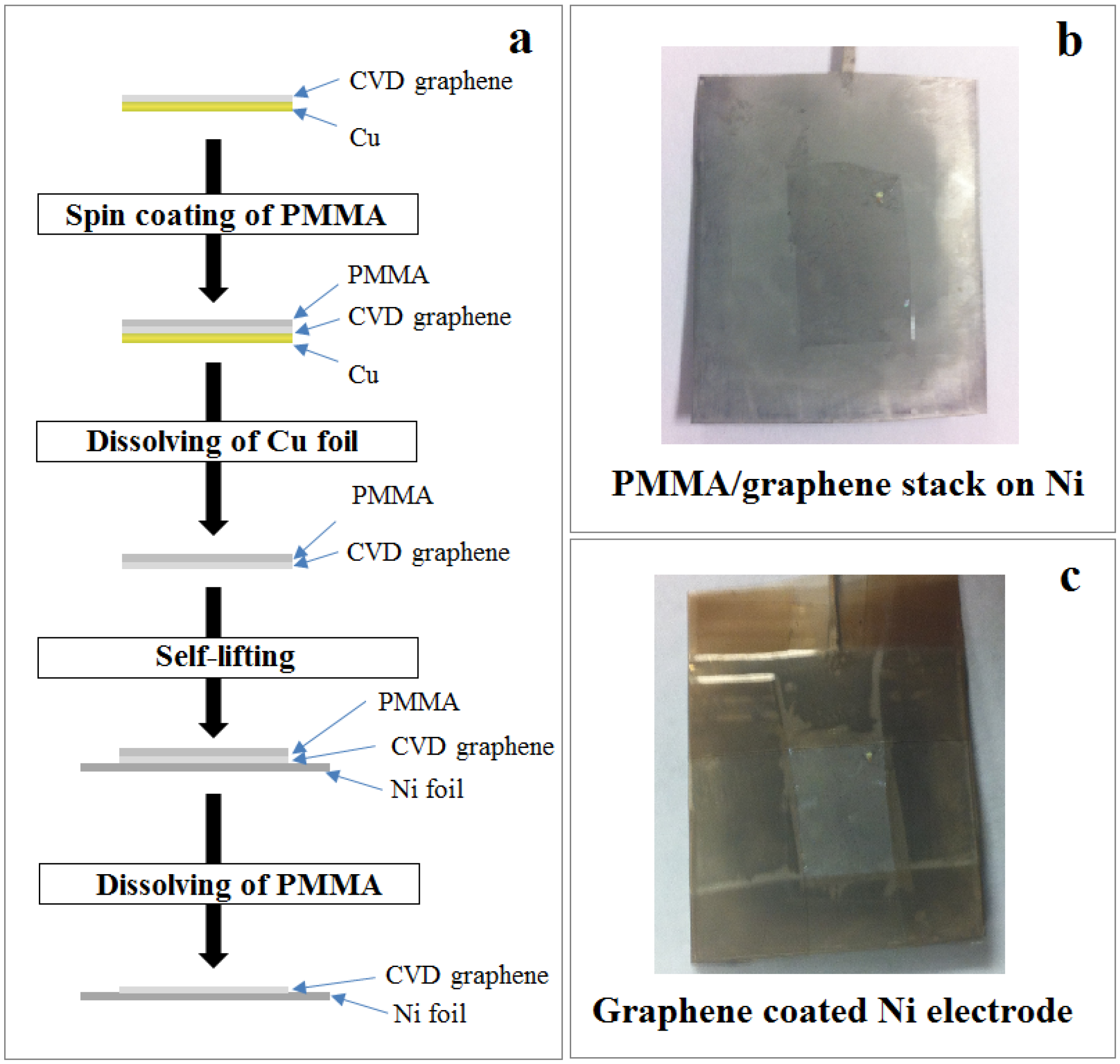
2.1. Preparation of Graphene-Coated Ni Electrode
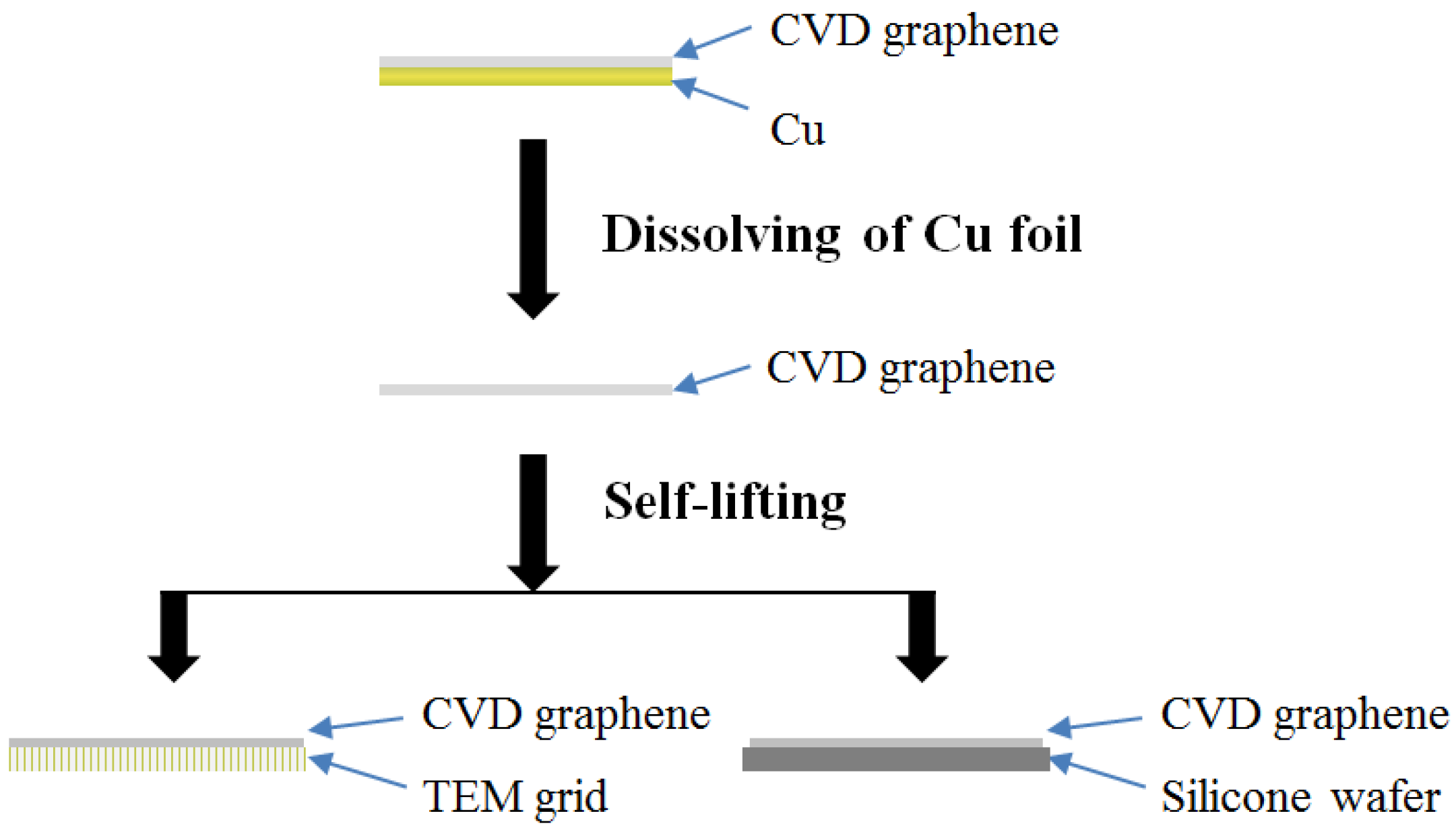
2.2. Phsysical Characterization of Graphene
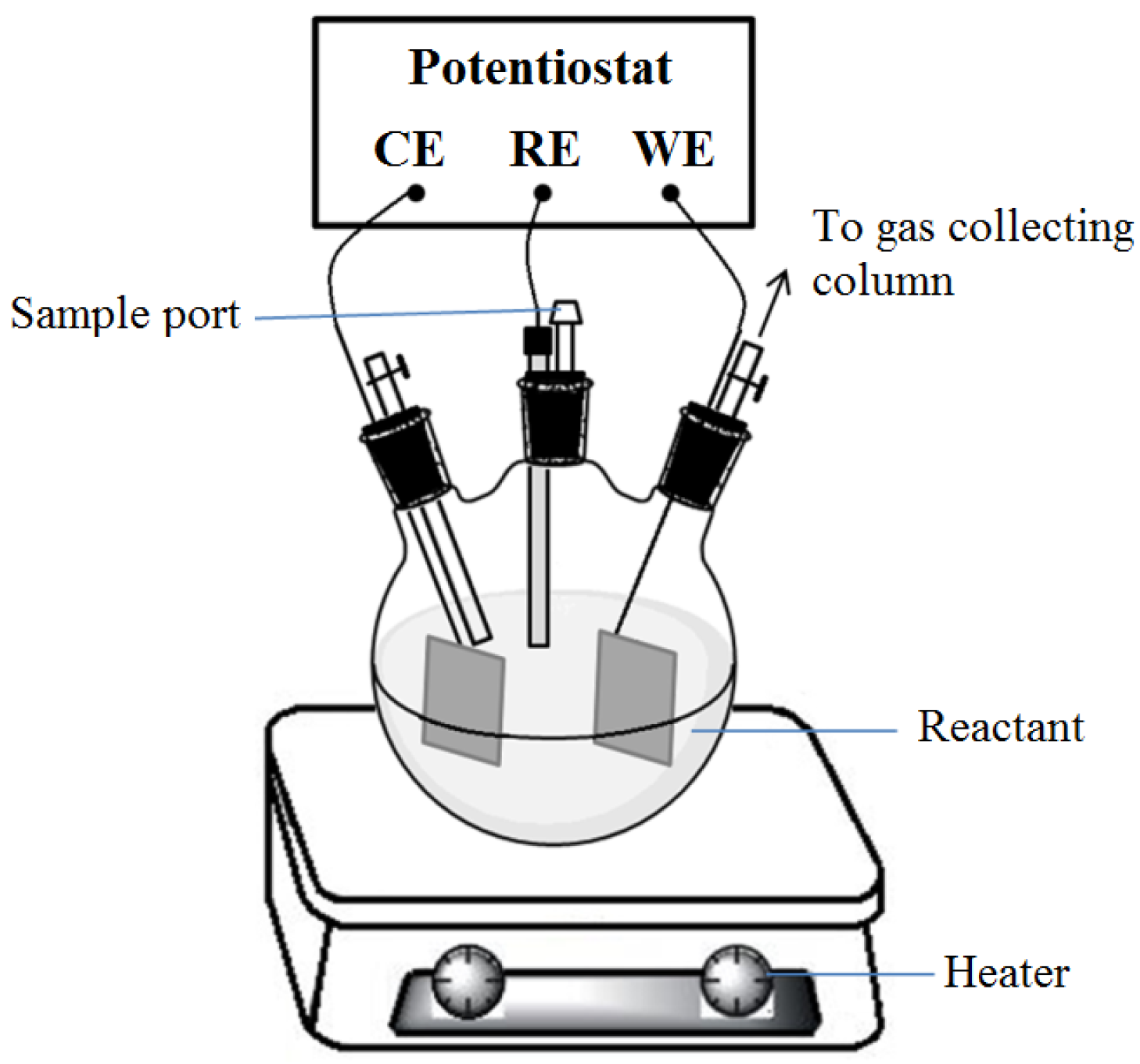
2.3. Cyclic Voltammetry and Urea to Ammonia
2.4. Quatification of Corrosion
3. Results
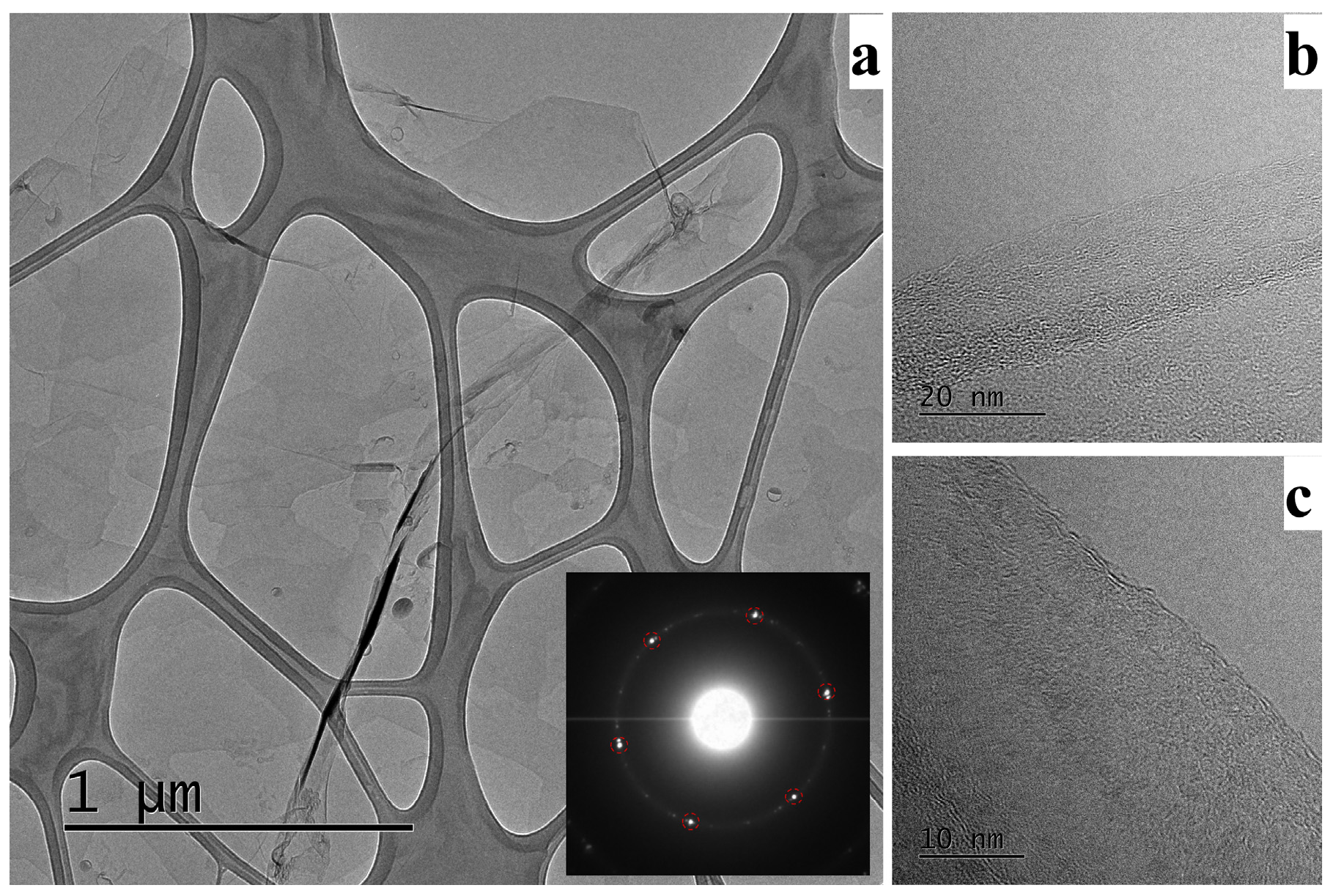
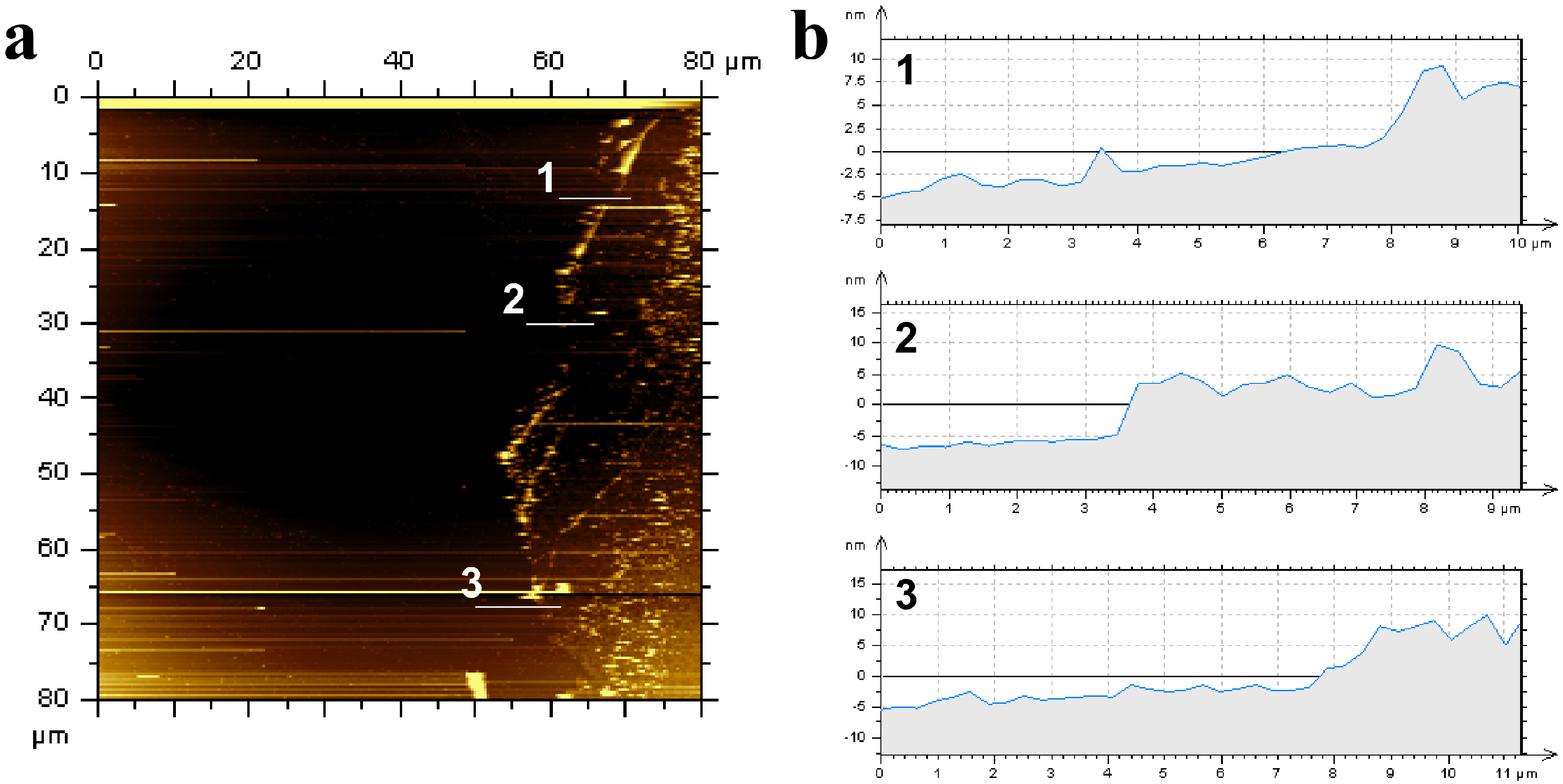
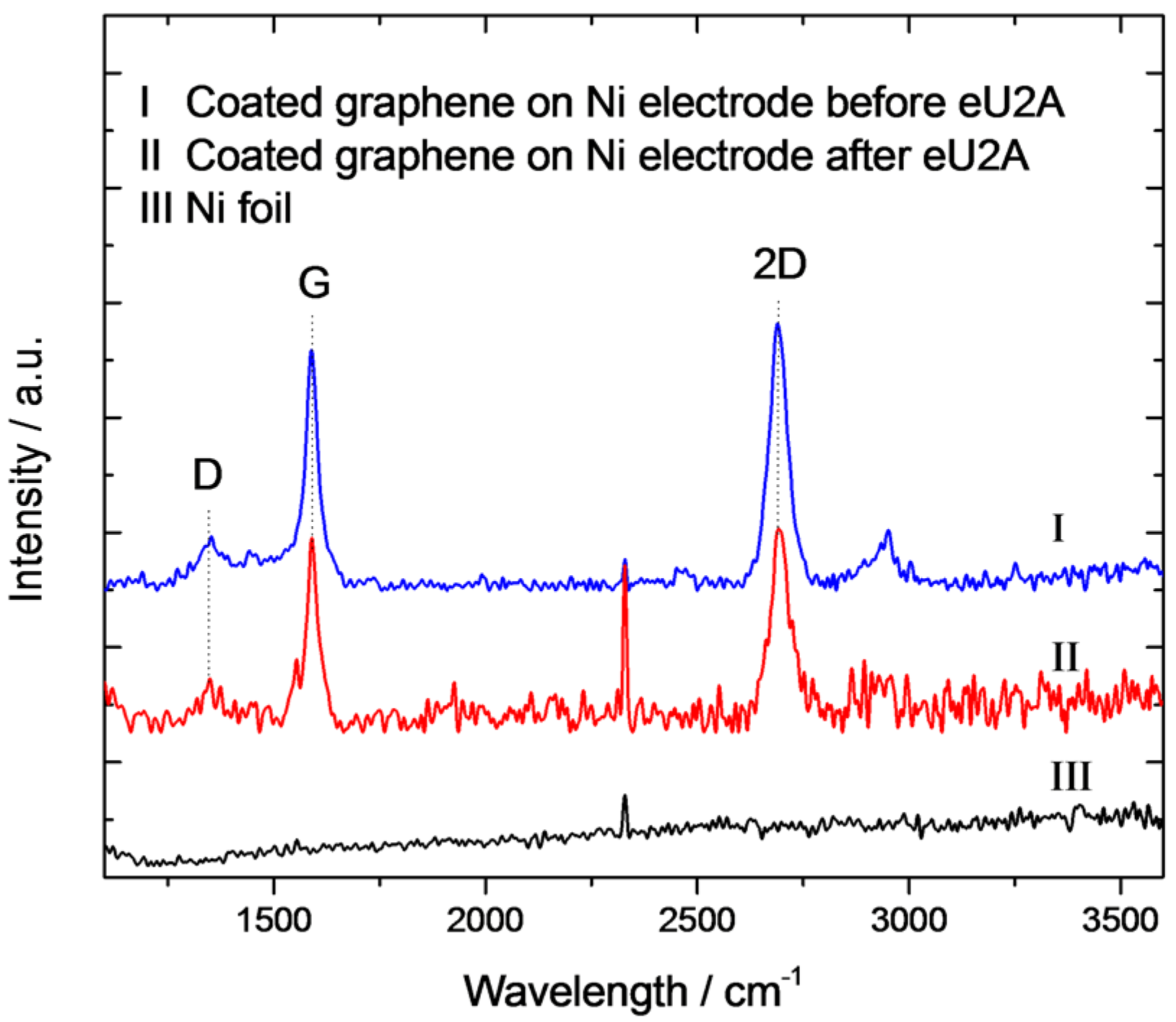
3.1. Physical Chracterization
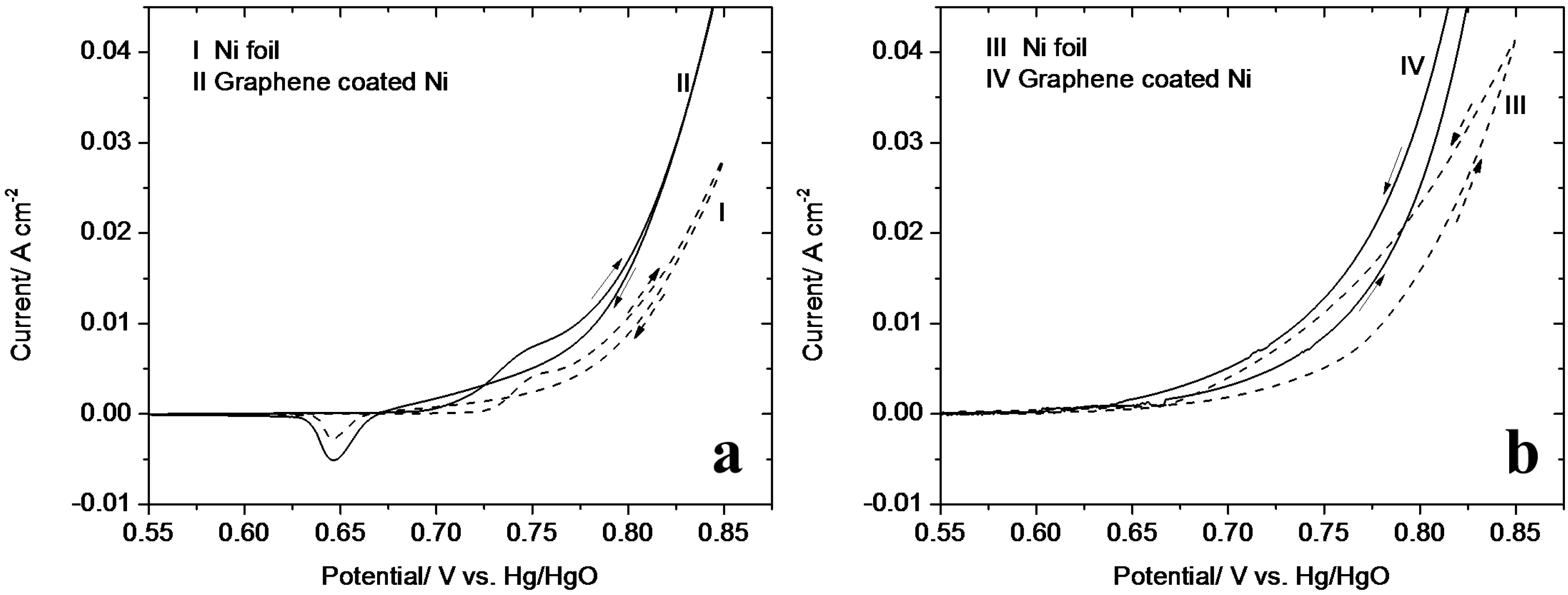
3.2. Cyclic Voltammetry
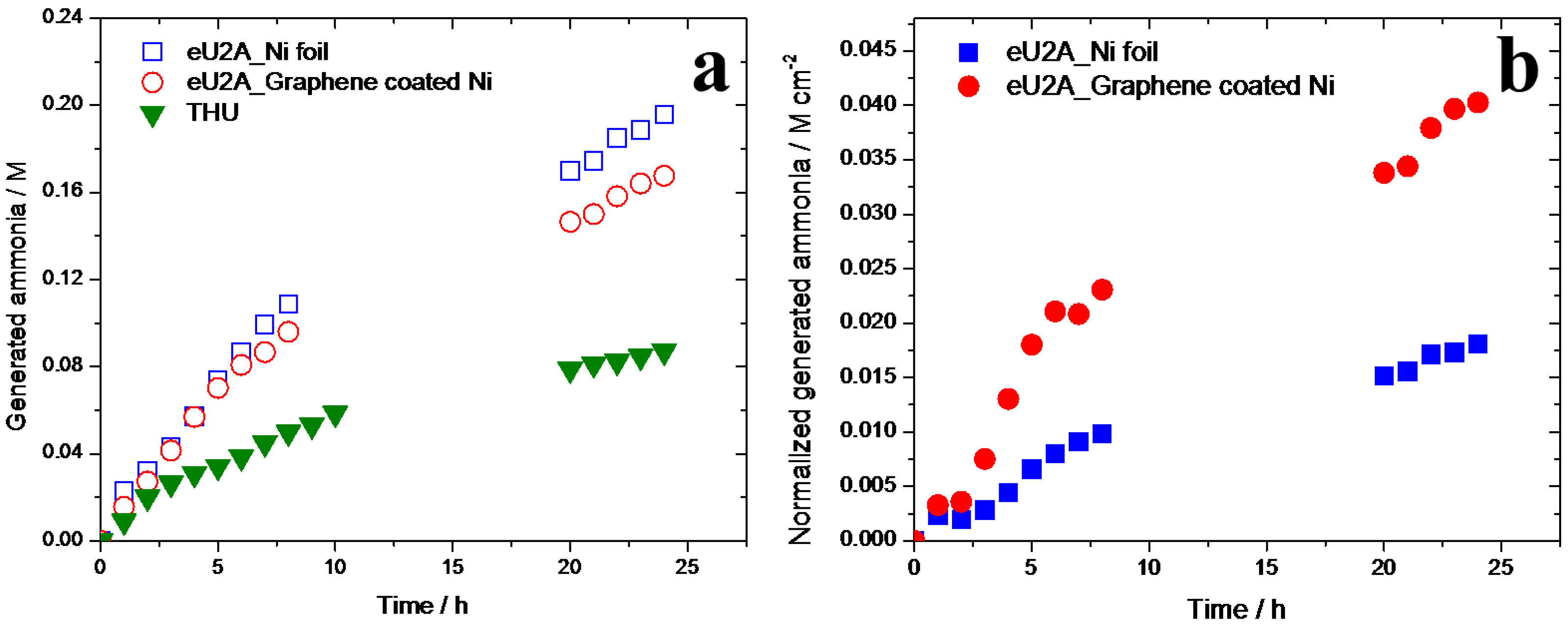
3.3. Generation of Ammonia in eU2A and THU Processes
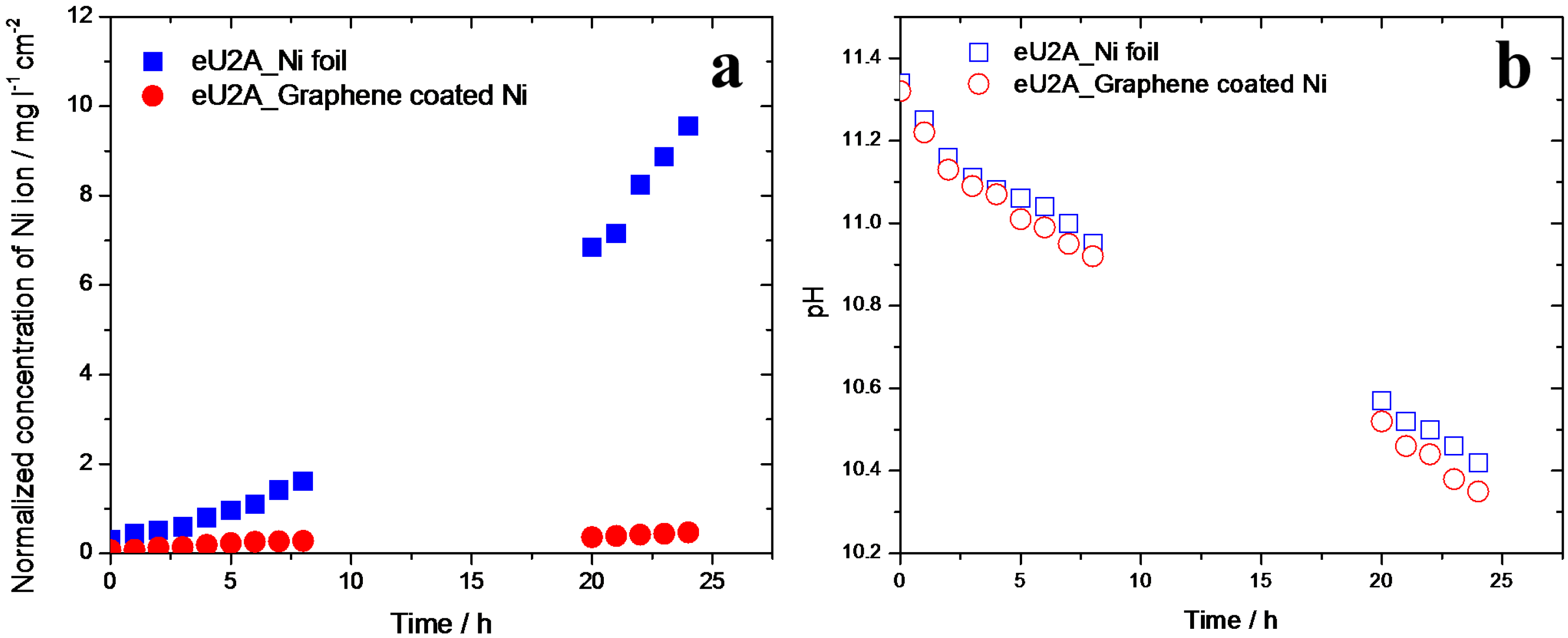
3.4. Ammonia Corrosion on Graphene-Coated Ni Electrode
4. Discussion and Conclusion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cichanowicz, J.E.; Muzio, L.J.; Hein, M.C. The First 100 GW of SCR in the U.S.—What Have We Learned? In Proceedings of the Mega Symposium, Baltimore, MD, USA, 28–31 August 2006. [Google Scholar]
- Botte, G.G. Electrolytic Cells and Methods for the Production of Ammonia and Hydrogen. U.S. Patent 9062382 B2, 2007. [Google Scholar]
- Lu, F.; Botte, G.G. Electrochemically Induced Conversion of Urea to Ammonia. ECS Electrochem. Lett. 2015, 4, E5–E7. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Hu, J.; Ji, Y.; Shi, Y.; Hui, F.; Duan, H.; Lanza, M. A Review on the use of Graphene as a Protective Coating Against Corrosion. Ann. J. Mater. Sci. Eng. 2014, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dumée, L.F.; He, L.; Wang, Z.; Sheath, P.; Xiong, J.; Feng, C.; Tan, M.Y.; She, F.; Duke, M.; Gray, S.; et al. Growth of nano-textured graphene coatings across highly porous stainless steel supports towards corrosion resistant coatings. Carbon 2015, 87, 395–408. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yan, W.; Vijapur, S.H.; Botte, G.G. Electrochemically reduced graphene oxide–nickel nanocomposites for urea electrolysis. Electrochim. Acta 2013, 89, 732–736. [Google Scholar] [CrossRef]
- Botte, G.G. Electrochemical Cell Containing a Graphene Coated Electrode. U.S. Patent 20160251765 A1, 2016. [Google Scholar]
- Politano, A.; Cattelan, M.; Boukhvalov, D.W.; Campi, D.; Cupolillo, A.; Agnoli, S.; Apostol, N.G.; Lacovig, P.; Lizzit, S.; Farías, D.; et al. Unveiling the Mechanisms Leading to H2 Production Promoted by Water Decomposition on Epitaxial Graphene at Room Temperature. ACS Nano 2016, 10, 4543–4549. [Google Scholar] [CrossRef] [PubMed]
- Vijapur, S.H.; Wang, D.; Botte, G.G. Raw Coal Derived Large Area and Transparent Graphene Films. ECS Solid State Lett. 2013, 2, M45–M47. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.N.; Ishihara, M.; Tiwari, J.N.; Yoshimura, M. Synthesis of graphene film from fullerene rods. Chem. Commun. 2012, 48, 3003–3005. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.H.; Boyle, J.M.; Carmignani, P.G. Small Scale Test Results From New Selective Catalytic NOx Reduction Process Using Urea. In Proceedings of the MEGA Symposium, Chicago, IL, USA, 20–23 August 2001. [Google Scholar]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Wang, Y.; Yu, T.; Shen, Z. Raman spectroscopy and imaging of graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Direct evidence of the mechanism for the electro-oxidation of urea on Ni(OH)2 catalyst in alkaline medium. Electrochim. Acta 2013, 108, 660–665. [Google Scholar] [CrossRef]
- Scridhar, N.; Hodge, F.G. Nickel and High Nickel Alloys. In Corrosion and Corrosion Protection Handbook, 2nd ed.; Schweitzer, P.A., Ed.; CRC Press: Chester, NJ, USA, 1989; p. 95. [Google Scholar]
- Grujicic, D.; Pesic, B. Electrochemical and AFM study of nickel nucleation mechanisms on vitreous carbon from ammonium sulfate solutions. Electrochim. Acta 2006, 51, 2678–2690. [Google Scholar] [CrossRef]
- Drodten, P. Ammonia and Ammonium Hydroxide. In Corrosion Resistance of Nickel and Nickel Alloys Against Acids and Lyes, 1st ed.; Schutze, M., Rebak, R.B., Bender, R., Eds.; Wiley-VCH: Weinheim, Germany, 2014; pp. 249–250. [Google Scholar]
- Haynes, W.P.; Benson, H.E.; Field, J.H.; Tosh, J.S. Equilibrium Study of the System Potassium Carbonate, Potassium Biocarbonate, Carbon Dioxide, and Water; U.S. Dept. of the Interior, Bureau of Mines: Washington, DC, USA, 1959; pp. 13–15.
- Dahal, A.; Batzill, M. Graphene-nickel interfaces: A review. Nanoscale 2014, 6, 2548–2562. [Google Scholar] [CrossRef] [PubMed]
- Politano, A. Quasi-freestanding graphene on Ni(110): A graphene/metal contact with suppressed interface states. Nano Res. 2016, 9, 1795–1800. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, F.; Botte, G.G. Ammonia Generation via a Graphene-Coated Nickel Catalyst. Coatings 2017, 7, 72. https://doi.org/10.3390/coatings7060072
Lu F, Botte GG. Ammonia Generation via a Graphene-Coated Nickel Catalyst. Coatings. 2017; 7(6):72. https://doi.org/10.3390/coatings7060072
Chicago/Turabian StyleLu, Fei, and Gerardine G. Botte. 2017. "Ammonia Generation via a Graphene-Coated Nickel Catalyst" Coatings 7, no. 6: 72. https://doi.org/10.3390/coatings7060072