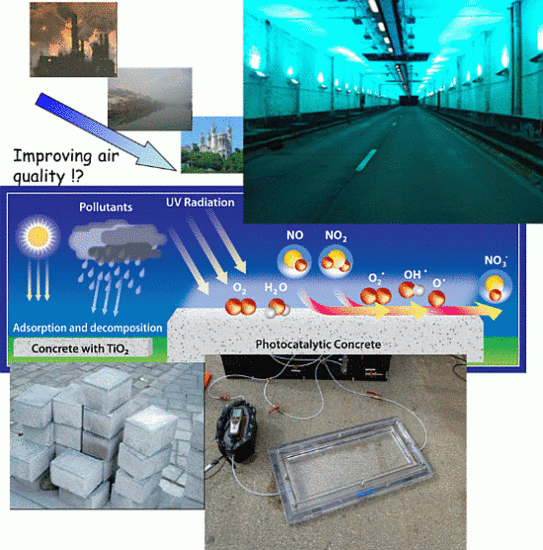
Recent Photocatalytic Applications for Air Purification in Belgium
Abstract
:1. Introduction
 with harmful consequences for building materials (corrosion of the surface) and vegetation.
with harmful consequences for building materials (corrosion of the surface) and vegetation.2. Photocatalytic Concrete: Purifying the Air through the Pavement
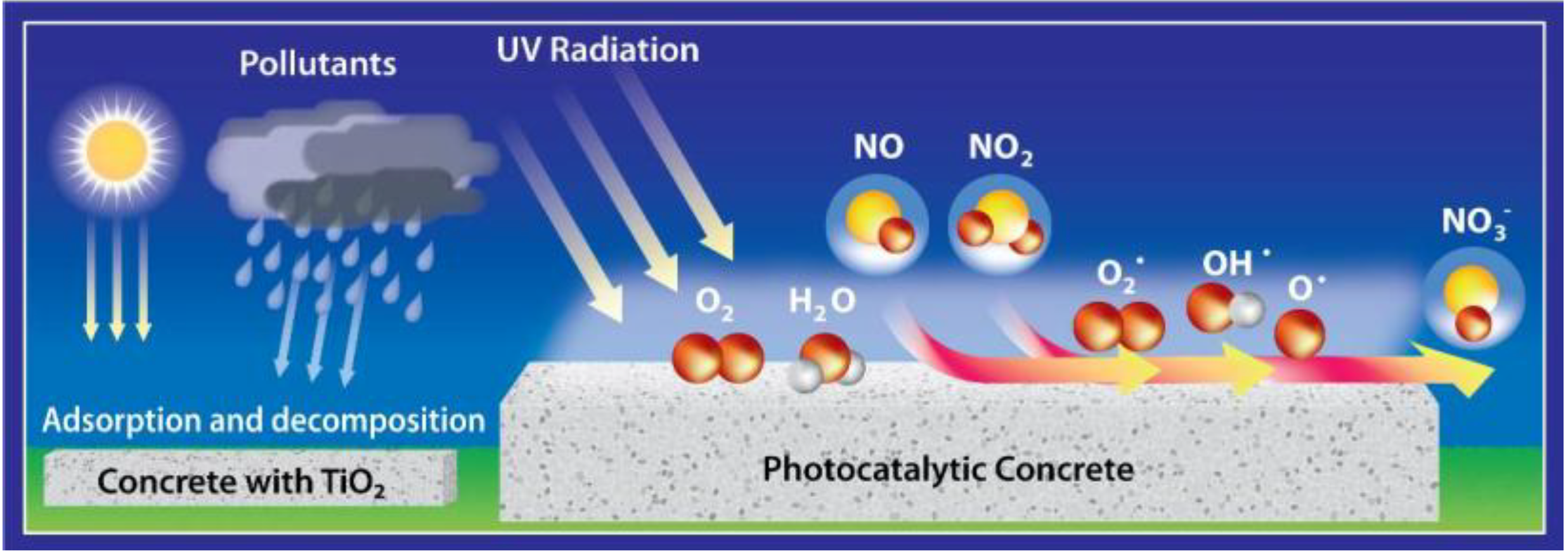
 , which is adsorbed at the surface due to the alkalinity of the concrete [11]. Thus, a synergetic effect is created in the presence of the cement matrix, which helps to effectively trap the reactant gases (NO and NO2) together with the nitrate salt formed. Subsequently, the deposited nitrate can be washed away by rain or washing with water. In addition, these nitrates pose no real threat towards pollution of body waters because the resulting concentrations in the waste water are very low, even below the current limit values for surface and ground water [12].
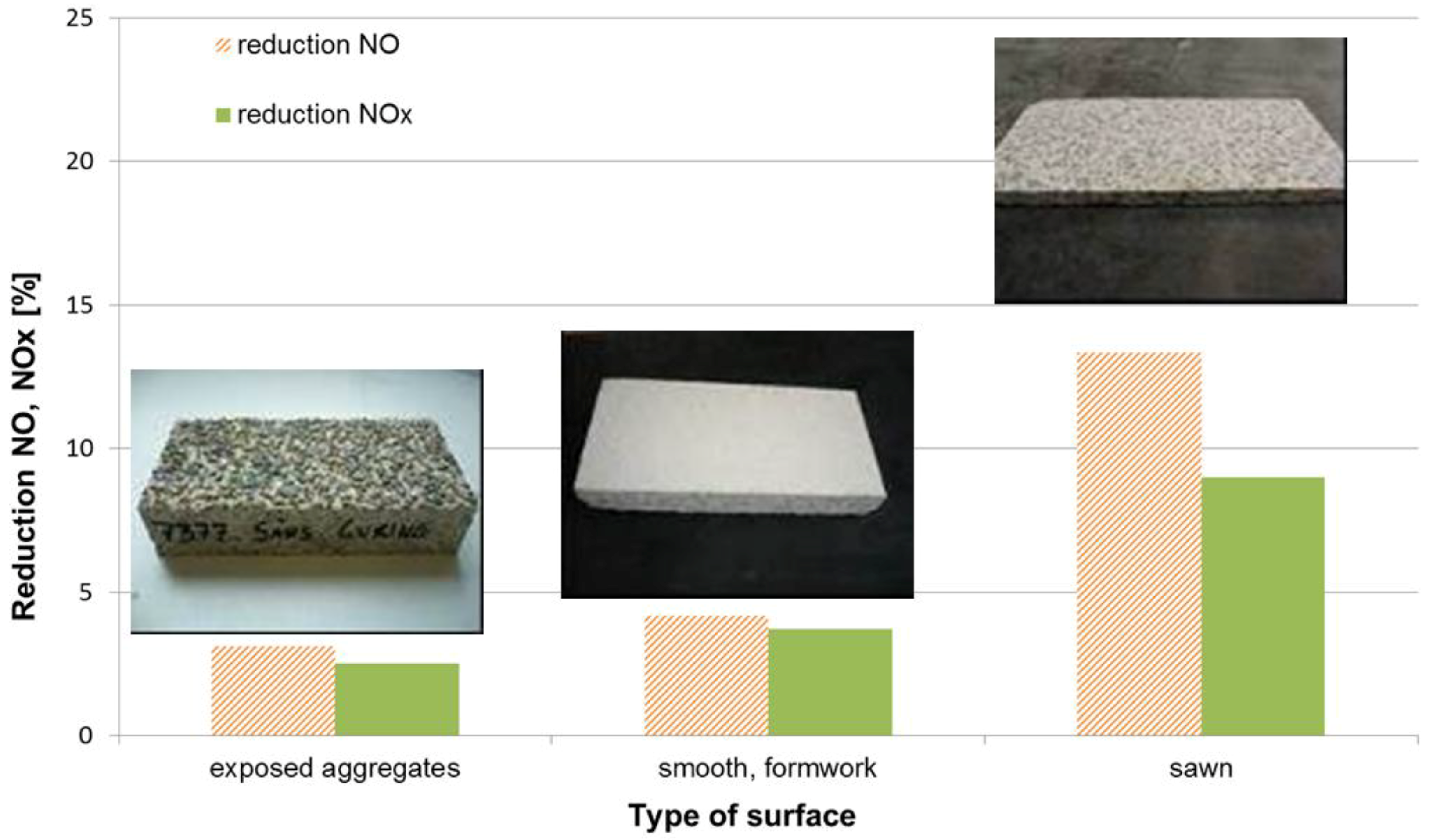
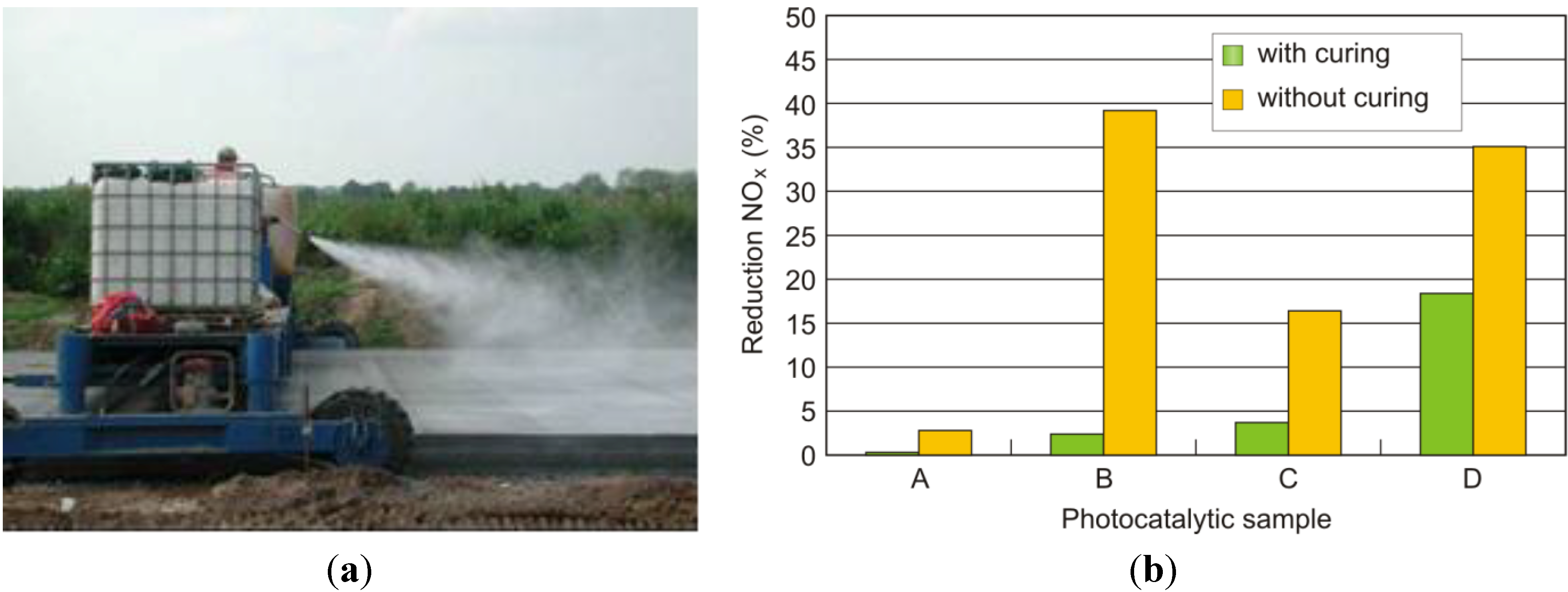
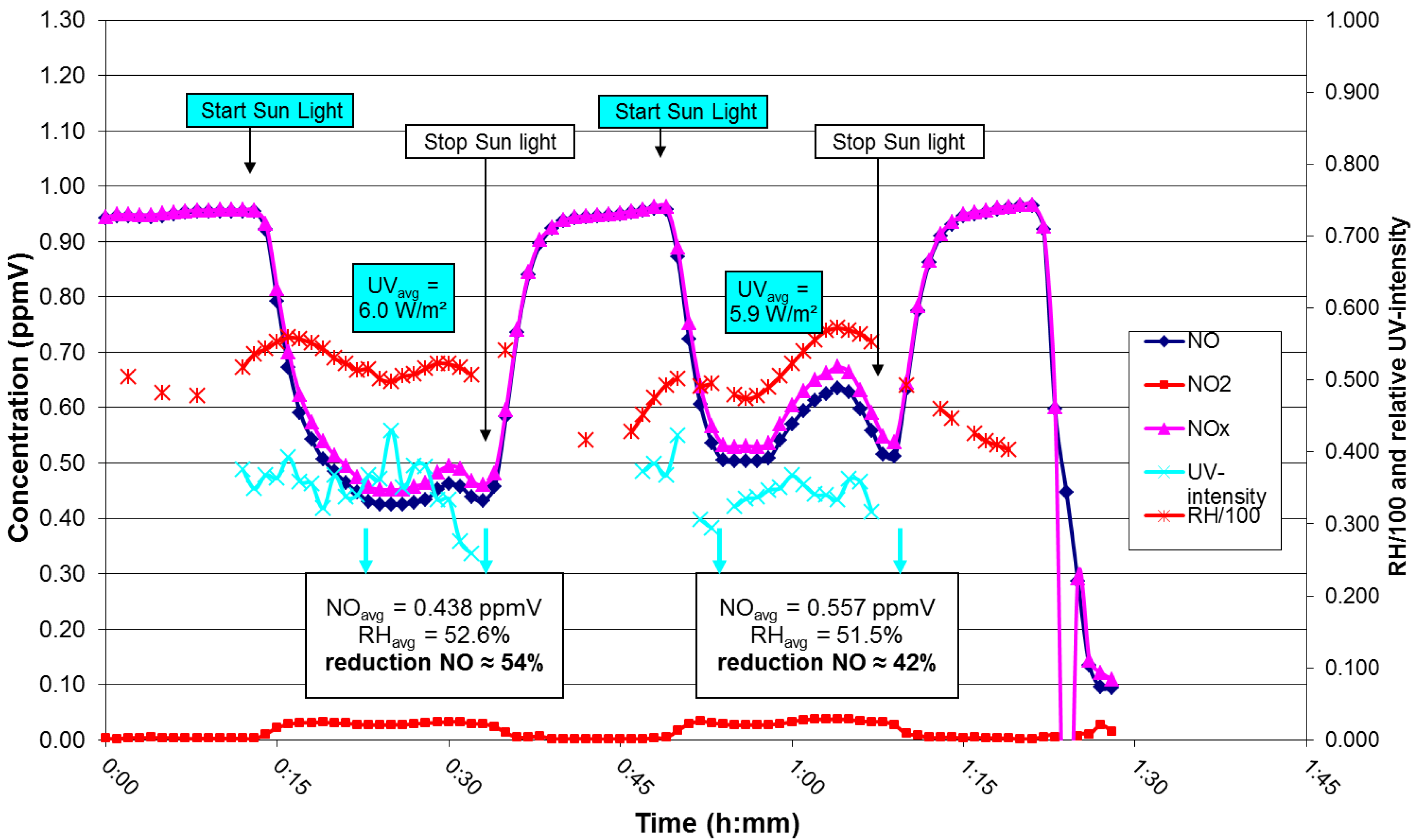
, which is adsorbed at the surface due to the alkalinity of the concrete [11]. Thus, a synergetic effect is created in the presence of the cement matrix, which helps to effectively trap the reactant gases (NO and NO2) together with the nitrate salt formed. Subsequently, the deposited nitrate can be washed away by rain or washing with water. In addition, these nitrates pose no real threat towards pollution of body waters because the resulting concentrations in the waste water are very low, even below the current limit values for surface and ground water [12].3. Laboratory Results: Parameter Evaluation

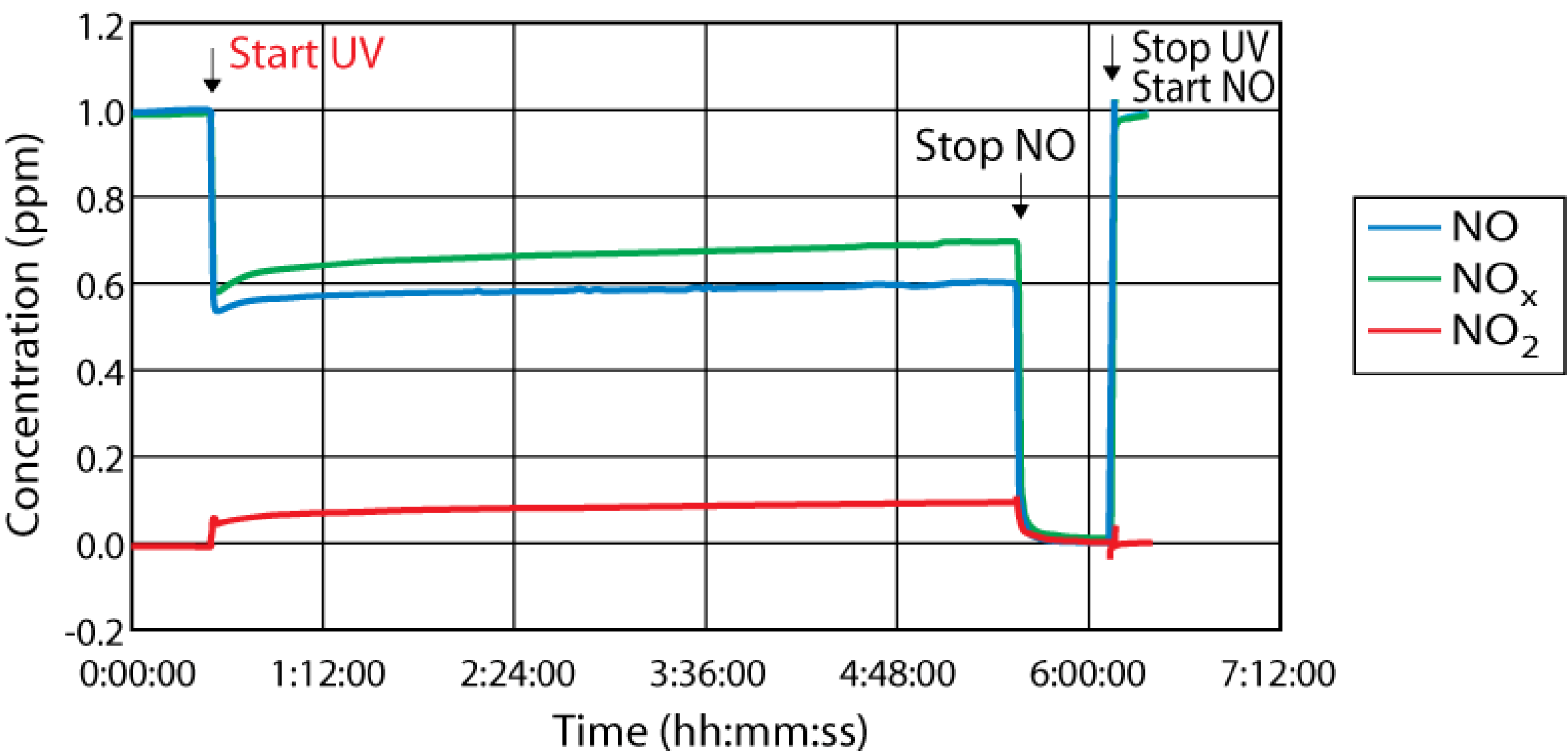
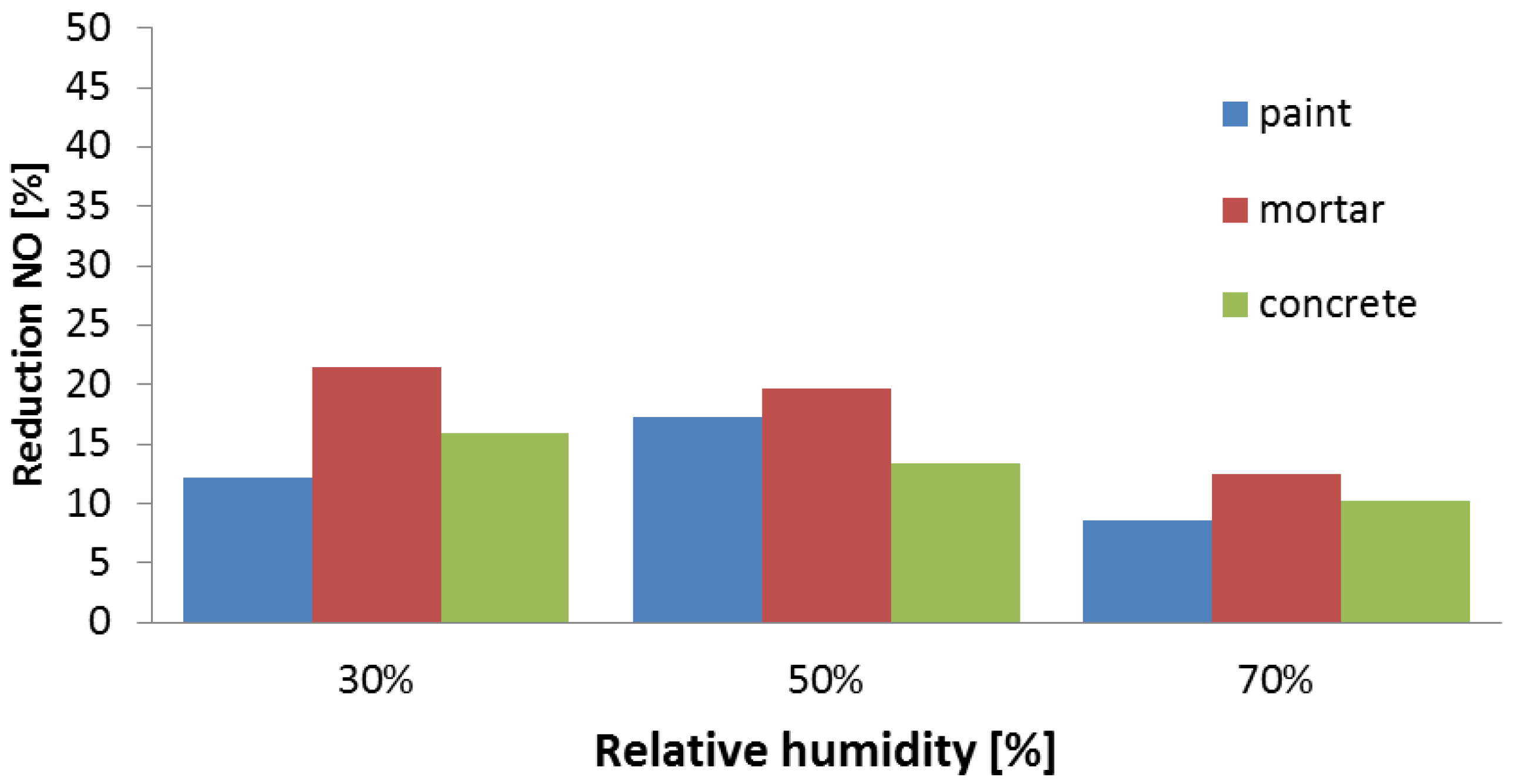
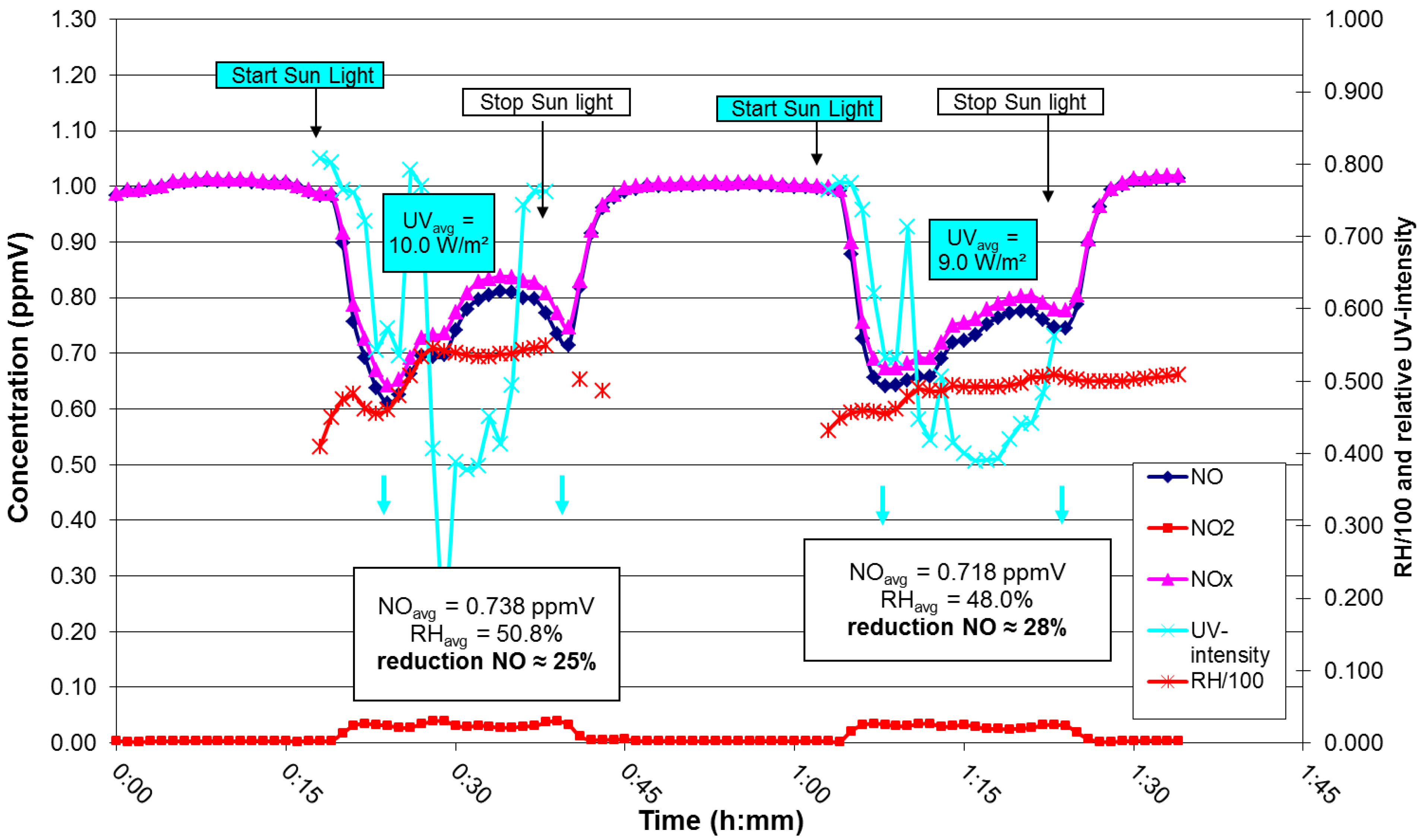
4. Pilot Project in Antwerp

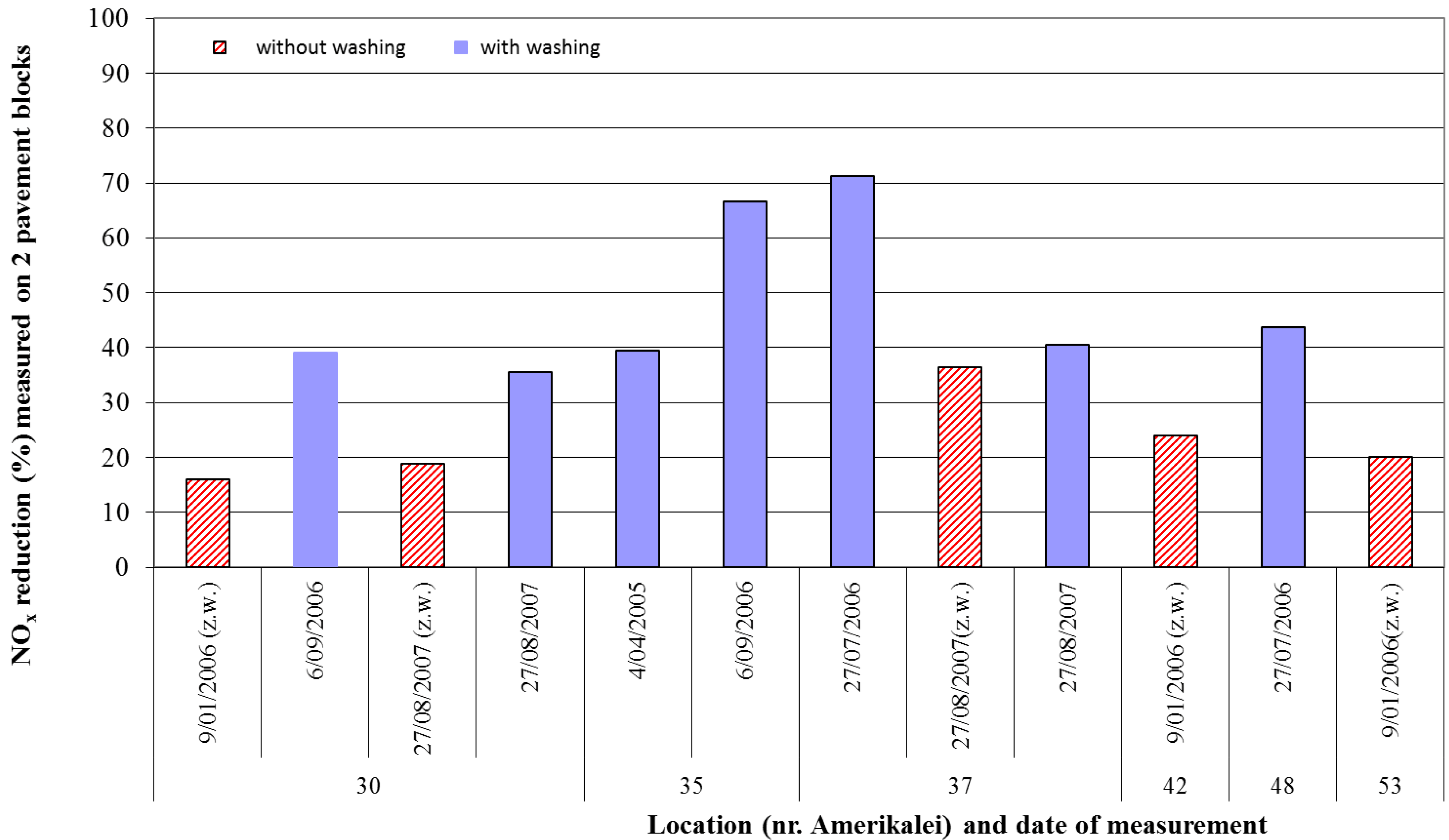
5. Recent Photocatalytic Applications in Belgium
5.1. Life+ Project PhotoPAQ


- -
- ±3% for the 160 m long test section;
- -
- ±12% for the entire Leopold II tunnel (ca. 3 km), if not affected by ventilation.
5.2. INTERREG Project ECO2PROFIT
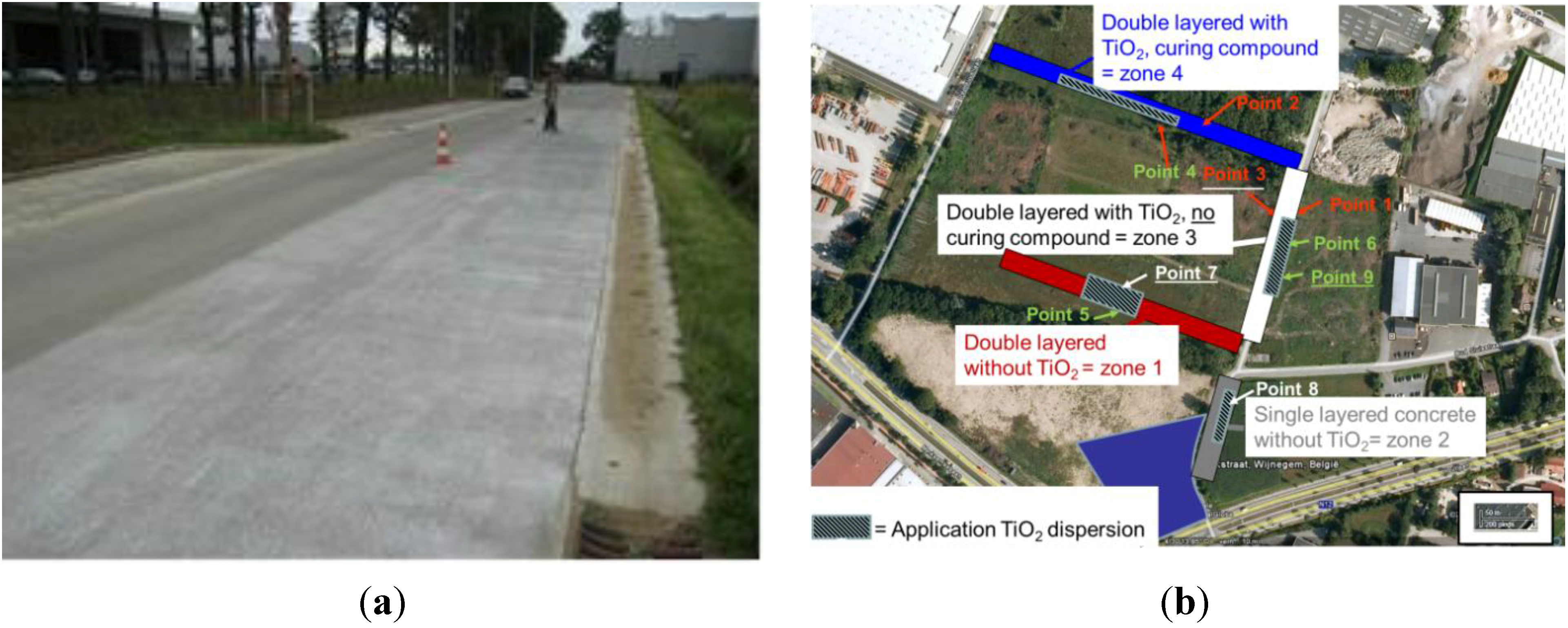
Double Layered Concrete at “Den Hoek 3” in Wijnegem




- -
- Zone 1 = double layered concrete (0/6.3 mm on top) without TiO2;
- -
- Zone 2 = single layered concrete (0/20 mm) without TiO2;
- -
- Zone 3 = double layered concrete with TiO2 (active cement) and without curing compound;
- -
- Zone 4 = double layered concrete with TiO2 (active cement) and with curing compound.
| Zone | kR,NO (kR, NOx) [m/h] | ||||
|---|---|---|---|---|---|
| Sun light | UV-lamp (10 W/m²) | ||||
| 2011 | 2012 | 2013 | 2012 | 2013 | |
| 4: with curing compound, active cement (point 2, 4) | 0.30 (0.26) | 0.09 (0.07) | – | 0.06 (0.04) | – |
| 3: without curing compound, active cement (point 1 and 3) | 0.70 (0.66) | 0.39 (0.34) | 0.38 (0.28) | 0.21 (0.19) | 0.22 (0.18) |
| 4: with curing, active cement +TiO2 dispersion (point 4) | – | – | 0.82 (0.62) | – | 0.28 (0.22) |
| 3: without curing, active cement +TiO2 dispersion (points 6 and 9) | – | – | 0.27 (0.20) | – | 0.21 (0.15) |
| 1: double layered, no active cement + TiO2 dispersion (point 7) | – | – | 0.32 (0.27) | – | 0.15 (0.13) |
| 2: single layered, no active cement + TiO2 dispersion (point 8) | – | – | 0.14 (0.13) | – | 0.08 (0.07) |

6. Conclusions and Perspectives
- -
- Optimized coating application for low surface roughness and minimizing dust adsorption;
- -
- High UV light intensity levels in the order of magnitude of 10 W/m²;
- -
- Low average relative humidity of tunnel air (≤ 60%);
- -
- High enough photocatalytic activity, with threshold values defined from lab studies;
- -
- Low average wind speed (< 2 m/s) in the tunnel for increased reaction time of pollutants;
- -
- High surface to volume ratio (smaller sized tunnel tubes).
Acknowledgements
Author Contributions
Conflicts of Interest
References
- European Commission. EU Energy and Transport in Figures, Statistical Pocketbook; Publications Office of the European Union: Brussels, Belgium, 2011. [Google Scholar]
- Beeldens, A. Air purification by pavement blocks: Final results of the research at the BRRC. In Proceedings of Transport Research Arena—TRA 2008, Ljubljana, Slovenia, 21–24 April 2008.
- Directive 2008/50/EC of the European Parliament and of the Council on ambient air quality and cleaner air for Europe. Off. J. Eur. Union 2008, L152:1–L152:44.
- Chen, J.; Poon, C. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Renz, C. Lichtreaktionen der Oxyde des Titans, Cers und der Erdsäuren. Helv. Chim. Acta 1921, 4, 961–968. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Sopyan, I.; Watanabe, M.; Murasawa, S.; Hashimoto, K.; Fujishima, A. An efficient TiO2 thin-film photocatalyst: Photocatalytic properties in gas-phase acetaldehyde degradation. J. Photochem. Photobiol. A 1996, 98, 79–86. [Google Scholar] [CrossRef]
- Cassar, L.; Pepe, C. Paving Tile Comprising an Hydraulic Binder and Photocatalyst Particles. EP-Patent 1600430 A1, 1997. [Google Scholar]
- Murata, Y.; Tawara, H.; Obata, H.; Murata, K. NOx-Cleaning Paving Block. EP-Patent 0786283 A1, 1996. [Google Scholar]
- Ohama, Y.; Van Gemert, D. Application of Titanium Dioxide Photocatalysis to Construction Materials; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Saubere Luft Durch Pflastersteine Clean Air by Airclean®. Available online: http://www.ime.fraunhofer.de/content/dam/ime/de/documents/AOe/2009_2010_Saubere%20Luft%20durch%20Pflastersteine_s.pdf (accessed on 25 July 2014).
- Dillert, R.; Stötzner, J.; Engel, A.; Bahnemann, D.W. Influence of inlet concentration and light intensity on the photocatalytic oxidation of nitrogen(II) oxide at the surface of Aeroxide® TiO2 P25. J. Hazard. Mater. 2012, 211–212, 240–246. [Google Scholar] [CrossRef]
- Laufs, S.; Burgeth, G.; Duttlinger, W.; Kurtenbach, R.; Maban, M.; Thomas, C.; Wiesen, P.; Kleffmann, J. Conversion of nitrogen oxides on commercial photocatalytic dispersion paints. Atmos. Environ. 2010, 44, 2341–2349. [Google Scholar] [CrossRef]
- Devahasdin, S.; Fan, C.; Li, J.K.; Chen, D.H. TiO2 photocatalytic oxidation of nitric oxide: Transient behavior and reaction kinetics. J. Photochem. Photobiol. A 2003, 156, 161–170. [Google Scholar] [CrossRef]
- Ballari, M.M.; Yu, Q.L.; Brouwers, H.J.H. Experimental study of the NO and NO2 degradation by photocatalytically active concrete. Catal. Today 2011, 161, 175–180. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- PhotoPAQ (2010–2014) Life+ Project. Available online: http://photopaq.ircelyon.univ-lyon1.fr/ (accessed on 25 July 2014).
- ISO 22197-1:2007 Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air-Purification Performance of Semi Conducting Photocatalytic Materials—Part 1: Removal of Nitric Oxide; International Standards Organization (ISO): Geneva, Switzerland, 2007.
- CEN Technical Committee 386 “Photocatalysis” Business Plan—(internet) Draft BUSINESS PLAN CEN/TC386 PHOTOCATALYSIS. Available online: http://standards.cen.eu/BP/653744.pdf (accessed on 28 July 2014).
- Hüsken, G.; Hunger, M.; Brouwers, H.J.H. Experimental study of photocatalytic concrete products for air purification. Build. Environ. 2009, 44, 2463–2474. [Google Scholar] [CrossRef]
- Beeldens, A.; Boonen, E. Photocatalytic applications in Belgium, purifying the air through the pavement. In Proceedings of the XXIVth World Road Conference, Mexico City, Mexico, 26–30 September 2011.
- Maggos, Th.; Plassais, A.; Bartzis, J.G.; Vasilakos, Ch.; Moussiopoulos, N.; Bonafous, L. Photocatalytic degradation of NOx in a pilot street canyon configuration using TiO2-mortar panels. Environ. Monit. Assess. 2008, 136, 35–44. [Google Scholar]
- Gignoux, L.; Christory, J.P.; Petit, J.F. Concrete roadways and air quality—Assessment of trials in Vanves in the heart of the Paris region. In Proceedings of the 12th International Symposium on Concrete Roads, Sevilla, Spain, 13–15 October 2010.
- Guerrini, G.L. Photocatalytic performances in a city tunnel in Rome: NOx monitoring results. Constr. Build. Mater. 2012, 27, 165–175. [Google Scholar] [CrossRef]
- Boonen, E.; Akylas, V.; Barmpas, F.; Boréave, A.; Bottalico, L.; Cazaunau, M.; Chen, H.; Daële, V.; De Marco, T.; Doussin, J.F.; et al. Photocatalytic de-pollution in the Leopold II tunnel in Brussels, Part I: Construction of the field site. Constr. Build. Mater. 2014. Submitted. [Google Scholar]
- Gallus, M.; Akylas, V.; Barmpas, F.; Beeldens, A.; Boonen, E.; Boréave, A.; Bottalico, L.; Cazaunau, M.; Chen, H.; Daële, V.; et al. Photocatalytic de-pollution in the Leopold II tunnel in Brussels, Part II: NOx abatement results. Constr. Build. Mater. 2014. Submitted. [Google Scholar]
- Boonen, E.; Beeldens, A. Photocatalytic roads: From lab testing to real scale applications. Eur. Transp. Res. Rev. 2013, 5, 79–89. [Google Scholar] [CrossRef]
- Beeldens, A.; Boonen, E. A double layered photocatalytic concrete pavement: A durable application with air-purifying properties. In Proceedings of 10th International Conference on Concrete Pavements (ICCP), Quebec, Canada, 8–12 July 2012.
- Ifang, S.; Gallus, M.; Liedtke, S.; Kurtenbach, R.; Wiesen, P.; Kleffmann, J. Standardization methods for testing photo-catalytic air remediation materials: Problems and solution. Atmos. Environ. 2014, 91, 154–161. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Boonen, E.; Beeldens, A. Recent Photocatalytic Applications for Air Purification in Belgium. Coatings 2014, 4, 553-573. https://doi.org/10.3390/coatings4030553
Boonen E, Beeldens A. Recent Photocatalytic Applications for Air Purification in Belgium. Coatings. 2014; 4(3):553-573. https://doi.org/10.3390/coatings4030553
Chicago/Turabian StyleBoonen, Elia, and Anne Beeldens. 2014. "Recent Photocatalytic Applications for Air Purification in Belgium" Coatings 4, no. 3: 553-573. https://doi.org/10.3390/coatings4030553
APA StyleBoonen, E., & Beeldens, A. (2014). Recent Photocatalytic Applications for Air Purification in Belgium. Coatings, 4(3), 553-573. https://doi.org/10.3390/coatings4030553