Insight into Microstructure Evolution and Corrosion Mechanisms of K2ZrF6/Al2O3-Doped Hot-Dip Aluminum/Micro-Arc Oxidation Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Coating Preparation
2.2. Characterization Methods
3. Results and Discussion
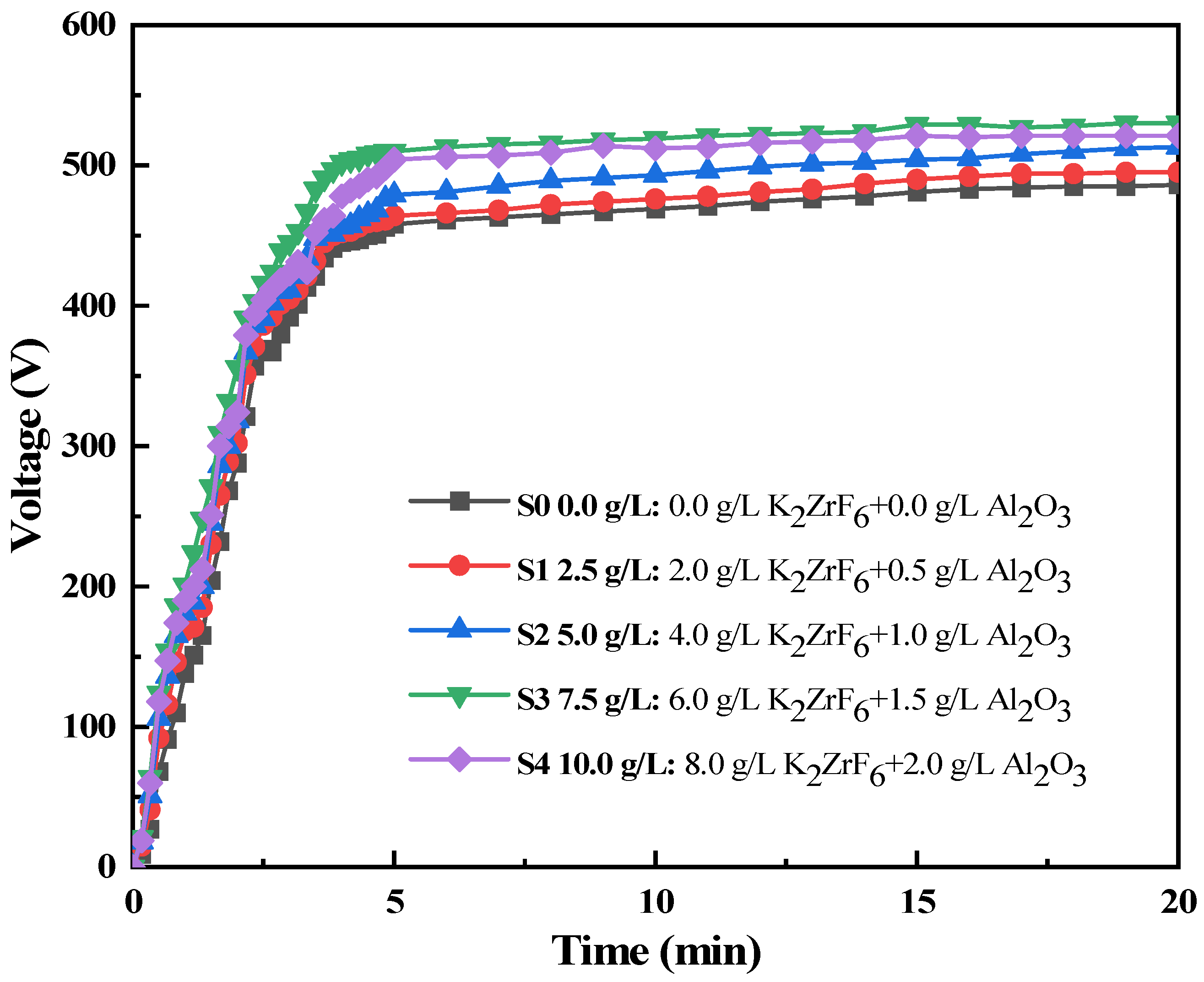
3.1. Analysis of Oxidation Voltage in Micro-Arc Oxidation Process
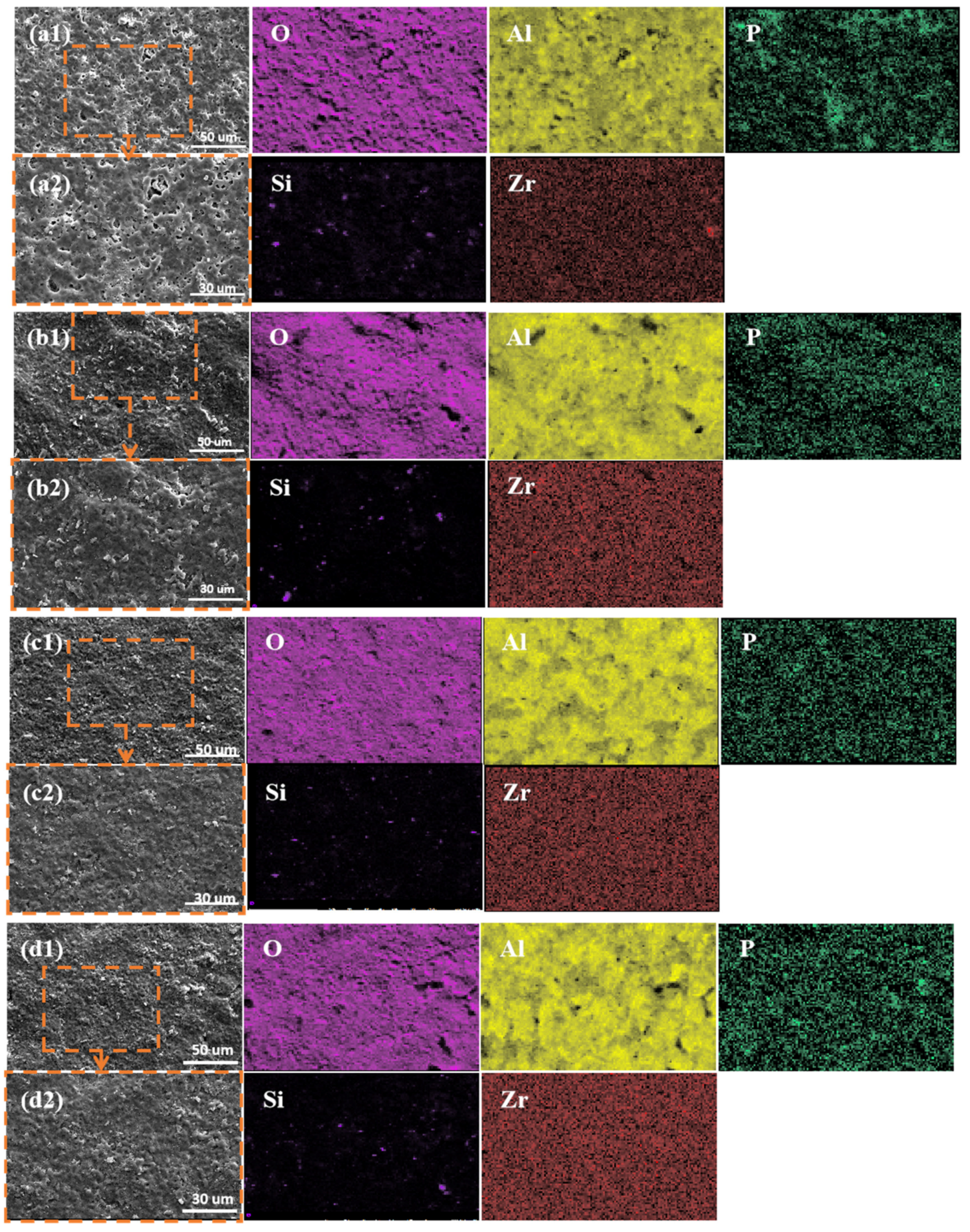
3.2. Microstructure and Elemental Analysis of Micro-Arc Oxidation Ceramic Coatings
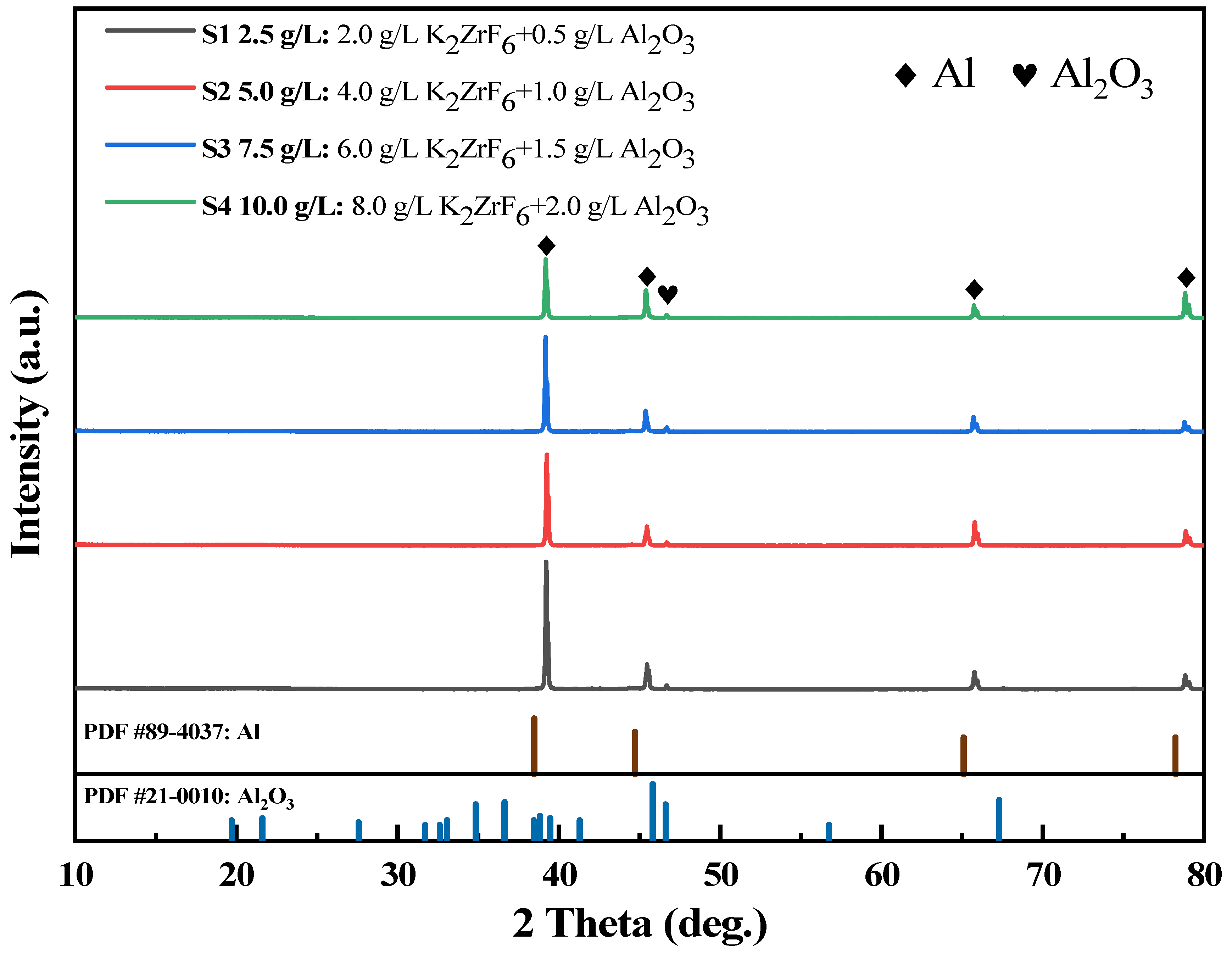
3.3. Phase Analysis of Micro-Arc Oxidation Ceramic Coatings
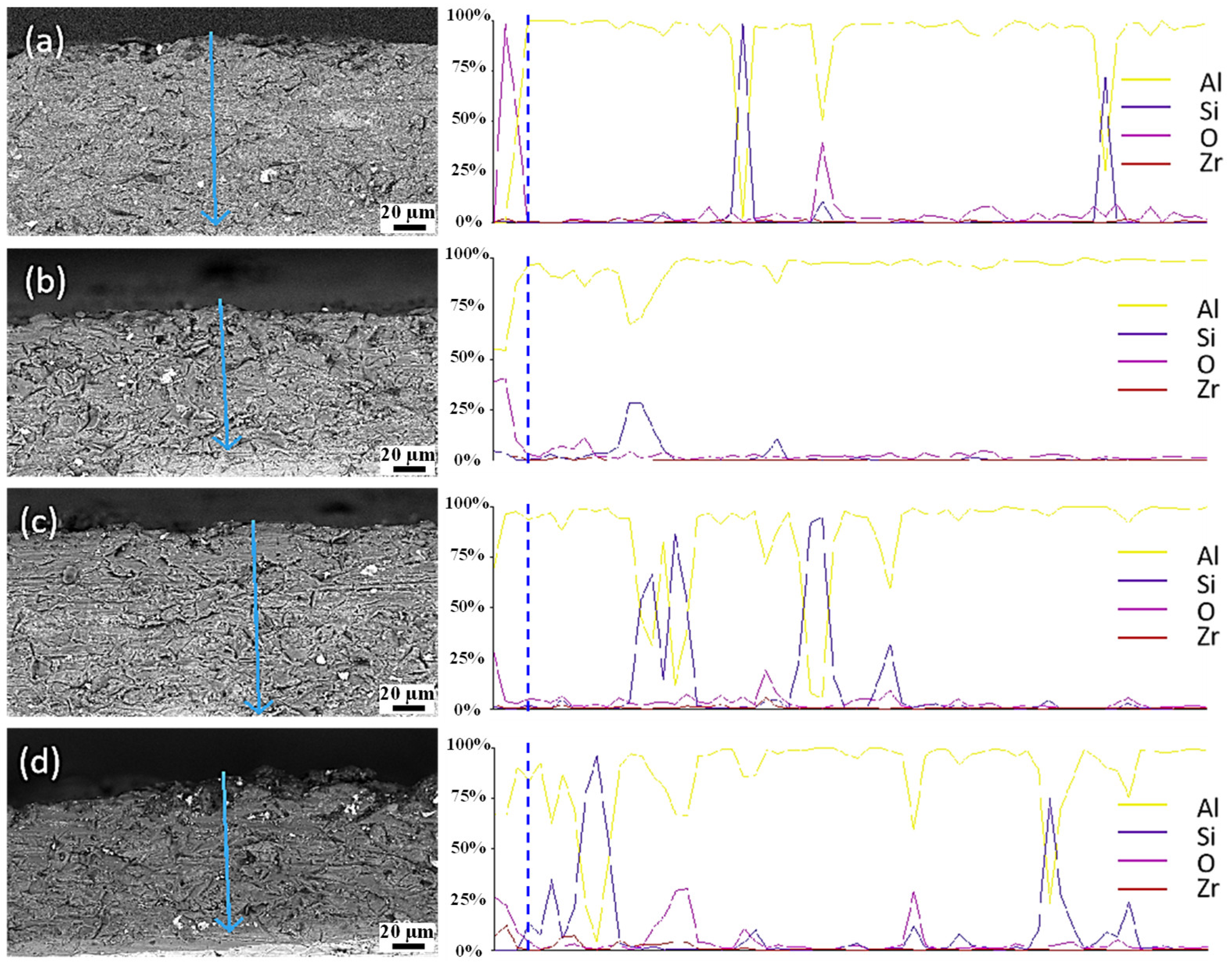
3.4. Analysis of Thickness and Hardness of Micro-Arc Oxidation Ceramic Coatings
3.5. Electrochemical Response and Corrosion Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, K.M.; Ruggles, T.H.; Rinaldi, K.; Dowling, J.A.; Duan, L.; Caldeira, K.; Lewis, N.S. The role of concentrated solar power with thermal energy storage in least-cost highly reliable electricity systems fully powered by variable renewable energy. Adv. Appl. Energy 2022, 6, 100091. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, Z. Solar Thermal Power Generation Technology in a New Generation of Energy System Positioning. Adv. Energy Power Eng. 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Singh, G.K. Solar power generation by PV (photovoltaic) technology: A review. Energy 2013, 53, 1–13. [Google Scholar] [CrossRef]
- Hayat, M.B.; Ali, D.; Monyake, K.C.; Alagha, L.; Ahmed, N. Solar energy–A look into power generation, challenges, and a solar-powered future. Int. J. Energ Res. 2019, 43, 1049–1067. [Google Scholar] [CrossRef]
- Devabhaktuni, V.; Alam, M.; Depuru, S.S.S.R.; Green II, R.C.; Nims, D.; Near, C. Solar energy: Trends and enabling technologies. Renew. Sustain. Energy Rev. 2013, 19, 555–564. [Google Scholar] [CrossRef]
- Mummana, S.S.; Anne, S.B.; Vooradi, R. A simple unit specific event based modeling framework for short term scheduling and heat integration of batch plants: Design and optimization of heat storage vessels. Comput. Chem. Eng. 2021, 145, 107155. [Google Scholar] [CrossRef]
- Budyko, M.I. The effect of solar radiation variations on the climate of the Earth. Tellus 1969, 21, 611–619. [Google Scholar] [CrossRef]
- Maruoka, D.; Sato, K.; Miura, S.; Murakami, T.; Kasai, E. Development of High Temperature Oxidation Resistant Iron-Based Heat Storage Materials for Rapid Carbonization and Pulverization Process of Biomass. Tetsu-to-Hagane 2020, 106, 527–533. [Google Scholar] [CrossRef]
- Chatterjee, U.; Bose, S.K.; Roy, S.K. Environmental Degradation of Metals: Corrosion Technology Series/14; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Czerwinski, F. Thermal Stability of Aluminum Alloys. Materials 2020, 13, 3441. [Google Scholar] [CrossRef]
- Zhu, M.; Yi, H.; Lu, J.; Huang, C.; Zhang, H.; Bo, P.; Huang, J. Corrosion of Ni–Fe based alloy in chloride molten salts for concentrating solar power containing aluminum as corrosion inhibitor. Sol. Energ. Mat. Sol. C 2022, 241, 111737. [Google Scholar] [CrossRef]
- Wei, G.; Huang, P.; Xu, C.; Liu, D.; Ju, X.; Du, X.; Xing, L.; Yang, Y. Thermophysical property measurements and thermal energy storage capacity analysis of aluminum alloys. Sol. Energy 2016, 137, 66–72. [Google Scholar] [CrossRef]
- García-Romero, A.; Delgado, A.; Urresti, A.; Martín, K.; Sala, J.M. Corrosion behaviour of several aluminium alloys in contact with a thermal storage phase change material based on Glauber’s salt. Corros. Sci. 2009, 51, 1263–1272. [Google Scholar] [CrossRef]
- Wang, S.; Niazi, H.; Lamborn, L.; Chen, W. Strain-shock-induced early stage high pH stress corrosion crack initiation and growth of pipeline steels. Corros. Sci. 2021, 178, 109056. [Google Scholar] [CrossRef]
- Li, M.C.; Wang, S.D.; Ma, R.Y.; Han, P.H.; Bi, H.Y. Effect of cyclic oxidation on electrochemical corrosion of type 409 stainless steel in the simulated muffler condensates. J. Solid State Electrochem. 2012, 16, 3059–3067. [Google Scholar] [CrossRef]
- Yao, X.; Xia, S.; Lv, Y.; Yang, W.; Chen, J. Effects of hot dipping temperature on microstructure and mechanical properties of Pb40Sn60 alloy coating on copper wires. Mater. Sci. Eng. A 2021, 810, 140991. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, S.; Song, R.; Cai, C. Effect of Mg and Si contents on hot-dip 55Al-Zn plating: Experimental and molecular dynamics simulation. Mater. Today Commun. 2023, 35, 106131. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, T.; Yu, X.; Sun, X.; Yang, H. Functionalization treatment of micro-arc oxidation coatings on magnesium alloys: A review. J. Alloys Compd. 2021, 879, 160453. [Google Scholar] [CrossRef]
- Golubkov, P.E.; Pecherskaya, E.A.; Karpanin, O.V.; Shepeleva, Y.V.; Zinchenko, T.O.; Artamonov, D.V. Automation of the micro-arc oxidation process. J. Phys. Conf. Ser. 2017, 917, 092021. [Google Scholar] [CrossRef]
- Likhanov, V.A.; Skryabin, M.L. The study of oxide films on the surface of a piston of aluminum alloy after micro-arc oxidation. IOP Conf. Ser. Earth Environ. Sci. 2019, 315, 032045. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Yao, W.; Chen, Y.; Zhong, Z.; Ci, W.; Wu, J.; Xie, Z.; Yuan, Y.; Pan, F. A self-healing corrosion protection coating with graphene oxide carrying 8-hydroxyquinoline doped in layered double hydroxide on a micro-arc oxidation coating. Corros. Sci. 2022, 194, 109941. [Google Scholar] [CrossRef]
- Yang, J.; Fang, K.; Xu, K.; Shen, X.; Xu, X. Effect of zinc or copper doping on corrosion resistance and anti-oxidative stress of strontium-based micro-arc oxidation coatings on titanium. Appl. Surf. Sci. 2023, 626, 157229. [Google Scholar] [CrossRef]
- Shen, X.; Fang, K.; Ru Yie, K.H.; Zhou, Z.; Shen, Y.; Wu, S.; Zhu, Y.; Deng, Z.; Ma, P.; Ma, J.; et al. High proportion strontium-doped micro-arc oxidation coatings enhance early osseointegration of titanium in osteoporosis by anti-oxidative stress pathway. Bioact. Mater. 2022, 10, 405–419. [Google Scholar] [CrossRef]
- Thukkaram, M.; Cools, P.; Nikiforov, A.; Rigole, P.; Coenye, T.; Van Der Voort, P.; Du Laing, G.; Vercruysse, C.; Declercq, H.; Morent, R.; et al. Antibacterial activity of a porous silver doped TiO2 coating on titanium substrates synthesized by plasma electrolytic oxidation. Appl. Surf. Sci. 2020, 500, 144235. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Zhang, J. Effect of ZrO2 particle on the performance of micro-arc oxidation coatings on Ti6Al4V. Appl. Surf. Sci. 2015, 342, 183–190. [Google Scholar] [CrossRef]
- Fatimah, S.; Kamil, M.P.; Kwon, J.H.; Kaseem, M.; Ko, Y.G. Dual incorporation of SiO2 and ZrO2 nanoparticles into the oxide layer on 6061 Al alloy via plasma electrolytic oxidation: Coating structure and corrosion properties. J. Alloys Compd. 2017, 707, 358–364. [Google Scholar] [CrossRef]
- Hu, C.-J.; Hsieh, M.-H. Preparation of ceramic coatings on an Al–Si alloy by the incorporation of ZrO2 particles in microarc oxidation. Surf. Coat. Technol. 2014, 258, 275–283. [Google Scholar] [CrossRef]
- Zhong, Y.; Shi, L.; Li, M.; He, F.; He, X. Characterization and thermal shock behavior of composite ceramic coating doped with ZrO2 particles on TC4 by micro-arc oxidation. Appl. Surf. Sci. 2014, 311, 158–163. [Google Scholar] [CrossRef]
- Kaseem, M.; Lee, Y.H.; Ko, Y.G. Incorporation of MoO2 and ZrO2 particles into the oxide film formed on 7075 Al alloy via micro-arc oxidation. Mater. Lett. 2016, 182, 260–263. [Google Scholar] [CrossRef]
- Liang, J.; Srinivasan, P.B.; Blawert, C.; Dietzel, W. Comparison of electrochemical corrosion behaviour of MgO and ZrO2 coatings on AM50 magnesium alloy formed by plasma electrolytic oxidation. Corros. Sci. 2009, 51, 2483–2492. [Google Scholar] [CrossRef]
- Wang, C.; Hao, J.; Xing, Y.; Guo, C.; Chen, H. High temperature oxidation behavior of TiO2+ZrO2 composite ceramic coatings prepared by microarc oxidation on Ti6Al4V alloy. Surf. Coat. Technol. 2015, 261, 201–207. [Google Scholar] [CrossRef]
- Selvi, E.; Muhaffel, F.; Yürektürk, Y.; Vanlı, A.S.; Baydoğan, M. Influence of Electrolyte Compositions and Electrical Parameters on Thermal Properties of Micro-Arc Oxidized AZ91 Alloy. J. Mater. Eng. Perform. 2021, 31, 1667–1678. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.; Negrea, D.; Andrei, V.; Coaca, E.; Mihailescu, C.N.; Lungu, C.P. Characterization of Al2O3/ZrO2 composite coatings deposited on Zr-2.5Nb alloy by plasma electrolytic oxidation. Appl. Surf. Sci. 2018, 451, 169–179. [Google Scholar] [CrossRef]
- Askarnia, R.; Sobhani, M.; Zare, M.; Aghamohammadi, H.; Staji, H. Incorporation of Al2O3 and ZrO2 ceramics to AZ31 magnesium alloys composite coating using micro-arc oxidation method. J. Mech. Behav. Biomed. 2023, 141, 105784. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Zhang, J.-M.; Li, Y.; Bai, L.-J.; Zhang, G.-J. Enhanced corrosion resistance of micro-arc oxidation coated magnesium alloy by superhydrophobic Mg−Al layered double hydroxide coating. Trans. Nonferr. Met. Soc. 2019, 29, 2066–2077. [Google Scholar] [CrossRef]
- Xin, S.G.; Le, J.; Song, L.X. The Composition and Hardness of the Coating Containing Zirconia Produced by Micro-Arc Oxidation on Aluminium Alloy. Key Eng Mat 2012, 512, 1082–1088. [Google Scholar] [CrossRef]
- Tang, M.; Li, W.; Liu, H.; Zhu, L. Preparation Al2O3/ZrO2 composite coating in an alkaline phosphate electrolyte containing K2ZrF6 on aluminum alloy by microarc oxidation. Appl. Surf. Sci. 2012, 258, 5869–5875. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, Y.; Kang, S.-H.; Tu, W.; Cheng, Y. A re-understanding of the breakdown theory from the study of the plasma electrolytic oxidation of a carbon steel—A non-valve metal. Electrochim. Acta 2018, 284, 681–695. [Google Scholar] [CrossRef]
- Qi, X.; Jiang, B.; Song, R. Effects of ageing treatment on corrosion behavior of 7075 aluminum alloy coated by micro arc oxidation (MAO). Corros. Sci. 2022, 199, 110164. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Z.; Wang, Y.; Li, J. Tribological mechanism of micro-arc oxidation coatings prepared by different electrolyte systems in artificial seawater. Ceram. Int. 2021, 47, 7344–7352. [Google Scholar] [CrossRef]
- Yang, W.; Xu, D.; Guo, Q.; Chen, T.; Chen, J. Influence of electrolyte composition on microstructure and properties of coatings formed on pure Ti substrate by micro arc oxidation. Surf. Coat. Technol. 2018, 349, 522–528. [Google Scholar] [CrossRef]
- Al Bosta, M.M.; Ma, K.-J.; Chien, H.-H. The effect of MAO processing time on surface properties and low temperature infrared emissivity of ceramic coating on aluminium 6061 alloy. Infrared Phys. Technol. 2013, 60, 323–334. [Google Scholar] [CrossRef]
- Lihong, L.; Dejiu, S.; Jingwu, Z.; Jian, S.; Liang, L. Evolution of micro-arc oxidation behaviors of the hot-dipping aluminum coatings on Q235 steel substrate. Appl. Surf. Sci. 2011, 257, 4144–4150. [Google Scholar] [CrossRef]
- Song, S.; Chen, B.; Li, H.; Shi, R.; Liu, C.; Yang, B.; de la Fuente, G. Growth behavior and insulation property of the oxide layer during micro-arc oxidation of aluminium in “soft” regime condition. J. Mater. Sci. 2023, 58, 7136–7148. [Google Scholar] [CrossRef]
- Wang, S.D.; Xu, D.K.; Wang, B.J.; Sheng, L.Y.; Qiao, Y.X.; Han, E.-H.; Dong, C. Influence of phase dissolution and hydrogen absorption on the stress corrosion cracking behavior of Mg-7% Gd-5% Y-1% Nd-0.5% Zr alloy in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 142, 185–200. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Li, Q.; Wang, S.; Wu, M.; Yang, S.; Xiang, D. Effects of Al or Mo Addition on Microstructure and Mechanical Properties of Fe-Rich Nonequiatomic FeCrCoMnNi High-Entropy Alloy. Metals 2022, 12, 191. [Google Scholar] [CrossRef]
- Yang, C.; Xu, W.; Meng, X.; Shi, X.; Shao, L.; Zeng, X.; Yang, Z.; Li, S.; Liu, Y.; Xia, X. A pH-responsive hydrophilic controlled release system based on ZIF-8 for self-healing anticorrosion application. Chem. Eng. J. 2021, 415, 128985. [Google Scholar] [CrossRef]
- Zhong, F.; He, Y.; Wang, P.; Chen, C.; Wu, Y. Novel pH-responsive self-healing anti-corrosion coating with high barrier and corrosion inhibitor loading based on reduced graphene oxide loaded zeolite imidazole framework. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128641. [Google Scholar] [CrossRef]
- Wang, S.; Lamborn, L.; Chevil, K.; Gamboa, E.; Chen, W. Dense and Sparse Stress Corrosion Crack Initiation in an X65 Pipeline Steel with Mill Scale. In Proceedings of the 2020 13th International Pipeline Conference, Virtual, Online, 28–30 September 2020. [Google Scholar]
- Wang, S.; Lamborn, L.; Chen, W. Near-neutral pH corrosion and stress corrosion crack initiation of a mill-scaled pipeline steel under the combined effect of oxygen and paint primer. Corros. Sci. 2021, 187, 109511. [Google Scholar] [CrossRef]
- Wang, S.; Lamborn, L.; Chevil, K.; Gamboa, E.; Chen, W. On the formation of stress corrosion crack colonies with different crack population. Corros. Sci. 2020, 168, 108592. [Google Scholar] [CrossRef]
- Wang, S.; Lamborn, L.; Chevil, K.; Gamboa, E.; Chen, W. Near-neutral pH corrosion of mill-scaled X-65 pipeline steel with paint primer. J. Mater. Sci. Technol. 2020, 49, 166–178. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, L.; Wang, S.; Lin, Y.; Sun, Y.; Xia, R. Effect of simulated pore solution on passivation characteristic of P110 steel. J. Petrol. Sci. Eng. 2018, 167, 949–956. [Google Scholar] [CrossRef]
- Durdu, S.; Usta, M. Characterization and mechanical properties of coatings on magnesium by micro arc oxidation. Appl. Surf. Sci. 2012, 261, 774–782. [Google Scholar] [CrossRef]
- Wang, S.D.; Wu, M.Y.; Xu, D.K.; Han, E.-h. Improving corrosive wear resistance of Mg-Zn-Y-Zr alloys through heat treatment. J. Magnes. Alloys 2023, 11, 1981–1995. [Google Scholar] [CrossRef]
- Wang, S.; Yao, M.; He, X.; Wu, B.; Liu, L.; Wang, S.; Wu, M.; Zhang, X.; Xiang, D. Corrosion Evolution of a Concrete/Casing Steel in Simulated Formation Water under Different CO2 Partial Pressures. Int. J. Electrochem. Sci. 2020, 15, 9948–9970. [Google Scholar] [CrossRef]
- Wang, S.D.; Xu, D.; Chen, X.; Han, E.; Dong, C. Effect of heat treatment on the corrosion resistance and mechanical properties of an as-forged Mg–Zn–Y–Zr alloy. Corros. Sci. 2015, 92, 228–236. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, C.; Wang, Q.; Bu, S. Growth characteristics and corrosion resistance of micro-arc oxidation coating on pure magnesium for biomedical applications. Corros. Sci. 2010, 52, 2228–2234. [Google Scholar] [CrossRef]
- Chen, X.W.; Li, M.L.; Zhang, D.F.; Cai, L.P.; Ren, P.; Hu, J.; Liao, D.D. Corrosion resistance of MoS2-modified titanium alloy micro-arc oxidation coating. Surf. Coat. Technol. 2022, 433, 128127. [Google Scholar] [CrossRef]
- Yan, Y.; Han, Y.; Li, D.; Huang, J.; Lian, Q.J.A.S.S. Effect of NaAlO2 concentrations on microstructure and corrosion resistance of Al2O3/ZrO2 coatings formed on zirconium by micro-arc oxidation. Appl. Surf. Sci. 2010, 256, 6359–6366. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Xu, T.; Li, H.; Jiang, B.; Miao, X. Preparation and corrosion resistance of a self-sealing hydroxyapatite-MgO coating on magnesium alloy by microarc oxidation. Ceram. Int. 2022, 48, 13676–13683. [Google Scholar] [CrossRef]
- Wang, S.; Lamborn, L.; Chevil, K.; Gamboa, E.; Chen, W. Strain-Aging-Assisted Localized Corrosion of a Mill-Scaled X-65 Pipeline Steel. Corrosion 2021, 77, 792–808. [Google Scholar] [CrossRef]
- Li, C.; Goei, R.; Li, Y.; Shi, J.; Liu, F.; Li, B.; Gao, Y.; Li, Y.; Li, S.; Tok, A.I.Y. Effect of chromium on erosion-corrosion properties of ZrO2-Al2O3 particles reinforced Fe-based composites in artificial seawater slurries. Corros. Sci. 2022, 198, 110138. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Wang, J.; Bian, D.; Zhao, Y. Effect of pore content and pH on the corrosion behavior of hydrophobic ceramic coatings. Int. J. Appl. Ceram. Technol. 2023, 20, 1624–1635. [Google Scholar] [CrossRef]


| Element | C | Si | Mn | S | P | Cr | Ni | Mo | N |
|---|---|---|---|---|---|---|---|---|---|
| Content | 0.02 | 0.8 | 1.5 | 0.03 | 0.03 | 16.5 | 10.8 | 2.2 | 0.08 |
| Condition | Total Concentration (g/L) | K2ZrF6 (g/L) | Al2O3 (g/L) |
|---|---|---|---|
| S0 | 0.0 | 0.0 | 0.0 |
| S1 | 2.5 | 2.0 | 0.5 |
| S2 | 5.0 | 4.0 | 1.0 |
| S3 | 7.5 | 6.0 | 1.5 |
| S4 | 10.0 | 8.0 | 2.0 |
| Composite Additive Concentration (g/L) | O (wt.%) | Al (wt.%) | P (wt.%) | Si (wt.%) | Zr (wt.%) |
|---|---|---|---|---|---|
| 2.5 | 35.165 | 61.538 | 0.200 | 1.399 | 1.698 |
| 5.0 | 31.900 | 64.900 | 0.300 | 1.100 | 1.800 |
| 7.5 | 30.400 | 66.300 | 0.300 | 0.800 | 2.200 |
| 10.0 | 30.800 | 65.500 | 0.300 | 1.100 | 2.300 |
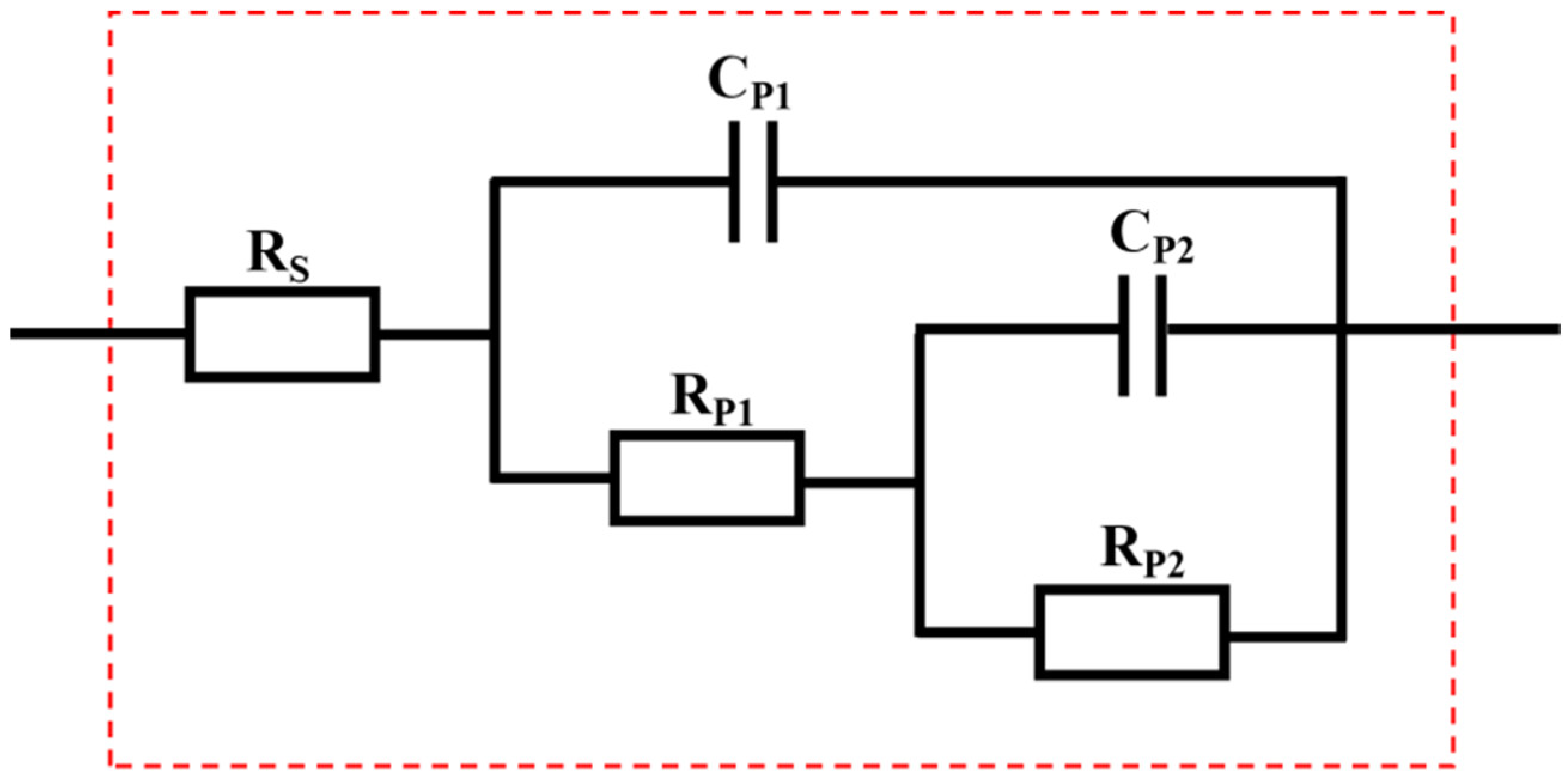
| Sample | Rs (Ω·cm2) | Cp1 (F·cm−2) | Rp1 (Ω·cm2) | Cp2 (F·cm−2) | Rp2 (Ω·cm2) |
|---|---|---|---|---|---|
| S0 | 20.37 | 9.707 × 10−5 | 545.3 | 3.856 × 10−3 | 1134 |
| S1 | 14.56 | 1.746 × 10−4 | 580.0 | 2.871 × 10−3 | 1353 |
| S2 | 52.13 | 2.242 × 10−5 | 1473.0 | 1.430 × 10−3 | 2779 |
| S3 | 52.70 | 1.263 × 10−5 | 3240.0 | 6.439 × 10−4 | 7488 |
| S4 | 18.26 | 7.745 × 10−7 | 2571.0 | 3.112 × 10−5 | 3207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Peng, X.; Yang, Y.; Wang, S.; Wu, M.; Hu, P.; Fu, C. Insight into Microstructure Evolution and Corrosion Mechanisms of K2ZrF6/Al2O3-Doped Hot-Dip Aluminum/Micro-Arc Oxidation Coatings. Coatings 2023, 13, 1543. https://doi.org/10.3390/coatings13091543
Wang S, Peng X, Yang Y, Wang S, Wu M, Hu P, Fu C. Insight into Microstructure Evolution and Corrosion Mechanisms of K2ZrF6/Al2O3-Doped Hot-Dip Aluminum/Micro-Arc Oxidation Coatings. Coatings. 2023; 13(9):1543. https://doi.org/10.3390/coatings13091543
Chicago/Turabian StyleWang, Shuliang, Xiaofei Peng, Yi Yang, Shidong Wang, Mingyu Wu, Ping Hu, and Chunyan Fu. 2023. "Insight into Microstructure Evolution and Corrosion Mechanisms of K2ZrF6/Al2O3-Doped Hot-Dip Aluminum/Micro-Arc Oxidation Coatings" Coatings 13, no. 9: 1543. https://doi.org/10.3390/coatings13091543






