Morphological and Mechanical Characterization of Films Incorporating Porphyran Extracted from Porphyra Dioica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Formulation and Processing
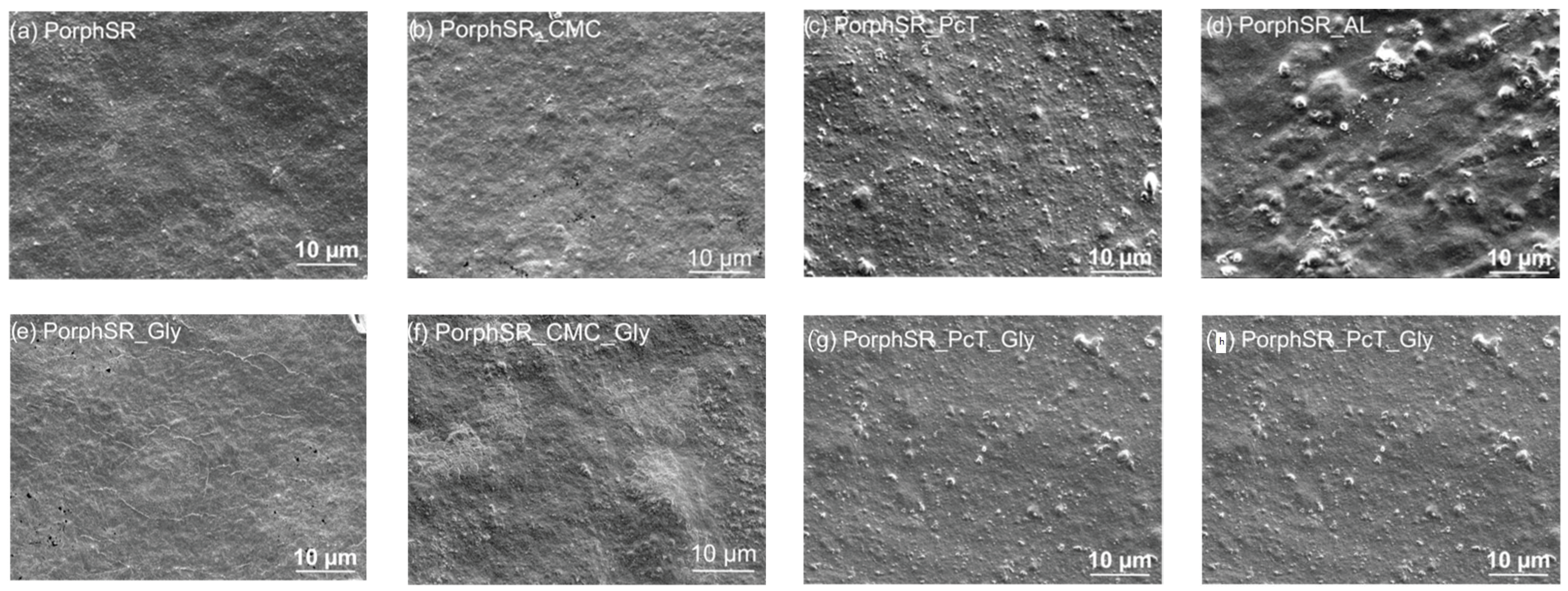
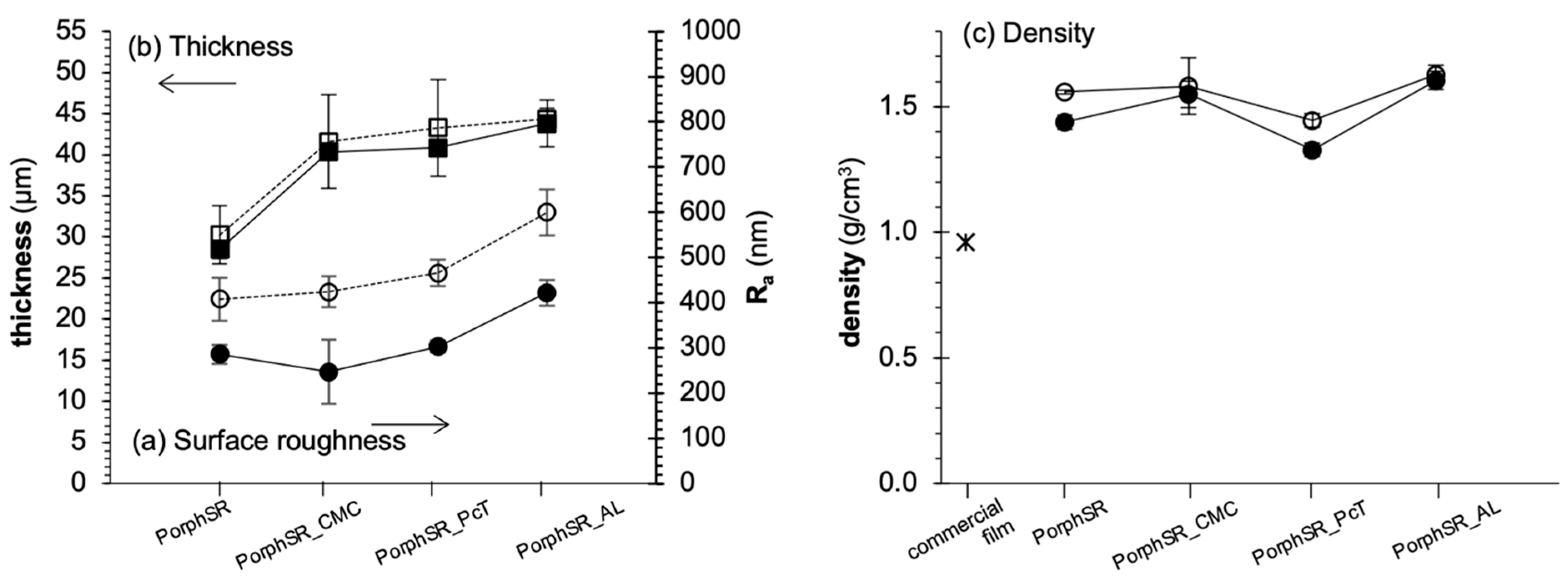
2.2. Morphological Characterization
2.3. Thermal Characterization
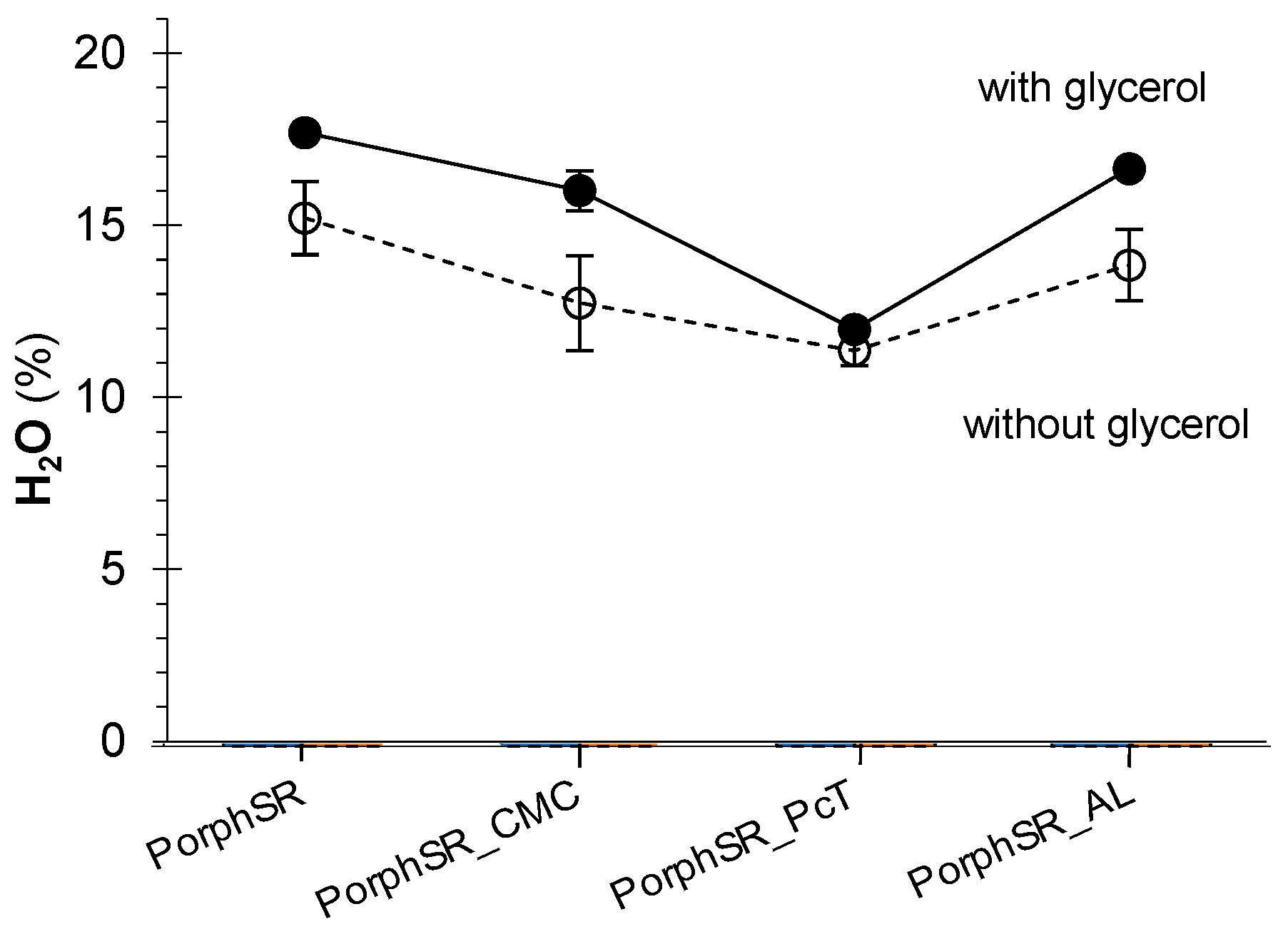
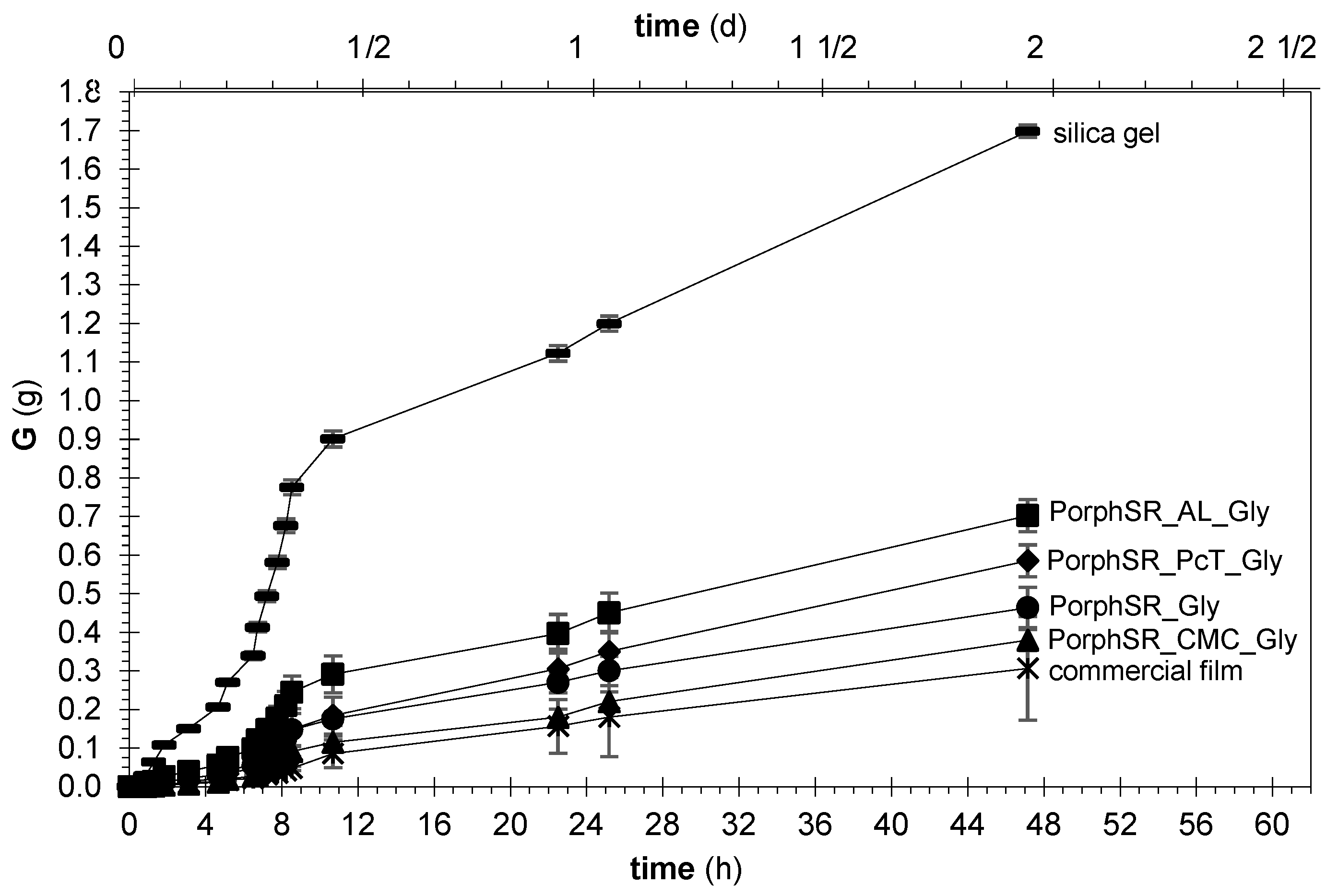
2.4. Characterization of Film Interactions with Water
2.5. Mechanical Testing
3. Results
3.1. Film Morphology
3.2. Film Composition
3.3. Thermal Properties
3.4. Interactions with Water
3.5. Mechanical Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Interactions with Water
| PorphSR_Gly | PorphSR_AL_Gly | PorphSR_CMC_Gly | PorphSR_PcT_Gly | Commercial Film | |
| PorphSR_AL_Gly | 1 | - | - | - | - |
| PorphSR_CMC_Gly | 1 | 1 | - | - | - |
| PorphSR_PcT_Gly | 9.39 × 10−2 | 7.01 × 10−1 | 1.74 × 10−2 | - | - |
| Commercial film | 1.05 × 10−2 | 1.70 × 10−3 | 5.49 × 10−2 | 7.02 × 10−5 | - |
| No film | 2.93 × 10−7 | 7.81 × 10−7 | 1.35 × 10−7 | 7.42 × 10−6 | 9.70 × 10−9 |
| PorphSR_Gly | PorphSR_AL_Gly | PorphSR_CMC_Gly | PorphSR_PcT_Gly | |
| PorphSR_AL_Gly | 2.00 × 10−10 | - | - | - |
| PorphSR_CMC_Gly | 1.10 × 10−2 | 5.40 × 10−11 | - | - |
| PorphSR_PcT_Gly | 4.50 × 10−10 | 2.98 × 10−1 | 1.10 × 10−10 | - |
| Commercial film | 7.90 × 10−14 | 1.90 × 10−15 | 1.50 × 10−13 | 2.40 × 10−15 |
| PorphSR_Gly | PorphSR_AL_Gly | PorphSR_CMC_Gly | PorphSR_PcT_Gly | |
| PorphSR_AL_Gly | 3.20 × 10−13 | - | - | - |
| PorphSR_CMC_Gly | 1.50 × 10−07 | 7.30 × 10−12 | - | - |
| PorphSR_PcT_Gly | 1.80× 10−12 | 2.00 × 10−05 | 8.30 × 10−11 | - |
| Commercial film | 7.80 × 10−12 | 1.40 × 10−15 | 3.40 × 10−13 | 3.60 × 10−15 |
| PorphSR | PorphSR _Gly | PorphSR _AL | PorphSR _AL_Gly | PorphSR _CMC | PorphSR _CMC_Gly | PorphSR _PcT | PorphSR _PcT_Gly | |
| PorphSR_Gly | 1 | - | - | - | - | - | - | - |
| PorphSR_AL | 4.10 × 10−2 | 4.10 × 10−2 | - | - | - | - | - | - |
| PorphSR_AL_Gly | 1 | 1 | 1 | - | - | - | - | - |
| PorphSR_CMC | 1 | 1 | 5.71 × 10−1 | 1 | - | - | - | - |
| PorphSR_CMC_Gly | 1 | 1 | 3.33 × 10−1 | 1 | 1 | - | - | - |
| PorphSR_PcT | 1 | 1 | 2.50 × 10−1 | 1 | 1 | 1 | - | - |
| PorphSR_PcT_Gly | 1 | 1 | 5.90 × 10−1 | 1 | 1 | 1 | 1 | - |
| Commercial film | <2 × 10−16 | <2 × 10−16 | <2 × 10−16 | <2 × 10−16 | <2 × 10−16 | <2 × 10−16 | <2 × 10−16 | <2 × 10−16 |
Appendix B. Statistical Analysis of Mechanical Properties
| PorphSR | PorphSR _Gly | PorphSR _PcT | PorphSR _PcT_Gly | PorphSR _CMC | PorphSR _CMC_Gly | PorphSR _AL | PorphSR _AL_Gly | |
| PorphSR_Gly | 1.60 × 10−6 | - | - | - | - | - | - | - |
| PorphSR_PcT | 3.44 × 10−3 | 1.30 × 10−12 | - | - | - | - | - | - |
| PorphSR_PcT_Gly | 2.19 × 10−3 | 1.0 | 4.10 × 10−9 | - | - | - | - | - |
| PorphSR_CMC | 8.50 × 10−4 | 1.0 | 5.60 × 10−10 | 1 | - | - | - | - |
| PorphSR_CMC_Gly | 1.10 × 10−3 | 8.99 × 10−1 | 7.10 × 10−10 | 1 | 1 | - | - | - |
| PorphSR_AL | 1.70 × 10−4 | 6.40 × 10−14 | 1 | 2.60 × 10−10 | 2.80 × 10−10 | 3.50 × 10−11 | - | - |
| PorphSR_AL_Gly | 5.00 × 10−4 | 1 | 3.50 × 10−10 | 1 | 1 | 1 | 1.80 × 10−11 | - |
| Commercial film | 2.00 × 10−10 | 2.25 × 10−3 | 1.90 × 10−15 | 4.30 × 10−5 | 1.20 × 10−5 | 9.10 × 10−6 | <2 × 10−16 | 2.10 × 10−5 |
| PorphSR | PorphSR _Gly | PorphSR _PcT | PorphSR _PcT_Gly | PorphSR _CMC | PorphSR _CMC_Gly | PorphSR _AL | PorphSR _AL_Gly | |
| PorphSR_Gly | 2.18 × 10−1 | - | - | - | - | - | - | - |
| PorphSR_PcT | 1.17 × 10−2 | 1.34 × 10−2 | - | - | - | - | - | - |
| PorphSR_PcT_Gly | 1.16 × 10−1 | 1.62 × 10−5 | 1 | - | - | - | - | - |
| PorphSR_CMC | 1 | 1 | 1.32 × 10−3 | 1.41 × 10−2 | - | - | - | - |
| PorphSR_CMC_Gly | 1.32 × 10−1 | 4.33 × 10−6 | 1 | 1 | 1.26 × 10−2 | - | - | - |
| PorphSR_AL | 1 | 2.90 × 10−4 | 1.51 × 10−1 | 1 | 5.82 × 10−1 | 1 | - | - |
| PorphSR_AL_Gly | 1 | 1 | 2.30 × 10−4 | 2.92 × 10−3 | 1 | 1.85 × 10−3 | 1.33 × 10−1 | - |
| Commercial film | 6.47 × 10−2 | 1 | 6.70 × 10−7 | 7.03 × 10−6 | 5.44 × 10−1 | 2.07 × 10−4 | <1.00 × 10−4 | 7.29 × 10−1 |
| PorphSR | PorphSR _Gly | PorphSR _PcT | PorphSR _PcT_Gly | PorphSR _CMC | PorphSR _CMC_Gly | PorphR _AL | |
| PorphSR_Gly | 1 | - | - | - | - | - | - |
| PorphSR_PcT | 1 | 1 | - | - | - | - | - |
| PorphSR_PcT_Gly | 5.39 × 10−7 | 9.00 × 10−8 | 5.65 × 10−6 | - | - | - | - |
| PorphSR_CMC | 1 | 1 | 1 | 1.04 × 10−5 | - | - | - |
| PorphSR_CMC_Gly | 1.55 × 10−7 | 2.60 × 10−8 | 1.51 × 10−6 | 1 | 2.72 × 10−6 | - | - |
| PorphSR_AL | 1 | 1 | 1 | 1.19 × 10−7 | 1 | 3.30 × 10−8 | - |
| PorphSR_AL_Gly | 1 | 1 | 1 | 3.10 × 10−6 | 1 | 8.42 × 10−7 | 1 |
References
- Marsh, K.; Bugusu, B. Food Packaging-Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible Films and Coatings for Food Packaging Applications: A Review. Environ. Chem. Lett. 2022, 20, 875–900. [Google Scholar] [CrossRef]
- Raheem, D. Application of Plastics and Paper as Food Packaging Materials—An Overview. Emirates J. Food Agric. 2017, 25, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Hamed, I.; Jakobsen, A.N.; Lerfall, J. Sustainable Edible Packaging Systems Based on Active Compounds from Food Processing Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 198–226. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Jamróz, E.; Gonçalves, S.d.Á.; da Silva, R.G.; Alves, R.M.V.; Vieira, R.P. Preparation and Characterization of Sodium Alginate Films with Propolis Extract and Nano-SiO2. Food Hydrocoll. Health 2022, 2, 100094. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid-Based Natural Edible Films in Food Packaging: A Review. Carbohydr Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Anderson, N.S.; Rees, D.A. Porphyran: A Polysaccharide with a Masked Repeating Structure. J. Chem. Soc. 1965, 5880–5887. [Google Scholar] [CrossRef]
- Teles, M.; Adão, P.; Afonso, C.; Bernardino, R.; Guedes, M.; Baptista, R.; Bernardino, S. Development and Characterization of Films for Food Application Incorporating Porphyran Extracted from Porphyra Dioica. Coatings 2022, 12, 148. [Google Scholar] [CrossRef]
- Morrice, L.M.; McLean, M.W.; Long, W.F.; Williamson, F.B. Porphyran Primary Structure. Hydrobiologia 2004, 116–117, 572–575. [Google Scholar]
- Bhatia, S.; Namdeo, A.G.; Nanda, S. Factors Effecting the Gelling and Emulsifying Properties of a Natural Polymer. Syst. Rev. Pharm. 2010, 1, 86–92. [Google Scholar] [CrossRef]
- Fernando, S.; Kim, K.-N.; Kim, D.; Jeon, Y.-J. Algal Polysaccharides: Potential Bioactive Substances for Cosmeceutical Applications. Crit. Rev. Biotechnol. 2019, 39, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.A.; Conway, E. The Structure and Biosynthesis of Porphyran: A Comparison of Some Samples. Biochem. J. 1962, 84, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.; Yoon, W.B.; Matsukawa, S. Effect of Moisture Uptake on the Texture of Dried Laver Porphyra. (Nori) Studied by Mechanical Characterization and NMR Measurements. Food Hydrocoll. 2022, 124, 107223. [Google Scholar] [CrossRef]
- Bhatia, S.; Sharma, K.; Nagpal, K.; Bera, T. Investigation of the Factors Influencing the Molecular Weight of Porphyran and Its Associated Antifungal Activity. Bioact. Carbohydr. Diet. Fibre 2015, 5, 153–168. [Google Scholar] [CrossRef]
- Cassani, L.; Lourenço-Lopes, C.; Barral-Martinez, M.; Chamorro, F.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Thermochemical Characterization of Eight Seaweed Species and Evaluation of Their Potential Use as an Alternative for Biofuel Production and Source of Bioactive Compounds. Int. J. Mol. Sci. 2022, 23, 2355. [Google Scholar] [CrossRef]
- Sinurat, E.; Fransiska, D.; Livia. The Effect of Addition Glycerol Against Nori Characterization from Gracilaria sp. and Ulva sp. Seaweeds. IOP Conf. Ser. Earth Environ. Sci. 2021, 715, 12054. [Google Scholar] [CrossRef]
- Adão, P.; Reboleira, J.; Teles, M.; Santos, B.; Ribeiro, N.; Teixeira, C.M.; Guedes, M.; Pessoa, J.C.; Bernardino, S. Enhancement of the Antioxidant and Antimicrobial Activities of Porphyran through Chemical Modification with Tyrosine Derivatives. Molecules 2021, 26, 2916. [Google Scholar] [CrossRef]
- Cian, R.E.; Salgado, P.R.; Drago, S.R.; Mauri, A.N. Effect of Glycerol and Ca+2 Addition on Physicochemical Properties of Edible Carrageenan/Porphyran-Based Films Obtained from the Red Alga, Pyropia Columbina. J. Appl. Phycol. 2015, 27, 1699–1708. [Google Scholar] [CrossRef]
- Cian, R.E.; Alaiz, M.; Vioque, J.; Drago, S.R. Enzyme Proteolysis Enhanced Extraction of ACE Inhibitory and Antioxidant Compounds (Peptides and Polyphenols) from Porphyra Columbina Residual Cake. J. Appl Phycol. 2013, 25, 1197–1206. [Google Scholar] [CrossRef]
- Nieto, M.B. Structure and Function of Polysaccharide Gum-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Embuscado, M.E., Huber, K.C., Eds.; Springer: New York, NY, USA, 2009; pp. 57–112. [Google Scholar]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydro-gel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef]
- Han, J.H. Chapter 9—Edible Films and Coatings: A Review. In Food Science and Technology, 2nd ed.; Han, J.H., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 213–255. ISBN 978-0-12-394601-0. [Google Scholar]
- Begum, R.; Yusof, Y.A.; Aziz, M.G.; Uddin, M.B. Structural and Functional Properties of Pectin Extracted from Jackfruit (Artocarpus heterophyllus) Waste: Effects of Drying. Int. J. Food Prop. 2017, 20, S190–S201. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Kanmani, P.; Rhim, J.-W. Antimicrobial and Physical-Mechanical Properties of Agar-Based Films Incorporated with Grapefruit Seed Extract. Carbohydr. Polym. 2014, 102, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Morales-Sanchez, E.; Velazquez, G.; Vázquez, M. Measurement of the Water Vapor Permeability of Chitosan Films: A Laboratory Experiment on Food Packaging Materials. J. Chem. Educ. 2022, 99, 2403–2408. [Google Scholar] [CrossRef]
- Abdel-Galil, A.; Ali, H.E.; Atta, A.; Balboul, M.R. Influence of Nanostructured TiO2 Additives on Some Physical Characteristics of Carboxymethyl Cellulose (CMC). J. Radiat. Res. Appl. Sci. 2014, 7, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Jia, S.; Yu, D.; Zhu, Y.; Wang, Z.; Chen, L.; Fu, L. Morphology, Crystallization and Thermal Behaviors of PLA-Based Composites: Wonderful Effects of Hybrid GO/PEG via Dynamic Impregnating. Polymers 2017, 9, 528. [Google Scholar] [CrossRef] [Green Version]
- Suryadi, H.; Sutriyo; Fauziah, G. Characterization Sodium Carboxymethyl Cellulose from Alpha Cellulose Betung Bamboo (Dendrocalamus asper). Pharmacogn. J. 2019, 11, 894–900. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Li, Q.; Che, Y.; Liu, X.; Dong, C.; Chen, X.; Wang, C. Effect of Na2CO3 on the Microstructure and Macroscopic Properties and Mechanism Analysis of PVA/CMC Composite Film. Polymers 2020, 12, 453. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Chen, S.; Wu, D.; Zheng, J.; Ye, X. Ultrasonic-Assisted Citrus Pectin Modification in the Bicarbonate-Activated Hydrogen Peroxide System: Chemical and Microstructural Analysis. Ultrason. Sonochem. 2019, 58, 104576. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Krupa, I. Alginate-Mediated Synthesis of Hetero-Shaped Silver Nanoparticles and Their Hydrogen Peroxide Sensing Ability. Molecules 2020, 25, 435. [Google Scholar] [CrossRef] [Green Version]
- Hammer, A.; Fedelich, N.; Giani, S.; Hempel, E.; Jing, N.; Nijman, M.; Riesen, R.; Schawe, J.; Schubnell, M. Thermal Analysis of Polymers; Mettler-Toledo: Greifensee, Switzerland, 2013. [Google Scholar]
- Motta, M.V.L.; de Castro, E.V.R.; Muri, E.J.B.; Loureiro, B.V.; Costalonga, M.L.; Filgueiras, P.R. Thermal and Spectroscopic Analyses of Guar Gum Degradation Submitted to Turbulent Flow. Int. J. Biol. Macromol. 2019, 131, 43–49. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, H.; Fu, L.; Ci, F.; Mao, X. Porphyran and Oligo-Porphyran Originating from Red Algae Porphyra: Preparation, Biological Activities, and Potential Applications. Food Chem. 2021, 349, 129209. [Google Scholar] [CrossRef] [PubMed]
- Argon, A. The Physics of Deformation and Fracture of Polymers; Cambridge University Press: Cambridge, UK, 2013; pp. 1–511. [Google Scholar] [CrossRef] [Green Version]
- Cian, R.E.; Salgado, P.R.; Drago, S.R.; González, R.J.; Mauri, A.N. Development of Naturally Activated Edible Films with Antioxidant Properties Prepared from Red Seaweed Porphyra Columbina Biopolymers. Food Chem. 2014, 146, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Gelation of Polysaccharides. J. Intell. Mater. Syst. Struct. 1993, 4, 210–215. [Google Scholar] [CrossRef]
- Simoni, R.C.; Lemessandra, G.F.; Fialho, S.; Gonçalves, O.H.; Gozzo, A.M.; Chiaradia, V.; Sayer, C.; Shirai, M.A.; Leimman, F.V. Effect of Drying Method on Mechanical, Thermal and Water Absorption Properties of Enzymatically Crosslinked Gelatin Hydrogels. Eng. Sci. An. Acad. Bras. Ciênc 2017, 89 (Suppl. 1), 745–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; del Carmen Garrigós, M.; Jiménez, A. Active Edible Films: Current State and Future Trends. J. Appl. Polym. Sci. 2016, 133, 42631. [Google Scholar] [CrossRef] [Green Version]
- Kibar, E.A.A.; Ferhunde, U. Thermal, Mechanical and Water Adsorption Properties of Corn Starch–Carboxymethylcellulose/Methylcellulose Biodegradable Films. J. Food Eng. 2013, 114, 123–131. [Google Scholar] [CrossRef]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; Rios, A.D.O.; Flôres, S.H. Edible Film Production from Chia Seed Mucilage: Effect of Glycerol Concentration on Its Physicochemical and Mechanical Properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Peng, S.; Wen, C.; Wang, X.; Fan, L.; Deng, R.; Pang, J. Structural Characterization and Properties of Konjac Glucomannan/Curdlan Blend Films. Carbohydr. Polym. 2012, 89, 497–503. [Google Scholar] [CrossRef]
- Fox, M. Optical Properties of Solids; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Wang, S.; Xia, Z.; Hu, Y.; He, Z.; Uzoejinwa, B.B.; Wang, Q.; Cao, B.; Xu, S. Co-Pyrolysis Mechanism of Seaweed Polysaccharides and Cellulose Based on Macroscopic Experiments and Molecular Simulations. Bioresour. Technol. 2017, 228, 305–314. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y.; Uzoejinwa, B.B.; Cao, B.; He, Z.; Wang, Q.; Xu, S. Pyrolysis Mechanisms of Typical Seaweed Polysaccharides. J. Anal. Appl. Pyrolysis 2017, 124, 373–383. [Google Scholar] [CrossRef]
- Wang, S.; Xia, Z.; Wang, Q.; He, Z.; Li, H. Mechanism Research on the Pyrolysis of Seaweed Polysaccharides by Py-GC/MS and Subsequent Density Functional Theory Studies. J. Anal. Appl. Pyrolysis 2017, 126, 118–131. [Google Scholar] [CrossRef]





| Reagent (Supplier) | d (g/cm3) | Chemical Composition (wt%) | Observations |
|---|---|---|---|
| Porphyran extract [8] | 1.5 | 67.7% D-galactose undetectable protein contamination | Anionic polysaccharide [10]. Gelling properties and film-forming potential after suitable chemical treatment [2,10,21]. Tg between 35–38 °C [14]. |
| Sodium alginate (BDH) | 1.601 | n.a. | Anionic polysaccharide based on the sodium salt of alginic acid. Low water barrier properties [2]. |
| Sodium carboxymethylcellulose (Sigma) | 1.59 | Degree of anhydroglucose substitution by carboxymethyl: 65–90 Na = 6.5–9.5 | Water-soluble, anionic polysaccharide with gelling, thickening, moisture retention, emulsification, and stabilization ability [2,22] |
| Amidated pectin (BioSynth) | 1.16−1.50 [23] | Degree of amidation: 10–16 Degree of esterification: 32–40 Contains β-TCP, SAPP and glycose | Anionic polysaccharide with galacturonic acid backbone. Gelling, thickening, moisture retention, emulsification, stabilization ability [2,22]. Antioxidant and antimicrobial abilities [23] |
| Glycerol (ThermoFisher) | 1.226 | Impurities ≤ 0.0047 H2O = 13.3 Glycerol = 86.9 | Plasticizer agent [2]. Tg = 78 °C; Tdecomposition > 290 °C [24] |
| Film | Solid Load (wt%) | Porphyran (wt%) | CMC (wt%) | PcT (wt%) | AL (wt%) | Glycerol (wt%) | Porphyran/ Polysaccharide * (wt ratio) | Glycerol/ Polysaccharides ** (wt ratio) |
| PorphSR | 1.0 | 100.0 | 1/0 | |||||
| PorphSR_Gly | 1.1 | 87.3 | 12.7 | 1/0 | 1/6.9 | |||
| PorphSR_CMC | 2.0 | 46.8 | 53.2 | 1/1.1 | ||||
| PorphSR_CMC_Gly | 2.1 | 43.8 | 49.8 | 6.4 | 1/1.1 | 1/14.7 | ||
| PorphSR_PcT | 1.0 | 46.8 | 53.2 | 1/1.1 | ||||
| PorphSR_PcT_Gly | 1.2 | 43.8 | 49.8 | 6.4 | 1/1.1 | 1/14.7 | ||
| PorphSR_AL | 1.5 | 30.6 | 69.4 | 1/2.3 | ||||
| PorphSR_AL_Gly | 1.6 | 28.1 | 63.8 | 8.1 | 1/2.3 | 1/11.3 |
| Elements (at/at) | PorphSR | PorphSR _Gly | PorphSR _CMC | PorphSR _CMC_Gly | PorphSR _PcT | PorphSR _PcT_Gly | PorphSR _AL | PorphSR _AL_Gly |
| N/C | 0.15 ± 0.00 | 0.08 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.09 ± 0.00 | 0.09 ± 0.00 | 0.05 ± 0.00 | 0.08 ± 0.00 |
| O/C | 1.22 ± 0.03 | 1.34 ± 0.03 | 0.83 ± 0.02 | 1.13 ± 0.02 | 0.79 ± 0.02 | 0.81 ± 0.02 | 1.12 ± 0.02 | 0.93 ± 0.02 |
| Na/C | 0.22 ± 0.01 | 0.10 ± 0.03 | 0.19 ± 0.02 | 0.18 ± 0.02 | 0.13 ± 0.02 | 0.09 ± 0.02 | 0.20 ± 0.02 | 0.09 ± 0.02 |
| Mg/C | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| P/C | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.05 ± 0.00 | 0.01 ± 0.00 | 0.10 ± 0.00 |
| S/C | 0.19 ± 0.00 | 0.11 ± 0.00 | 0.04 ± 0.00 | 0.07 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.08 ± 0.00 |
| Cl/C | 0.05 ± 0.01 | 0.02 ± 0.00 | 0.04 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | |||
| K/C | 0.25 ± 0.00 | 0.12 ± 0.00 | 0.03 ± 0.00 | 0.08 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.05 ± 0.00 |
| Ca/C | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Film Formulation | Desiccant Weight Gain (%) | Film | ||
| WVTR (g·d−1·m−2) | WVP (mm·g·d−1·m−2·kPa−1) | Solubility (%) | ||
| PorphSR | - | - | - | 100.0 ± 0.0 |
| PorphSR_Gly | 3.64 ± 0.37 | 443.77 ± 4.62 | 2.98 ± 0.10 | 100.0 ± 0.0 |
| PorphSR_AL | - | - | - | 98.8 ± 1.2 (a),(b) |
| PorphSR_AL_Gly | 4.32 ± 1.59 | 644.38 ± 13.86 (b) | 6.65 ± 0.12 (b) | 99.4 ± 0.1 |
| PorphSR_CMC | - | - | - | 99.6 ± 0.1 |
| PorphSR_CMC_Gly | 3.07 ± 0.23 | 415.40 ± 2.03 (b),(h) | 3.95 ± 0.01 (b),(h) | 99.7 ± 0.1 |
| PorphSR_PcT | - | - | - | 99.7 ± 0.0 |
| PorphSR_PcT_Gly | 5.70 ± 0.63 (f) | 628.16 ± 8.62 (b),(f) | 6.05 ± 0.06 (b),(f),(h) | 99.9 ± 0.0 |
| Reference commercial film | 0.83 ± 0.02 (b),(c),(f),(h) | 3.4 ± 0.06 (b),(c),(f),(h) | 0.32 ± 0.01 (b),(c),(f),(h) | 0.0 ± 0.0 (a)–(h) |
| No film | 11.73 ± 0.61 (b),(c),(f),(h),(i) | - | - | - |
| E’ (GPa) | UTS (MPa) | εf (%) | |
| PorphSR | 2.90 ± 0.52 | 12.9 ± 0.8 | 0.53 ± 0.20 |
| PorphSR_Gly | 1.12 ± 0.20 (a) | 4.3 ± 0.8 | 0.41 ± 0.14 |
| PorphSR_PcT | 3.99 ± 0.27 (a),(b) | 26.0 ± 5.9 (a),(b) | 0.82 ± 0.25 |
| PorphSR_PcT_Gly | 1.62 ± 0.14 (a),(c) | 23.2 ± 1.6 (b) | 2.94 ± 0.74 (a)–(c) |
| PorphSR_CMC | 1.62 ± 0.33 (a),(c) | 10.3 ± 2.6 (c),(d) | 0.90 ± 0.48 (d) |
| PorphSR_CMC_Gly | 1.65 ± 0.30 (a),(c) | 21.9 ± 4.3 (b),(e) | 3.10 ± 0.80 (a)–(c),(e) |
| PorphSR_AL | 4.21 ± 0.19 (a),(b),(d)–(f) | 17.4 ± 5.0 (b) | 0.52 ± 0.20 (d),(f) |
| PorphSR_AL_Gly | 1.58 ± 0.42 (a),(c),(g) | 9.3 ± 3.0 (c),(d),(f) | 0.75 ± 0.23 (d),(f) |
| Commercial film | 0.08 ± 0.00 (a)–(h) | 2.5 ± 0.1 (c),(d),(f),(g) | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baptista, R.S.; Teles, M.; Adão, P.; Afonso, C.; Bernardino, R.; Bernardino, S.; Ferro, A.C.; Elias, S.; Guedes, M. Morphological and Mechanical Characterization of Films Incorporating Porphyran Extracted from Porphyra Dioica. Coatings 2022, 12, 1720. https://doi.org/10.3390/coatings12111720
Baptista RS, Teles M, Adão P, Afonso C, Bernardino R, Bernardino S, Ferro AC, Elias S, Guedes M. Morphological and Mechanical Characterization of Films Incorporating Porphyran Extracted from Porphyra Dioica. Coatings. 2022; 12(11):1720. https://doi.org/10.3390/coatings12111720
Chicago/Turabian StyleBaptista, Ricardo S., Marco Teles, Pedro Adão, Clélia Afonso, Raul Bernardino, Susana Bernardino, Alberto C. Ferro, Sara Elias, and Mafalda Guedes. 2022. "Morphological and Mechanical Characterization of Films Incorporating Porphyran Extracted from Porphyra Dioica" Coatings 12, no. 11: 1720. https://doi.org/10.3390/coatings12111720






