Current Research Status on Cold Sprayed Amorphous Alloy Coatings: A Review
Abstract
:1. Introduction
- (1)
- The size of bulk amorphous alloys is limited. Due to the limited glass forming capacity and extremely high cooling rate (>105 K/s), the products of amorphous alloy materials are mainly powders, wires or ribbons [9]. The size of the bulk amorphous alloy materials obtained by the casting process is in the centimeter level, which greatly restricts its application range [10].
- (2)
- The room temperature plasticity of amorphous alloy materials is poor. Unlike crystals which have slip bands, it is difficult to deform due to the special atomic structure of amorphous alloys. Deformation is confined to a highly concentrated shear zone [11]. When in a tensile stress state, once the shear band is generated and rapidly expanded, a brittle fracture occurs before the yield limit is reached, thus it has limitations when used as a load-bearing structural material [12,13].
2. Principle and Advantages of CS Technology
3. Preparation and Properties of CS Amorphous Alloy Coatings
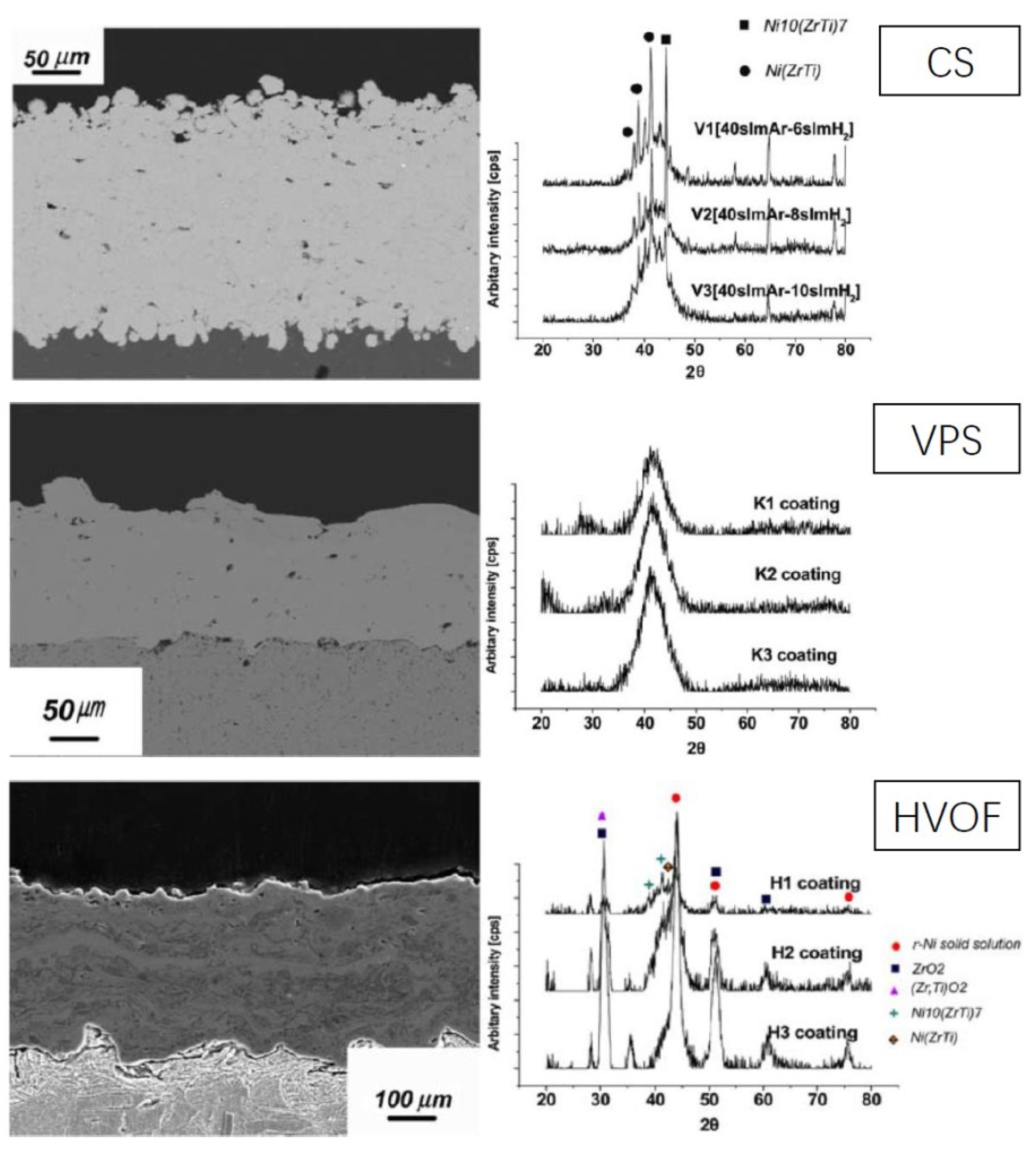
3.1. Fe-Based Amorphous Alloy Coatings
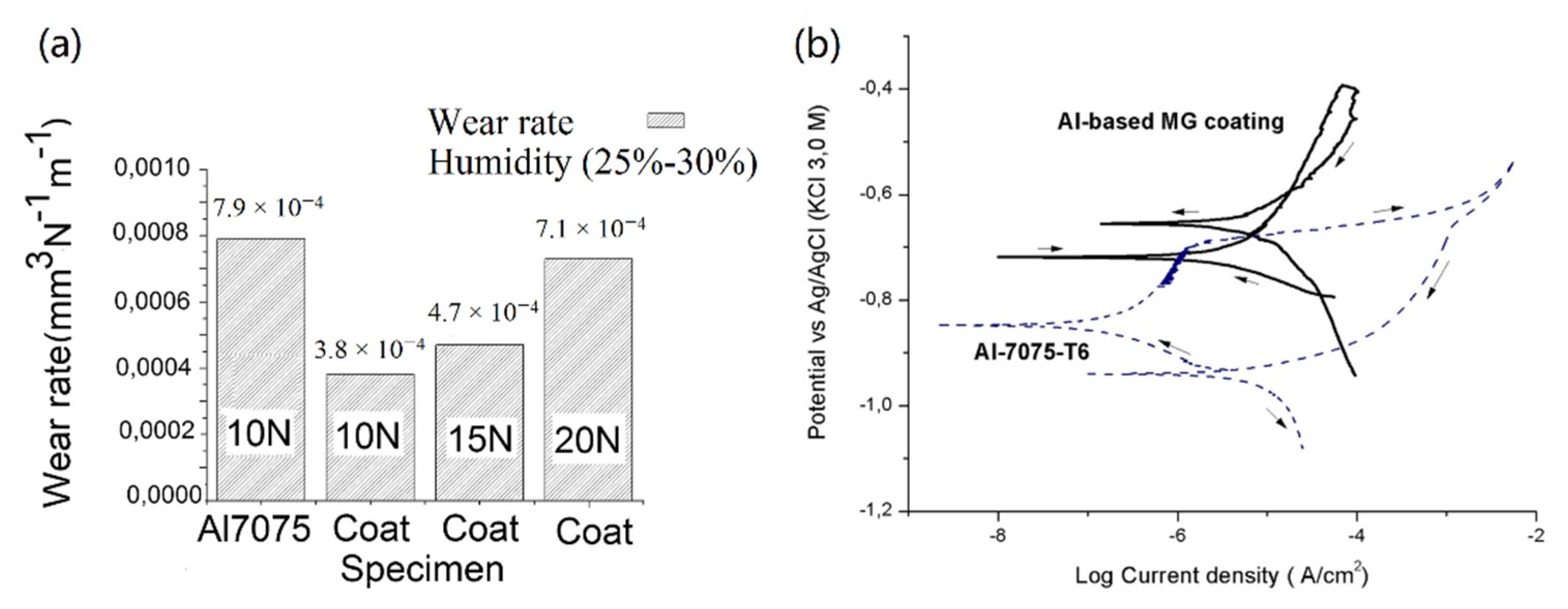
3.2. Al-Based Amorphous Alloy Coatings
3.3. Ni-Based Amorphous Alloy Coatings
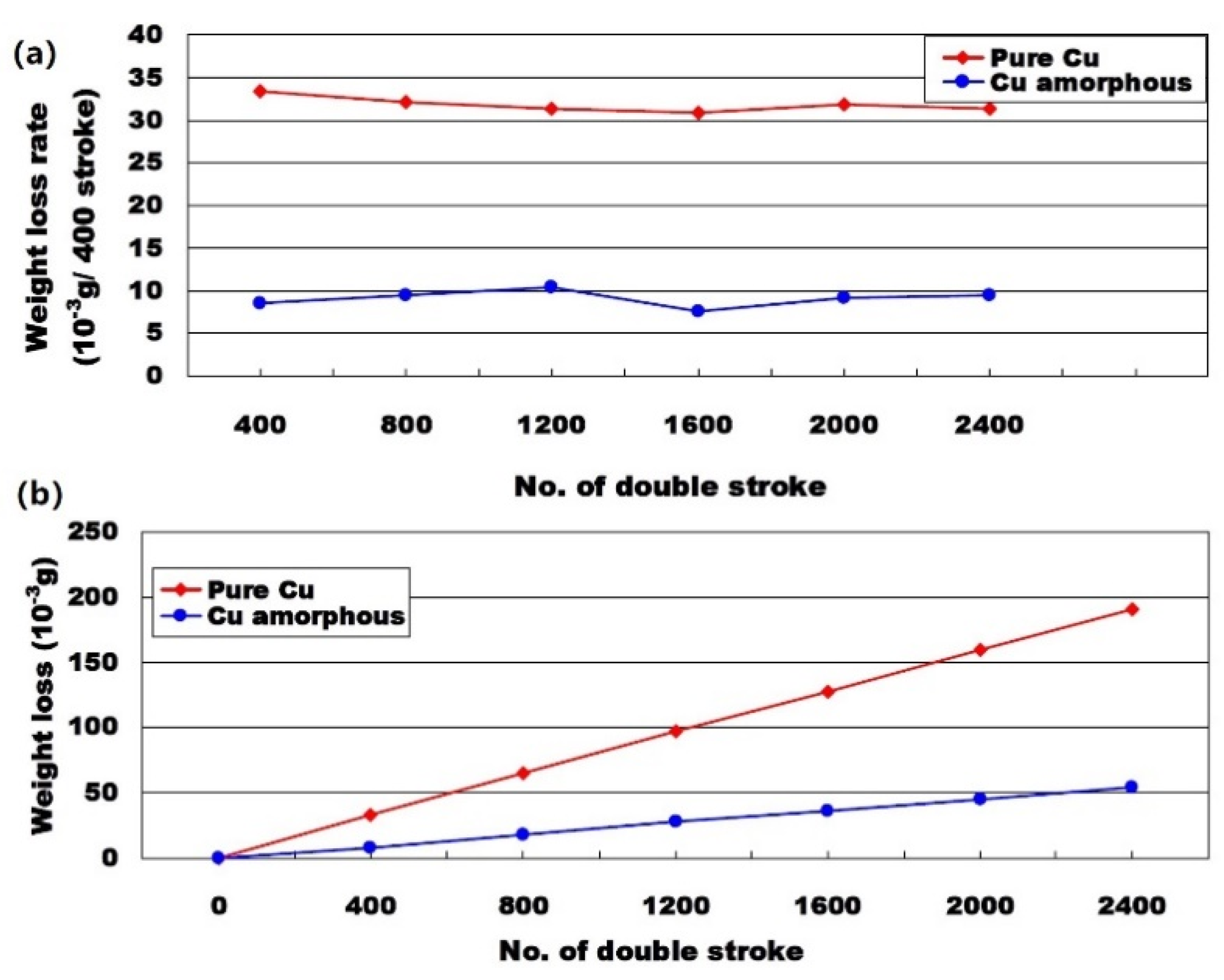
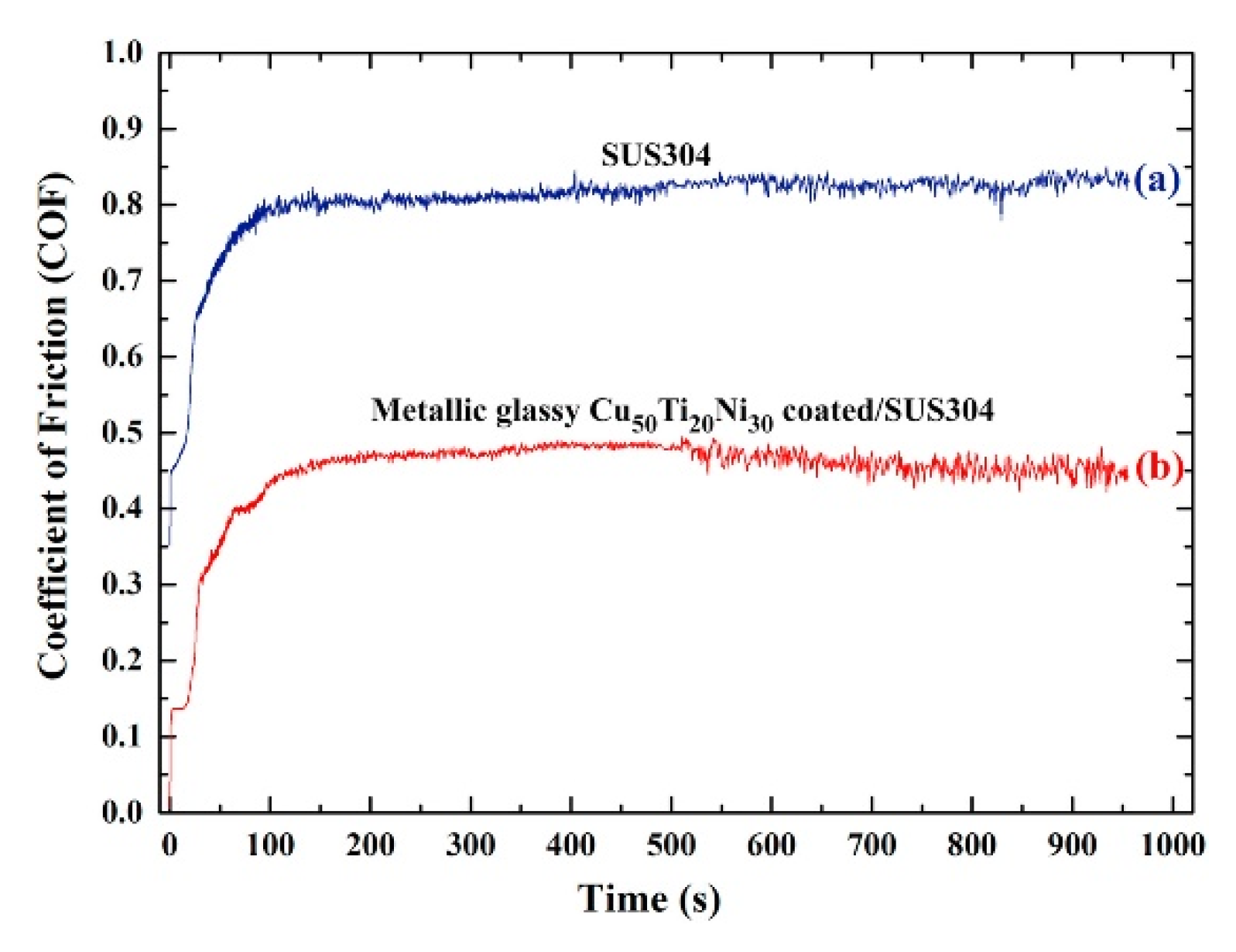
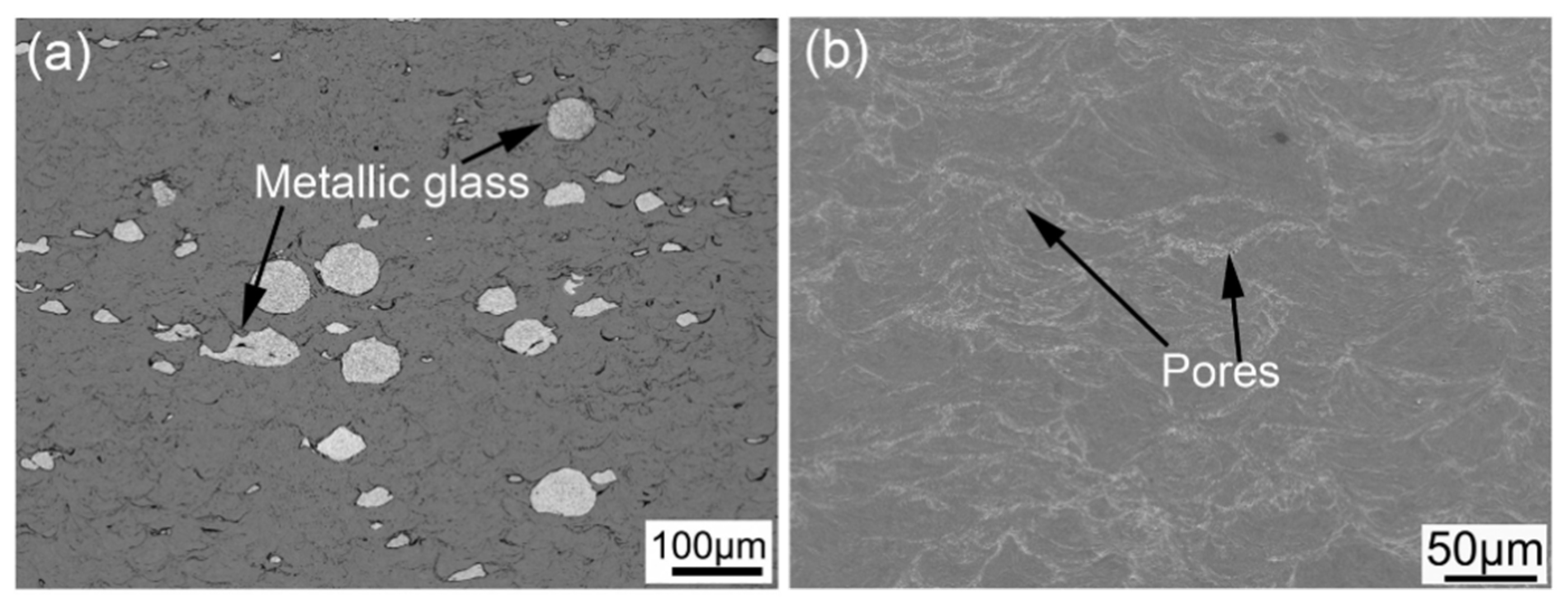
3.4. Cu-Based Amorphous Alloy Coatings
3.5. Zr-Based Amorphous Alloy Coatings
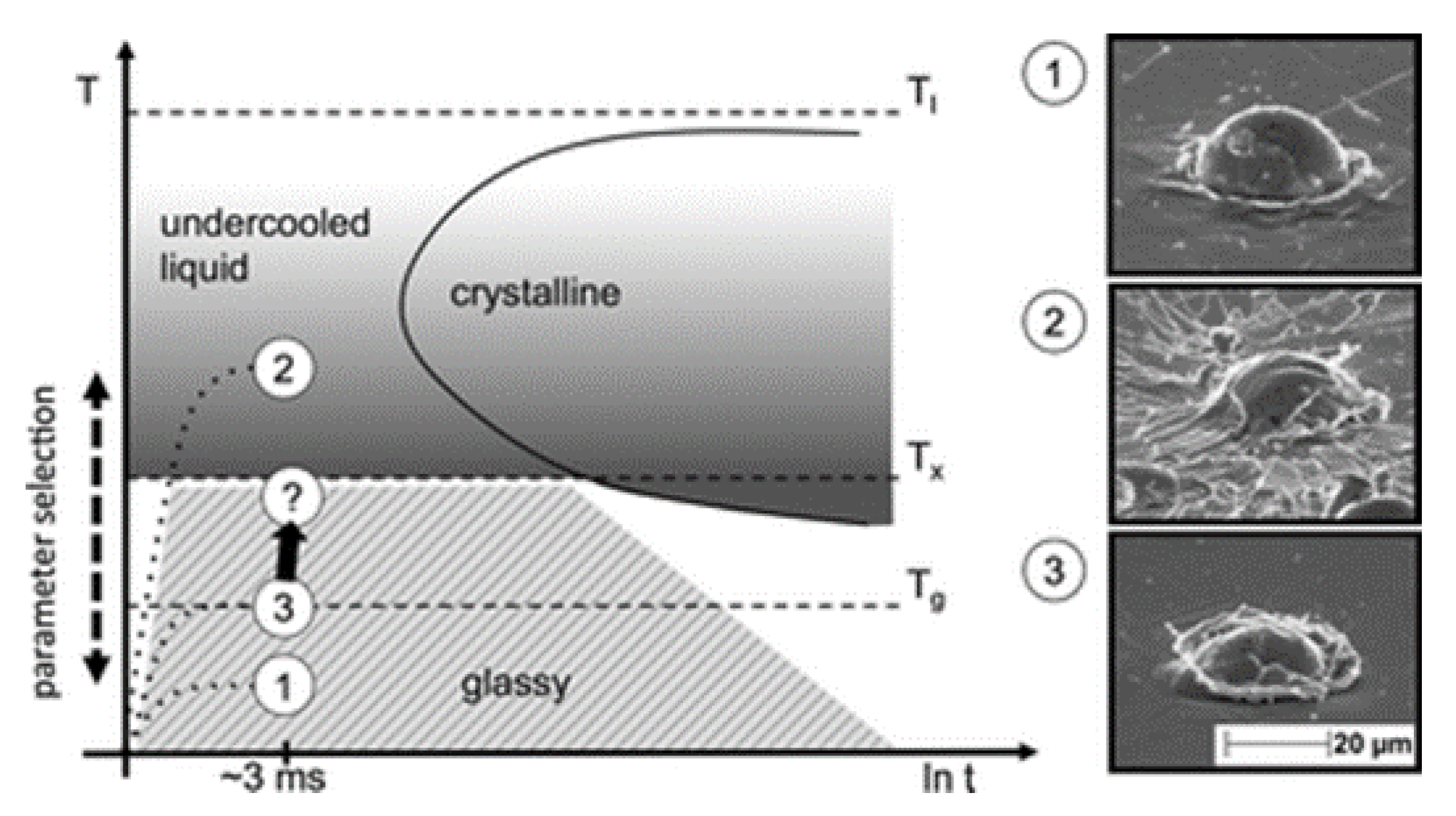
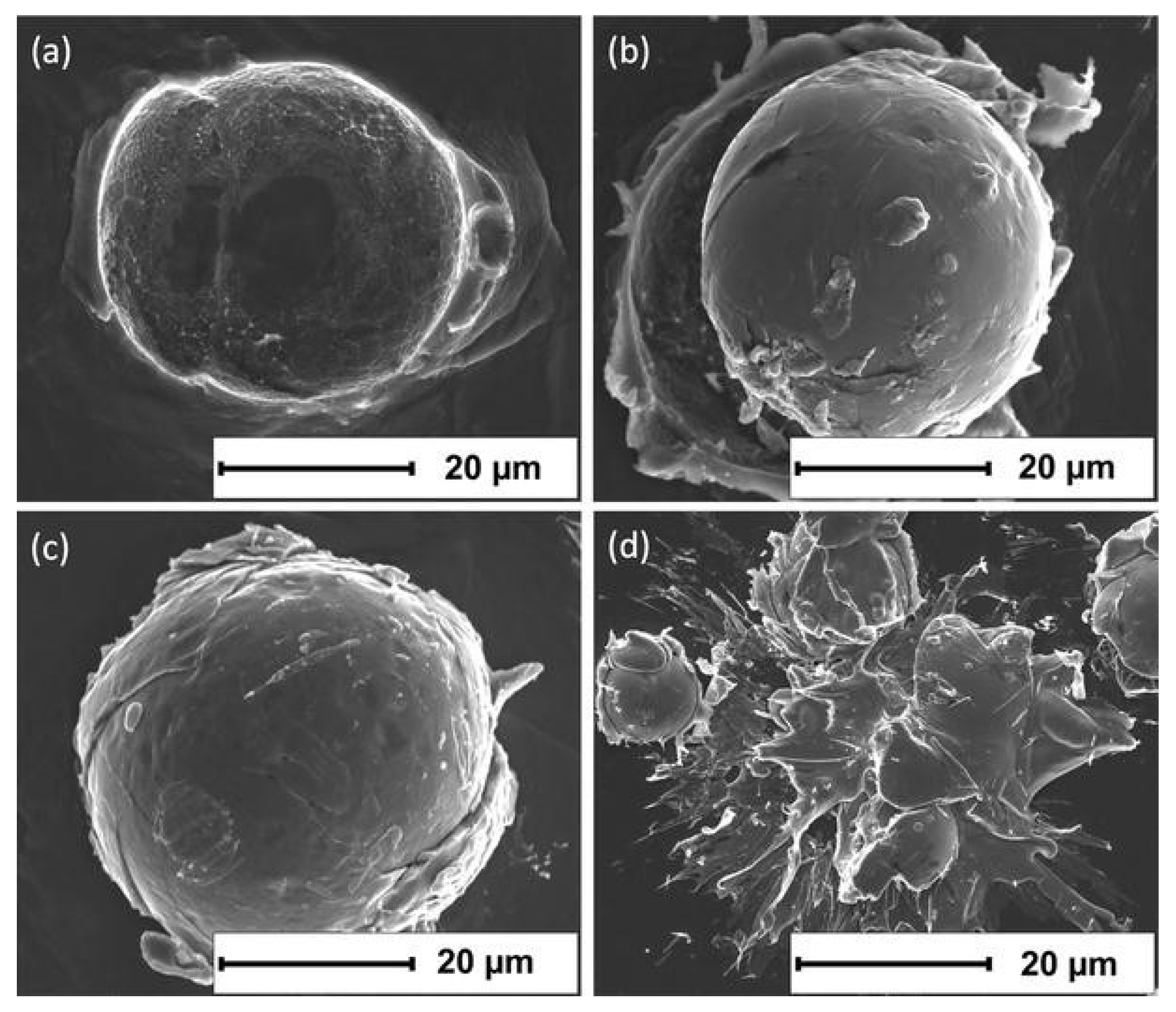
4. Deposition Mechanism of CS Amorphous Alloy Coatings
5. Influencing Factors on the Microstructure and Properties of Amorphous Alloy Coatings
5.1. Powder Characteristics
5.2. CS Process Parameters
- (1)
- Gas type and temperature
- (2)
- Gas pressure
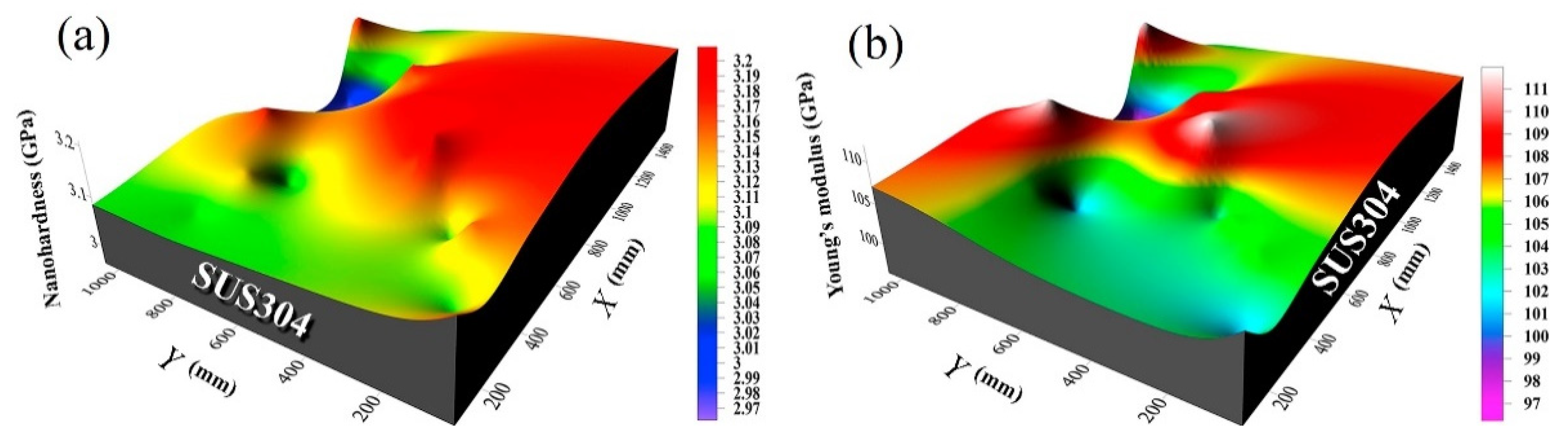
5.3. Substrate Material
5.4. Heat Treatment Process
6. Summary and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.H.; Dong, C.; Shek, C.H. Bulk metallic glasses. Mater. Sci. Eng. R Rep. 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Sheng, H.W.; Luo, W.K.; Alamgir, F.M.; Bai, J.M.; Ma, E. Atomic packing and short-to-medium-range order in metallic glasses. Nature 2006, 439, 419–425. [Google Scholar] [CrossRef]
- Nie, D.; Panfilova, E.; Samusenkov, V.; Mikhaylov, A. E-learning financing models in russia for sustainable development. Sustainability 2020, 12, 4412. [Google Scholar] [CrossRef]
- Yumashev, A.; Ślusarczyk, B.; Kondrashev, S.; Mikhaylov, A. Global indicators of sustainable development: Evaluation of the influence of the human development index on consumption and quality of energy. Energies 2020, 13, 2768. [Google Scholar] [CrossRef]
- Johnson, W.L. Bulk amorphous metal—An emerging engineering material. JOM 2002, 54, 40–43. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, G.; Wang, R.J.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Super plastic bulk metallic glasses at room temperature. Science 2007, 315, 1385–1388. [Google Scholar] [CrossRef]
- Kruzic, J.J. Bulk metallic glasses as structural materials: A review. Adv. Eng. Mater. 2016, 18, 1308–1331. [Google Scholar] [CrossRef]
- Greer, A.L.; Ma, E. Bulk metallic glasses: At the cutting edge of metals research. MRS Bull. 2011, 32, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Takeuchi, A. Recent development and application products of bulk glassy alloys. Acta Mater. 2011, 59, 2243–2267. [Google Scholar] [CrossRef]
- Miracle, D.B. A structural model for metallic glasses. Microsc. Microanal. 2004, 10, 786–787. [Google Scholar] [CrossRef] [Green Version]
- Greer, A.L.; Cheng, Y.Q.; Ma, E. Shear bands in metallic glasses. Mater. Sci. Eng. R Rep. 2013, 74, 71–132. [Google Scholar] [CrossRef]
- Das, J.; Tang, M.B.; Kim, K.B.; Theissmann, R.; Baier, F.; Wang, W.H.; Eckert, J. “Work-hardenable” ductile bulk metallic glass. Phys. Rev. Lett 2005, 94, 205501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer, J.R.; De Hosson, J.T.M. Plasticity in small-sized metallic systems: Intrinsic versus extrinsic size effect. Prog. Mater. Sci. 2011, 56, 654–724. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, C.; Choi, H. Evaluation of the effects of the crystallinity of kinetically sprayed Ni–Ti–Zr–Si–Sn bulk metallic glass on the scratch response. Mater. Sci. Eng. A 2007, 449–451, 285–289. [Google Scholar] [CrossRef]
- Joshi, S.S.; Katakam, S.; Arora, H.S.; Mukherjee, S.; Dahotre, N.B. Amorphous coatings and surfaces on structural materials. Crit. Rev. Solid State Mater. Sci. 2015, 41, 1–46. [Google Scholar] [CrossRef]
- Abrosimova, G.E. Evolution of the structure of amorphous alloys. Phys. Uspekhi 2011, 54, 1227–1242. [Google Scholar] [CrossRef]
- Sienicki, J.; Zórawski, W.; Dworak, A.; Koruba, P.; Jurewicz, P.; Reiner, J. Cold spraying and laser cladding as an alternative to electroplating processes. Aircr. Eng. Aerosp. Technol. 2019, 91, 205–215. [Google Scholar] [CrossRef]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2013, 57, 133–164. [Google Scholar] [CrossRef]
- Vardelle, A.; Moreau, C.; Themelis, N.J.; Chazelas, C. A perspective on plasma spray technology. Plasma Chem. Plasma Process. 2014, 35, 491–509. [Google Scholar] [CrossRef]
- Fu, W.; Chen, Q.-Y.; Yang, C.; Yi, D.-L.; Yao, H.-L.; Wang, H.-T.; Ji, G.-C.; Wang, F. Microstructure and properties of high velocity oxygen fuel sprayed (WC–Co)–Ni coatings. Ceram. Int. 2020, 46, 14940–14948. [Google Scholar] [CrossRef]
- Ding, P.; Liu, X.-J.; Liu, J.-J.; Li, J.-B.; Li, H.-Q.; Zhao, H.-Y.; Duan, J.-Y.; Jiao, Y.-Z. Study on the properties of FeCrNi/CBN composite coating with high velocity arc spraying. Arab. J. Chem. 2018, 11, 935–941. [Google Scholar] [CrossRef]
- Cheng, J.B.; Liang, X.B.; Chen, Y.X.; Wang, Z.H.; Xu, B.S. High-temperature erosion resistance of FeBSiNb amorphous coatings deposited by arc spraying for boiler applications. J. Therm. Spray Technol. 2012, 22, 820–827. [Google Scholar] [CrossRef]
- Ang, A.S.M.; Sanpo, N.; Sesso, M.L.; Kim, S.Y.; Berndt, C.C. Thermal spray maps: Material genomics of processing technologies. J. Therm. Spray Technol. 2013, 22, 1170–1183. [Google Scholar] [CrossRef]
- Guo, S.F.; Pan, F.S.; Zhang, H.J.; Zhang, D.F.; Wang, J.F.; Miao, J.; Su, C.; Zhang, C. Fe-based amorphous coating for corrosion protection of magnesium alloy. Mater. Des. 2016, 108, 624–631. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, W.T.; Park, E.S.; Mattern, N.; Eckert, J. Phase separation in metallic glasses. Prog. Mater. Sci. 2013, 58, 1103–1172. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Y.; Zhang, J.; Hong, S.; Li, G.; Ying, G.; Guo, J.; Qin, Y. Fabrication and characterization of thermal-sprayed Fe-based amorphous/nanocrystalline composite coatings: An overview. J. Therm. Spray Technol. 2014, 23, 1157–1180. [Google Scholar] [CrossRef]
- Alkhimov, A.P.; Klinkov, S.V.; Kosarev, V.F.; Papyrin, A.N. Gas-dynamic spraying study of a plane supersonic two-phase jet. J. Appl. Mech. Tech. Phys. 1997, 38, 324–330. [Google Scholar] [CrossRef]
- Winnicki, M.; Małachowska, A.; Dudzik, G.; Rutkowska-Gorczyca, M.; Marciniak, M.; Abramski, K.; Ambroziak, A.; Pawłowski, L. Numerical and experimental analysis of copper particles velocity in low-pressure cold spraying process. Surf. Coat. Technol. 2015, 268, 230–240. [Google Scholar] [CrossRef]
- Raoelison, R.N.; Xie, Y.; Sapanathan, T.; Planche, M.P.; Kromer, R.; Costil, S.; Langlade, C. Cold gas dynamic spray technology: A comprehensive review of processing conditions for various technological developments till to date. Addit. Manuf. 2018, 19, 134–159. [Google Scholar] [CrossRef]
- Assadi, H.; Schmidt, T.; Richter, H.; Kliemann, J.O.; Binder, K.; Gärtner, F.; Klassen, T.; Kreye, H. On parameter selection in cold spraying. J. Therm. Spray Technol. 2011, 20, 1161–1176. [Google Scholar] [CrossRef]
- Assadi, H.; Kreye, H.; Gärtner, F.; Klassen, T. Cold spraying–A materials perspective. Acta Mater. 2016, 116, 382–407. [Google Scholar] [CrossRef] [Green Version]
- Pattison, J.; Celotto, S.; Morgan, R.; Bray, M.; O’Neill, W. Cold gas dynamic manufacturing: A non-thermal approach to freeform fabrication. Int. J. Mach. Tools Manuf. 2007, 47, 627–634. [Google Scholar] [CrossRef]
- Moridi, A.; Hassani-Gangaraj, S.M.; Guagliano, M.; Dao, M. Cold spray coating: Review of material systems and future perspectives. Surf. Eng. 2014, 30, 369–395. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, M.X. Review on recent research and development of cold spray technologies. Key Eng. Mater. 2012, 533, 1–52. [Google Scholar] [CrossRef]
- List, A.; Gärtner, F.; Schmidt, T.; Klassen, T. Impact conditions for cold spraying of hard metallic glasses. J. Therm. Spray Technol. 2012, 21, 531–540. [Google Scholar] [CrossRef]
- Concustell, A.; Henao, J.; Dosta, S.; Cinca, N.; Cano, I.G.; Guilemany, J.M. On the formation of metallic glass coatings by means of Cold Gas Spray technology. J. Alloy. Compd. 2015, 651, 764–772. [Google Scholar] [CrossRef]
- Henao, J.; Concustell, A.; Cano, I.G.; Cinca, N.; Dosta, S.; Guilemany, J.M. Influence of cold gas spray process conditions on the microstructure of Fe-based amorphous coatings. J. Alloy. Compd. 2015, 622, 995–999. [Google Scholar] [CrossRef] [Green Version]
- Ajdelsztajn, L.; Lavernia, E.J.; Jodoin, B.; Richer, P.; Sansoucy, E. Cold gas dynamic spraying of iron-base amorphous alloy. J. Therm. Spray Technol. 2006, 15, 495–500. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, J.; Bae, G.; Kim, B.; Lee, C. Formation of coating and tribological behavior of kinetic sprayed Fe-based bulk metallic glass. J. Alloy. Compd. 2011, 509, 347–353. [Google Scholar] [CrossRef]
- Henao, J.; Concustell, A.; Cano, I.G.; Dosta, S.; Cinca, N.; Guilemany, J.M.; Suhonen, T. Novel Al-based metallic glass coatings by Cold Gas Spray. Mater. Des. 2016, 94, 253–261. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, S.; Kim, G.; Jo, H.; Lee, C. Phase evolutions of bulk amorphous NiTiZrSiSn feedstock during thermal and kinetic spraying processes. Scr. Mater. 2005, 53, 125–130. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, C.; Choi, H.; Jo, H. Kinetic spraying deposition behavior of bulk amorphous NiTiZrSiSn feedstock. Mater. Sci. Eng. A 2006, 415, 45–52. [Google Scholar] [CrossRef]
- List, A.; Gärtner, F.; Mori, T.; Schulze, M.; Assadi, H.; Kuroda, S.; Klassen, T. Cold spraying of amorphous Cu50Zr50 alloys. J. Therm. Spray Technol. 2014, 24, 108–118. [Google Scholar] [CrossRef]
- Yoon, S.; Bae, G.; Xiong, Y.; Kumar, S.; Kang, K.; Kim, J.-J.; Lee, C. Strain-enhanced nanocrystallization of a CuNiTiZr bulk metallic glass coating by a kinetic spraying process. Acta Mater. 2009, 57, 6191–6199. [Google Scholar] [CrossRef]
- Kang, N.; Coddet, P.; Liao, H.; Coddet, C. The effect of heat treatment on microstructure and tensile properties of cold spray Zr base metal glass/Cu composite. Surf. Coat. Technol. 2015, 280, 64–71. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C. Fe-based amorphous coatings: Structures and properties. Thin Solid Film. 2014, 561, 70–86. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Inoue, A. Iron-based bulk metallic glasses. Int. Mater. Rev. 2013, 58, 131–166. [Google Scholar] [CrossRef]
- Duarte, M.J.; Kostka, A.; Jimenez, J.A.; Choi, P.; Klemm, J.; Crespo, D.; Raabe, D.; Renner, F.U. Crystallization, phase evolution and corrosion of Fe-based metallic glasses: An atomic-scale structural and chemical characterization study. Acta Mater. 2014, 71, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.J.; Lee, H.S.; Jang, J.W.; Yi, S. Corrosion behavior in a 3.5 wt.% NaCl solution of amorphous coatings prepared through plasma-spray and cold-spray coating processes. Met. Mater. Int. 2014, 20, 1053–1057. [Google Scholar] [CrossRef]
- Shen, Y.; Perepezko, J.H. Al-based amorphous alloys: Glass-forming ability, crystallization behavior and effects of minor alloying additions. J. Alloy. Compd. 2017, 707, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.D.; Wang, Z.M.; Chang, X.C.; Hou, W.L.; Wang, J.Q. Identifying the role of nanoscale heterogeneities in pitting behaviour of Al-based metallic glass. Corros. Sci. 2011, 53, 3007–3015. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, S.; Jeon, J.-B.; Kim, S. Excessively high vapor pressure of Al-based amorphous alloys. Metals 2015, 5, 1878–1886. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, D.; Gill, P.K.; Scudino, S.; Zhang, C.; Singh, V.; Karthikeyan, J.; Munroe, N.; Seal, S.; Agarwal, A. Cold sprayed aluminum based glassy coating: Synthesis, wear and corrosion properties. Surf. Coat. Technol. 2013, 232, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Liu, M.; Cong, L.; Wang, L. A study on preparation and mechanism of Ni based ternary alloy. Mater. Express 2019, 9, 681–685. [Google Scholar] [CrossRef]
- Wang, A.P.; Zhang, T.; Wang, J.Q. Ni-based fully amorphous metallic coating with high corrosion resistance. Philos. Mag. Lett. 2006, 86, 5–11. [Google Scholar] [CrossRef]
- Rajaei, V.; Rashtchi, H.; Raeissi, K.; Shamanian, M. The study of Ni-based nano-crystalline and amorphous alloy coatings on AISI 304 stainless steel for PEM fuel cell bipolar plate application. Int. J. Hydrog. Energy 2017, 42, 14264–14278. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, H.J.; Lee, C. Deposition behavior of bulk amorphous NiTiZrSiSn according to the kinetic and thermal energy levels in the kinetic spraying process. Surf. Coat. Technol. 2006, 200, 6022–6029. [Google Scholar] [CrossRef]
- Wang, A.P.; Chang, X.C.; Hou, W.L.; Wang, J.Q. Preparation and corrosion behaviour of amorphous Ni-based alloy coatings. Mater. Sci. Eng. A 2007, 449–451, 277–280. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.W.; Ahn, J.P.; Fleury, E.; Kim, Y.C.; Lee, J.C. A Cu-based amorphous alloy with a simultaneous improvement in its glass forming ability and plasticity. Met. Mater. Int. 2007, 13, 21–24. [Google Scholar] [CrossRef]
- Kim, J.; Kang, K.; Yoon, S.; Lee, C. Enhancement of metallic glass properties of Cu-based BMG coating by shroud plasma spraying. Surf. Coat. Technol. 2011, 205, 3020–3026. [Google Scholar] [CrossRef]
- Wu, J.; Peng, Z. Effects of microadditions on glass transition and hardness of Cu-based bulk metallic glasses. Appl. Phys. A 2018, 124, 632. [Google Scholar] [CrossRef]
- Lee, K.A.; Jung, D.J.; Park, D.Y.; Kang, W.G.; Lee, J.K.; Kim, H.J. Study on the fabrication and physical properties of cold-sprayed, Cu-based amorphous coating. J. Phys. Conf. Ser. 2009, 144, 012113. [Google Scholar] [CrossRef]
- El-Eskandrany, M.S.; Al-Azmi, A. Potential applications of cold sprayed Cu50Ti20Ni30 metallic glassy alloy powders for antibacterial protective coating in medical and food sectors. J. Mech. Behav. Biomed. Mater. 2016, 56, 183–194. [Google Scholar] [CrossRef]
- Ma, Z.-C.; Ma, X.-X.; Zhao, H.-W.; Zhang, F.; Zhou, L.-M.; Ren, L.-Q. Novel crystallization behaviors of Zr-based metallic glass under thermo-mechanical coupled fatigue loading condition. Acta Metall. Sin. (Engl. Lett.) 2019, 32, 797–802. [Google Scholar] [CrossRef] [Green Version]
- Sugita, K.; Matsumoto, M.; Mizuno, M.; Araki, H.; Shirai, Y. Electron irradiation damage and the recovery in a Zr-based bulk amorphous alloy Zr55Cu30Al10Ni5. J. Phys. Conf. Ser. 2008, 106, 012024. [Google Scholar] [CrossRef]
- Grujicic, M.; Saylor, J.R.; Beasley, D.E.; DeRosset, W.S.; Helfritch, D. Computational analysis of the interfacial bonding between feed-powder particles and the substrate in the cold-gas dynamic-spray process. Appl. Surf. Sci. 2003, 219, 211–227. [Google Scholar] [CrossRef]
- Assadi, H.; Gärtner, F.; Stoltenhoff, T.; Kreye, H. Bonding mechanism in cold gas spraying. Acta Mater. 2003, 51, 4379–4394. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, C.; Choi, H.; Kim, H.; Bae, J. Impacting behavior of bulk metallic glass powder at an abnormally high strain rate during kinetic spraying. Mater. Sci. Eng. A 2007, 449–451, 911–915. [Google Scholar] [CrossRef]
- Hufnagel, T.C.; Schuh, C.A.; Falk, M.L. Deformation of metallic glasses: Recent developments in theory, simulations, and experiments. Acta Mater. 2016, 109, 375–393. [Google Scholar] [CrossRef] [Green Version]
- Henao, J.; Concustell, A.; Dosta, S.; Bolelli, G.; Cano, I.G.; Lusvarghi, L.; Guilemany, J.M. Deposition mechanisms of metallic glass particles by Cold Gas Spraying. Acta Mater. 2017, 125, 327–339. [Google Scholar] [CrossRef]
- Schmidt, T.; Gaertner, F.; Kreye, H. New developments in cold spray based on higher gas and particle temperatures. J. Therm. Spray Technol. 2006, 15, 488–494. [Google Scholar] [CrossRef]
- Song, J.; Liu, J.; Chen, Q.; Li, K. Effect of the shape factor on the cold-spraying dynamic characteristics of sprayed particles. J. Therm. Spray Technol. 2017, 26, 1851–1858. [Google Scholar] [CrossRef]
- Gu, S.; Kamnis, S. Numerical modelling of in-flight particle dynamics of non-spherical powder. Surf. Coat. Technol. 2009, 203, 3485–3490. [Google Scholar] [CrossRef]
- Ziemian, C.W.; Wright, W.J.; Cipoletti, D.E. Influence of impact conditions on feedstock deposition behavior of cold-sprayed Fe-based metallic glass. J. Therm. Spray Technol. 2018, 27, 843–856. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.; Gärtner, F.; Assadi, H.; Kreye, H. Development of a generalized parameter window for cold spray deposition. Acta Mater. 2006, 54, 729–742. [Google Scholar] [CrossRef]
- Prisco, U. Size-dependent distributions of particle velocity and temperature at impact in the cold-gas dynamic-spray process. J. Mater. Process. Technol. 2015, 216, 302–314. [Google Scholar] [CrossRef]
- Schmidt, T.; Assadi, H.; Gärtner, F.; Richter, H.; Stoltenhoff, T.; Kreye, H.; Klassen, T. From particle acceleration to impact and bonding in cold spraying. J. Therm. Spray Technol. 2009, 18, 794–808. [Google Scholar] [CrossRef] [Green Version]
- Bae, G.; Xiong, Y.; Kumar, S.; Kang, K.; Lee, C. General aspects of interface bonding in kinetic sprayed coatings. Acta Mater. 2008, 56, 4858–4868. [Google Scholar] [CrossRef]
- Yin, S.; Wang, X.-F.; Li, W.-Y.; Guo, X.-P. Examination on substrate preheating process in cold gas dynamic spraying. J. Therm. Spray Technol. 2011, 20, 852–859. [Google Scholar] [CrossRef]
- Henao, J.; Concustell, A.; Dosta, S.; Cinca, N.; Cano, I.G.; Guilemany, J.M. Influence of the substrate on the formation of metallic glass coatings by cold gas spraying. J. Therm. Spray Technol. 2016, 25, 992–1008. [Google Scholar] [CrossRef] [Green Version]
- Ievlev, V.M.; Kannykin, S.V.; Il’inova, T.N.; Vavilova, V.V.; Kushchev, S.B.; Serikov, D.V.; Baikin, A.S. Heat treatment- and lamp processing-induced structural transformations of an amorphous Fe77B7Nb2.1Si13Cu0.9 alloy and nonmonotonic behavior of its mechanical properties. Inorg. Mater. 2019, 55, 659–668. [Google Scholar] [CrossRef]
- Kozlov, I.V.; Elmanov, G.N.; Prikhodko, K.E.; Kutuzov, L.V.; Tarasov, B.A.; Mikhalchik, V.V.; Svetogorov, R.D.; Mashera, V.S.; Gorelikov, E.S.; Gudoshnikov, S.A. The evolution of structure and magnetoimpedance characteristics of amorphous Co69Fe4Cr4Si12B11 microwires under heat treatment. J. Magn. Magn. Mater. 2020, 493, 493. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, Y.G.; Sun, W.H.; Wang, J.Q. Effect of heat treatment on the structure, cavitation erosion and erosion–corrosion behavior of Fe-based amorphous coatings. Tribol. Int. 2015, 90, 393–403. [Google Scholar] [CrossRef]
- Pitchuka, S.B.; Boesl, B.; Zhang, C.; Lahiri, D.; Nieto, A.; Sundararajan, G.; Agarwal, A. Dry sliding wear behavior of cold sprayed aluminum amorphous/nanocrystalline alloy coatings. Surf. Coat. Technol. 2014, 238, 118–125. [Google Scholar] [CrossRef]
- Choi, H.; Jo, H.; An, K.; Yoon, S.; Lee, C. Tribological behavior of the kinetic sprayed Ni59Ti16Zr20Si2Sn3 bulk metallic glass. J. Alloy. Compd. 2007, 434–435, 64–67. [Google Scholar] [CrossRef]
- Babu, P.S.; Jha, R.; Guzman, M.; Sundararajan, G.; Agarwal, A. Indentation creep behavior of cold sprayed aluminum amorphous/nano-crystalline coatings. Mater. Sci. Eng. A 2016, 658, 415–421. [Google Scholar] [CrossRef]
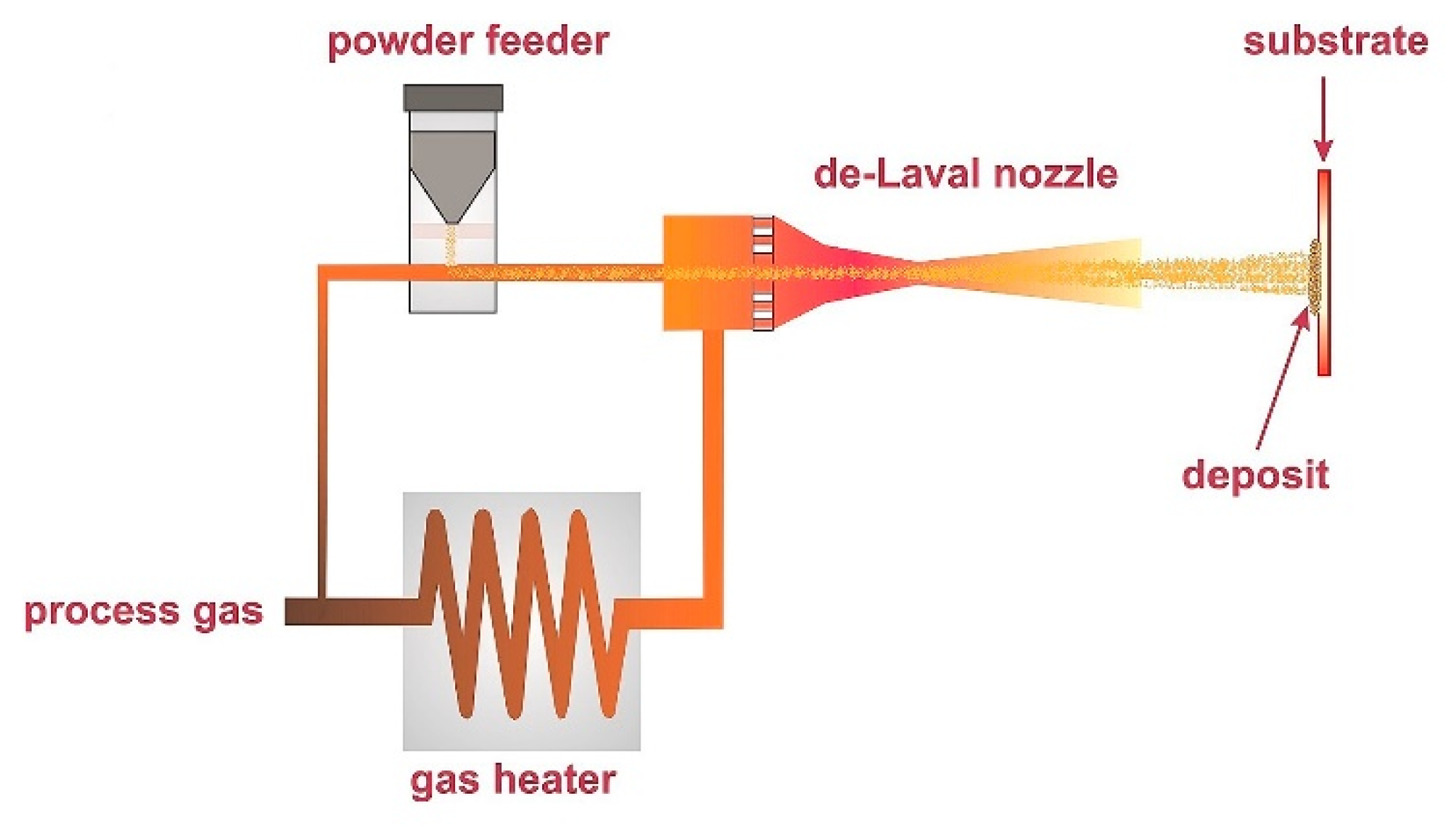
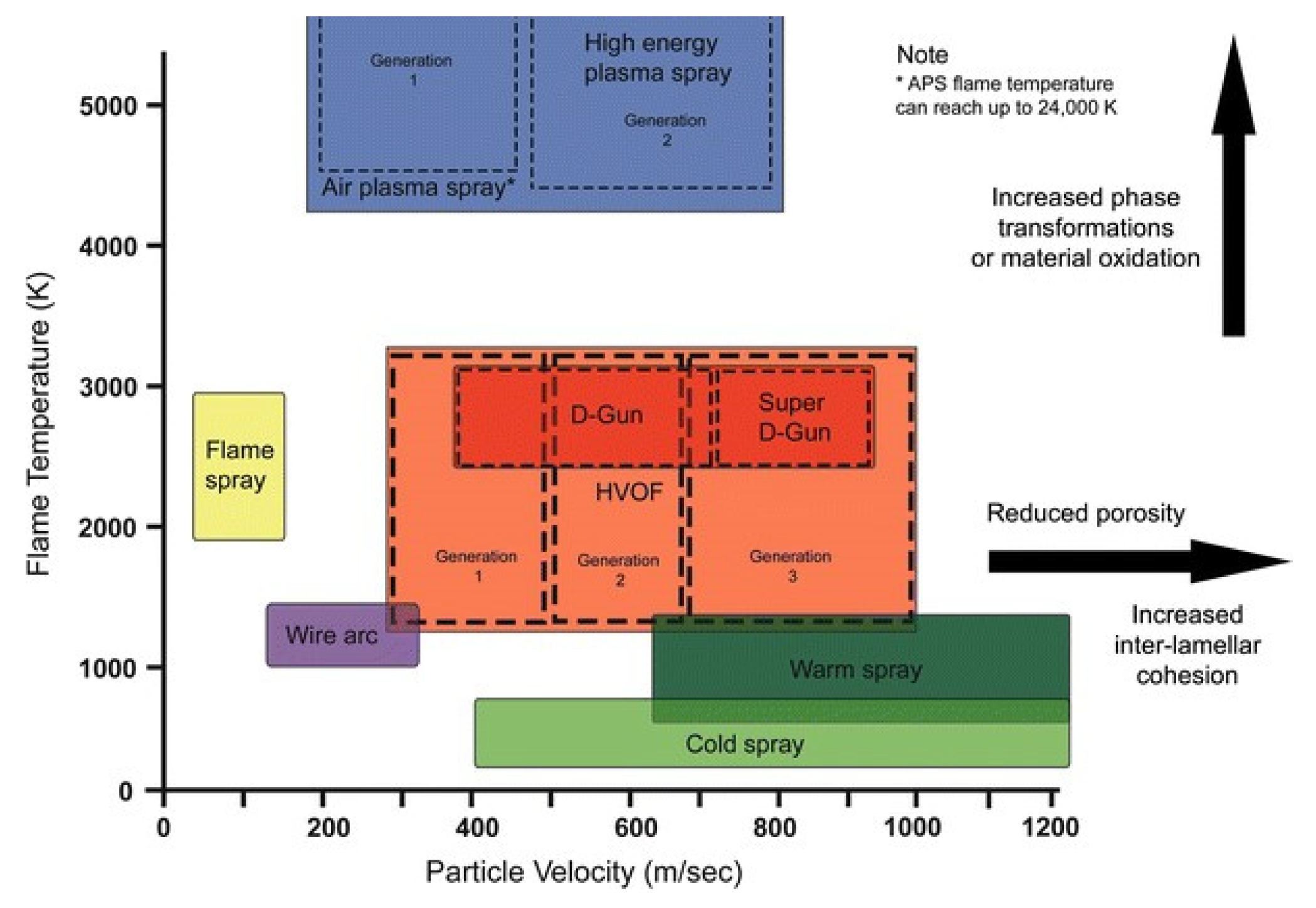
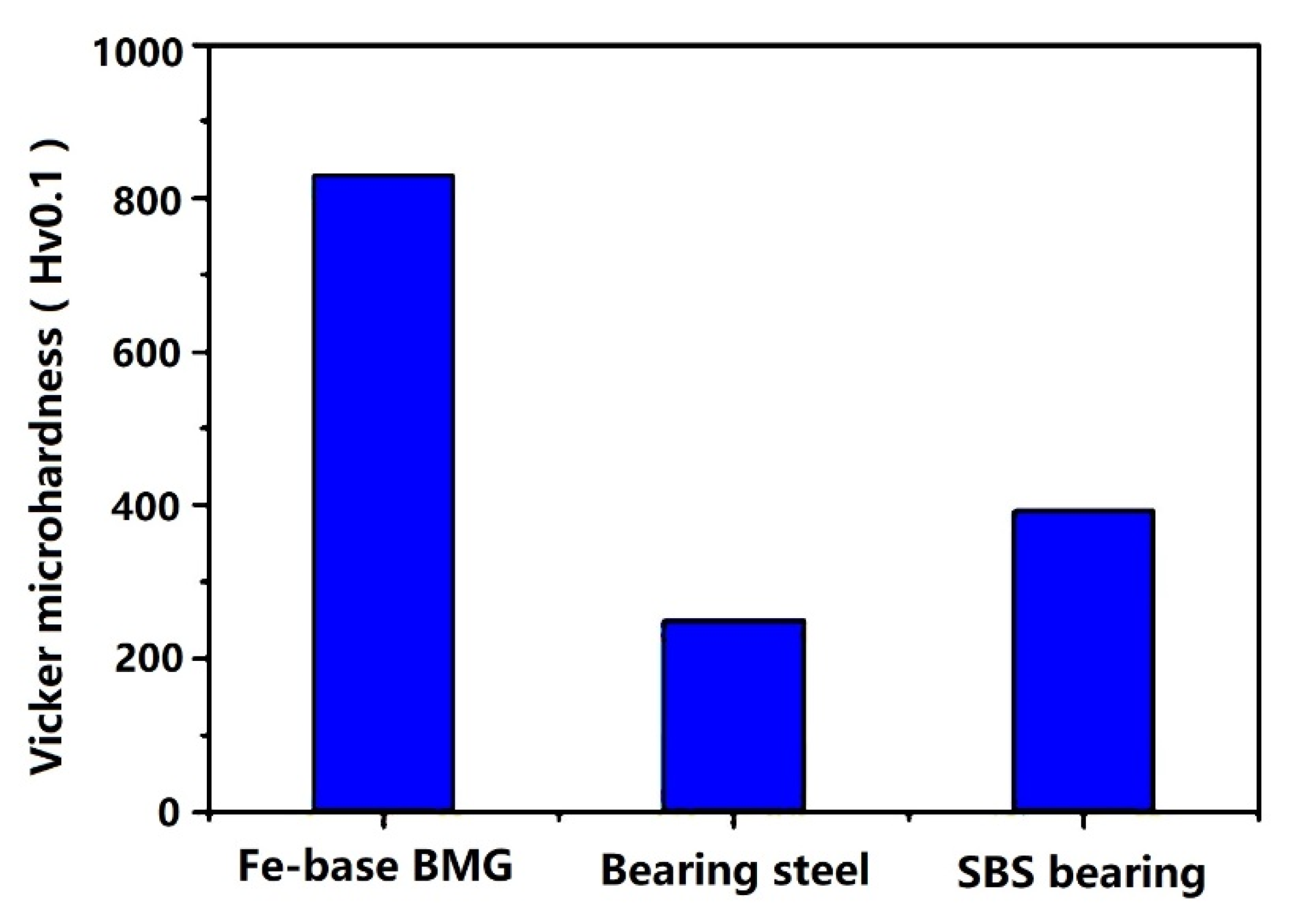

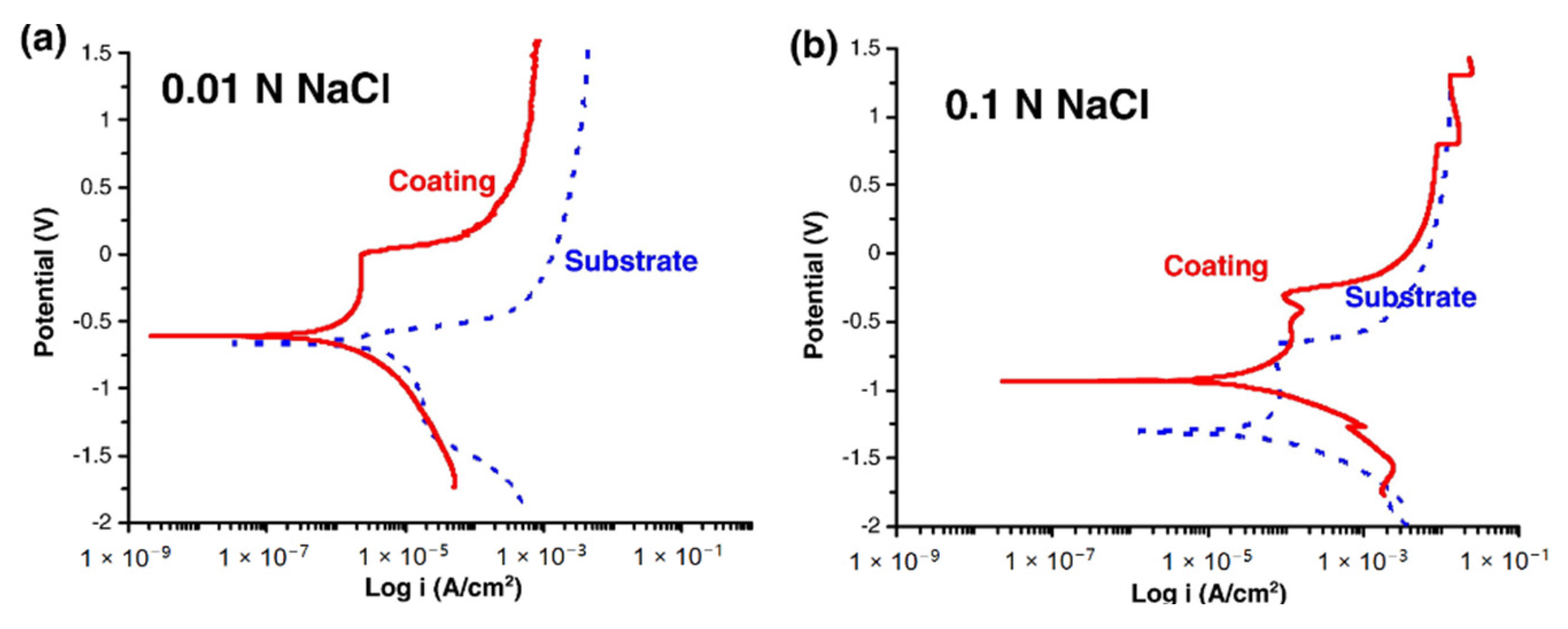
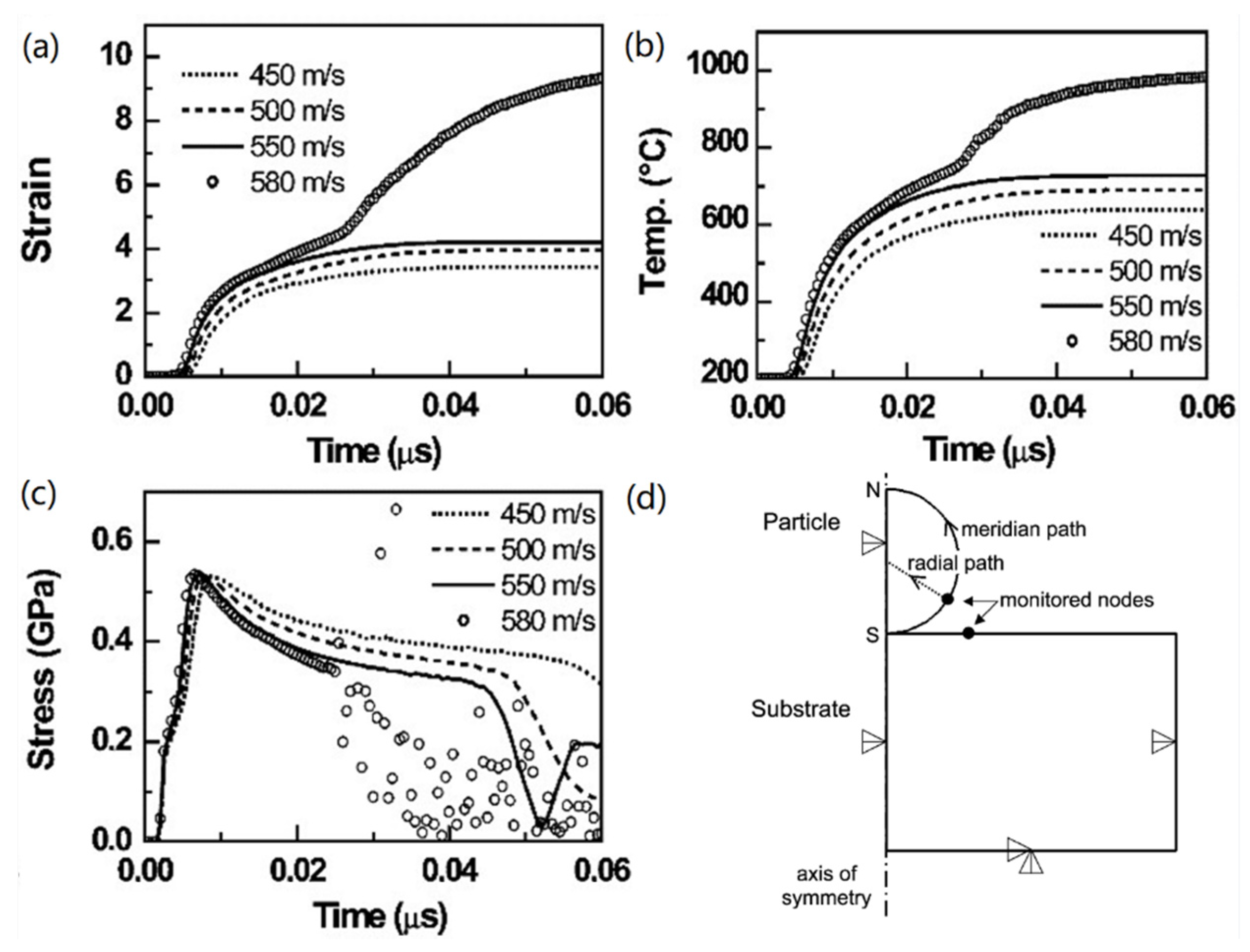

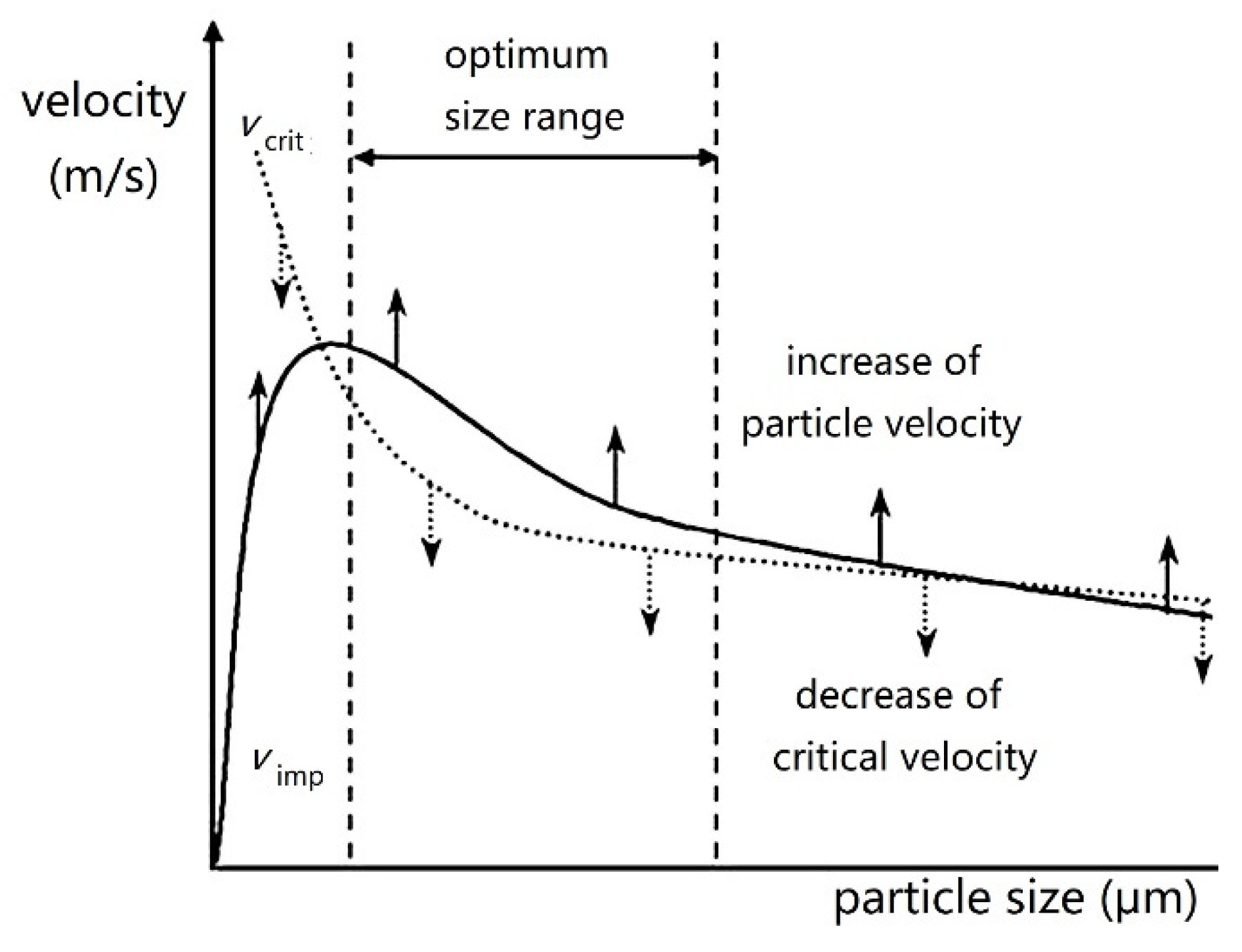
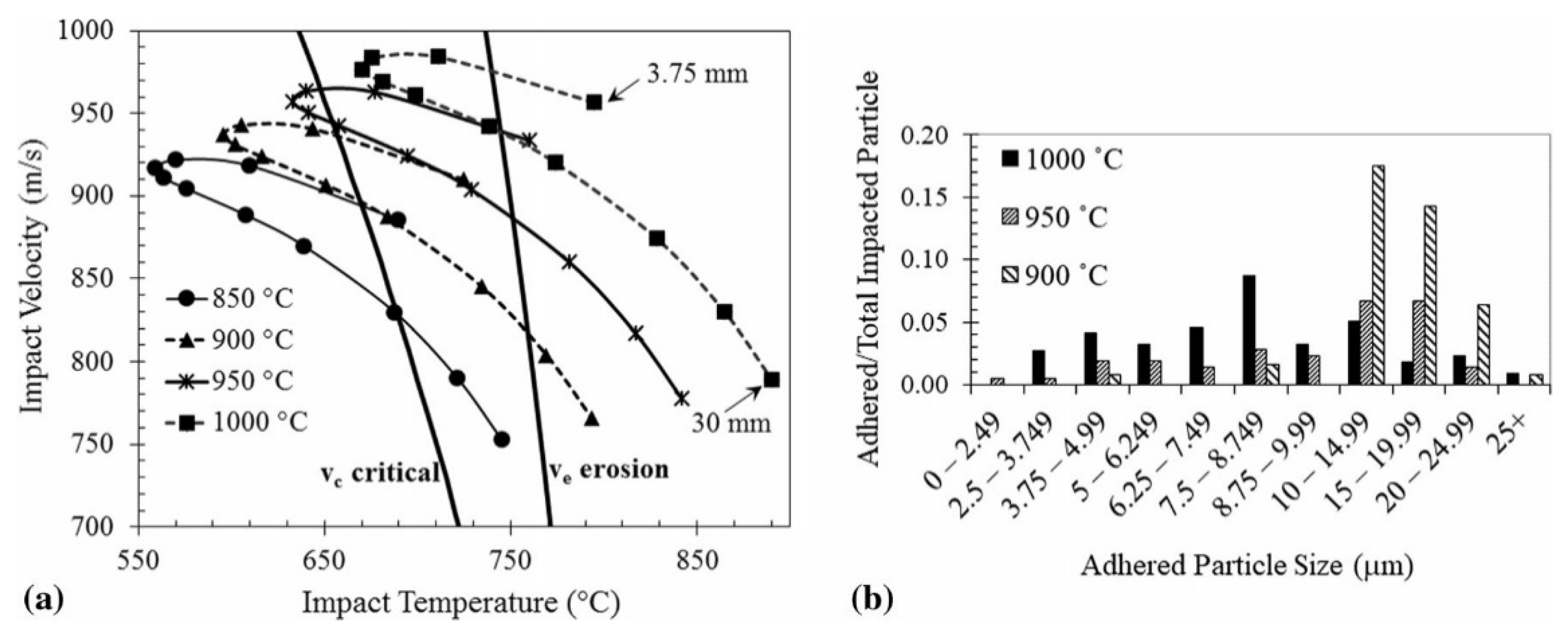
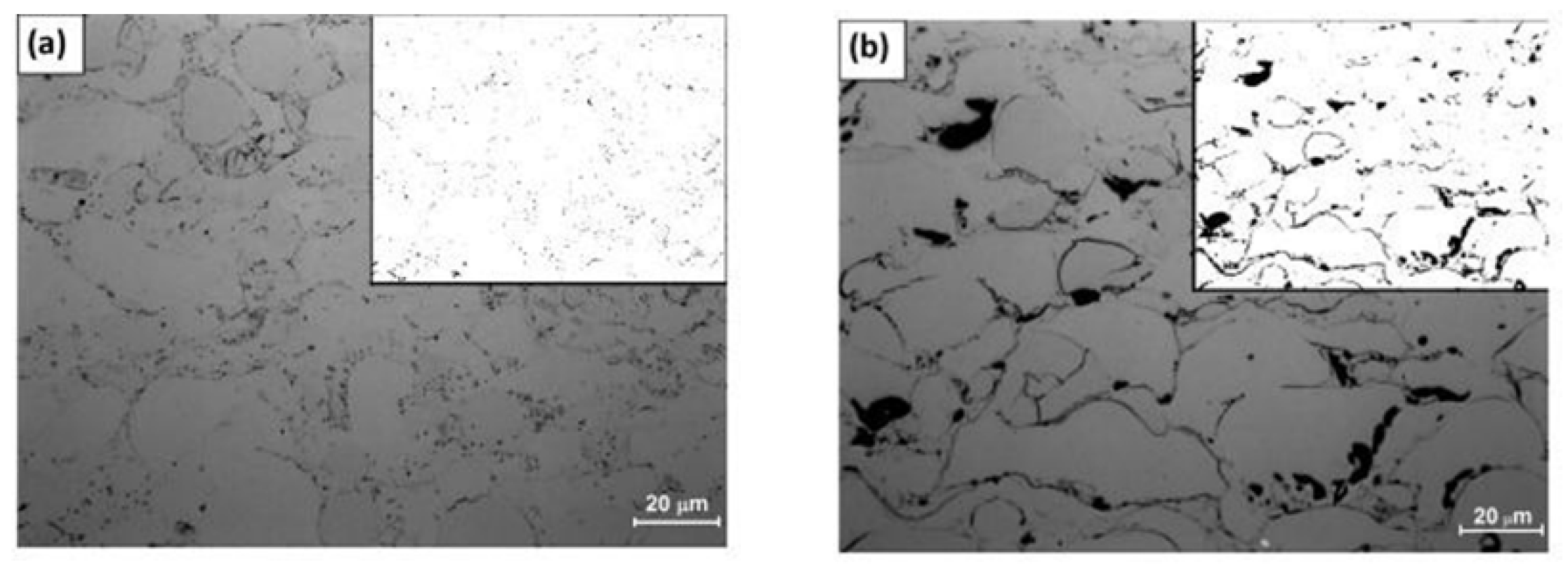
| Amorphous Alloy Coating Material | Substrate Material | Gas | Pressure/MPa | Temperature/°C | Ref |
|---|---|---|---|---|---|
| Fe44Co6Cr15Mo14C15B6 | Al/Cu/Ti | N2 | 4.0 | 900–950 | [35] |
| Fe73Cr2Si11B11C3 | Carbon Steel | N2 | 4.0–5.0 | 900–1000 | [36,37] |
| Fe–Cr–Mo–W–C–Mn–Si–Zr–B | Al 6061 | He | 1 | 300 | [38] |
| Fe68.8C7.0Si3.5B5.0P9.6Cr2.1Mo2.0Al2.0 | Mild Steel | He | 2.1–2.9 | 550 | [39] |
| Al88Ni6Y4.5Co1La0.5 | Al 7075 | N2 | 3.0–4.0 | 300–400 | [40] |
| Ni57Ti18Zr20Si3Sn2 | Mild Steel | He | 3.0 | 600 | [41,42] |
| Cu50Zr50 | Stainless Steel | N2 | 4.0 | 500–800 | [43] |
| Cu54Ni6Ti18Zr22 | Cu alloy | He | 1.5–3 | 550 | [44] |
| ZrCuAlNiTi | Cu | He | 2.4 | 500 | [45] |
| Sample/Bath | Ecorr, V | Icorr, μA | Corrosion Rate, Mpy |
|---|---|---|---|
| Coating/0.01 NaCl | −0.705 | 1.960 | 3.012 |
| Coating/0.1 NaCl | −0.869 | 6.260 | 9.593 |
| Substrate/0.01 NaCl | −0.661 | 9.870 | 15.14 |
| Substrate/0.1 NaCl | −1.310 | 28.7 | 44.0 |
| Gas Type | C (K) | Tref (K) | μref (μ·Pa·s) | Molecular Weight |
|---|---|---|---|---|
| Air | 120 | 291.15 | 18.27 | 28.96 (mean) |
| Nitrogen | 111 | 300.55 | 17.81 | 28.01 |
| Helium | 99 | 273 | 19 | 4.00 |
| Argon | 135 | 300 | 22.9 | 39.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Han, P.; Yin, S.; Niu, W.-J.; Zhai, L.; Li, X.; Mao, X.; Han, Y. Current Research Status on Cold Sprayed Amorphous Alloy Coatings: A Review. Coatings 2021, 11, 206. https://doi.org/10.3390/coatings11020206
Wang Q, Han P, Yin S, Niu W-J, Zhai L, Li X, Mao X, Han Y. Current Research Status on Cold Sprayed Amorphous Alloy Coatings: A Review. Coatings. 2021; 11(2):206. https://doi.org/10.3390/coatings11020206
Chicago/Turabian StyleWang, Qiang, Peng Han, Shuo Yin, Wen-Juan Niu, Le Zhai, Xu Li, Xuan Mao, and Yu Han. 2021. "Current Research Status on Cold Sprayed Amorphous Alloy Coatings: A Review" Coatings 11, no. 2: 206. https://doi.org/10.3390/coatings11020206






