Bulgecin A: The Key to a Broad‐Spectrum Inhibitor That Targets Lytic Transglycosylases
Abstract
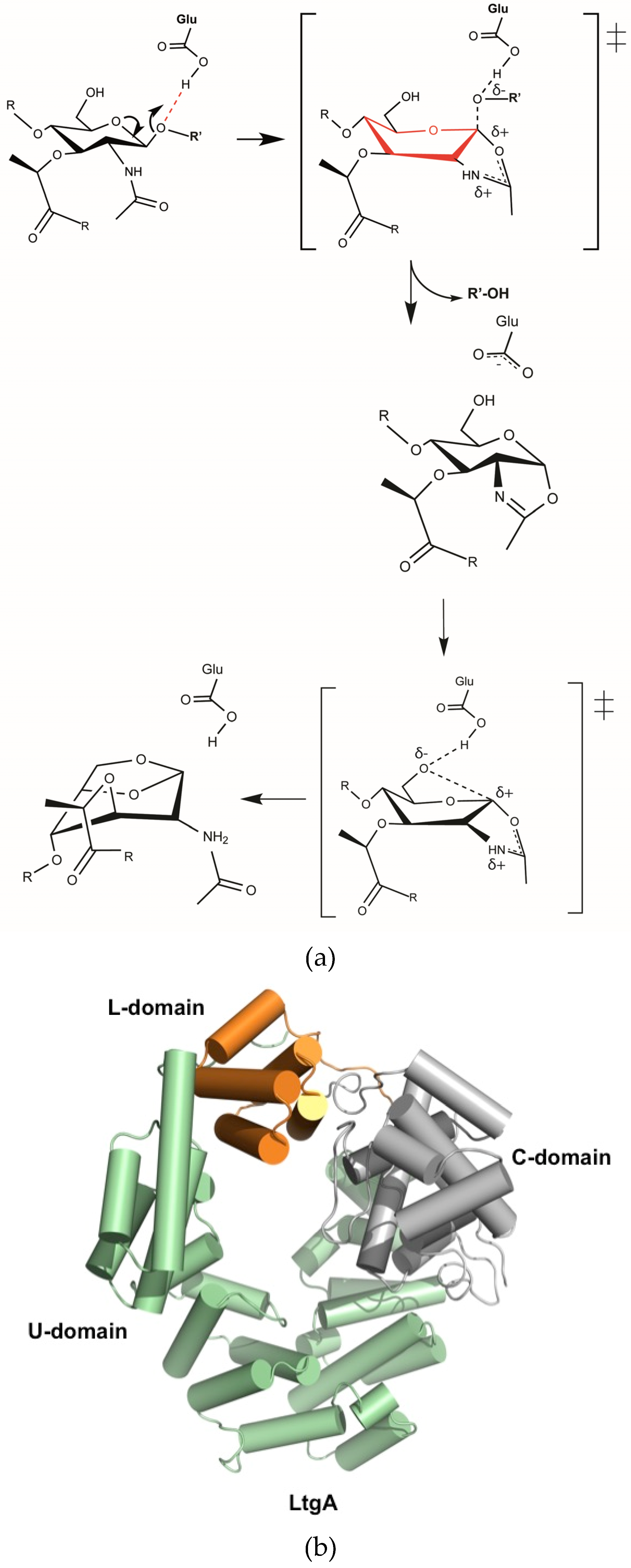
:1. Lytic Transglycosylases (Lts) as New Antibiotic Targets
2. Results
2.1. The Native Structure of LtgA
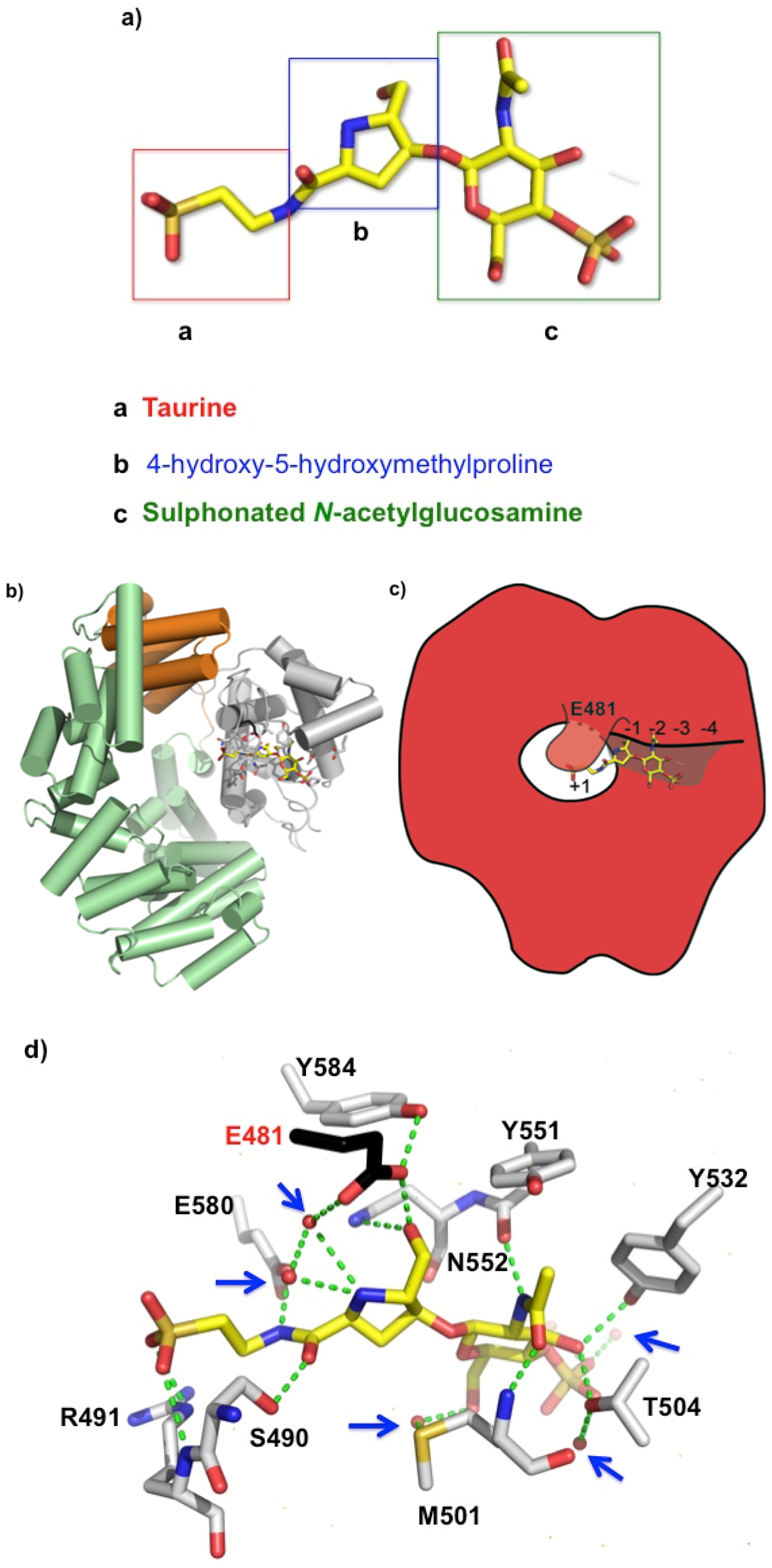
2.2. Bulgecin A Occupies the Active Site of LtgA
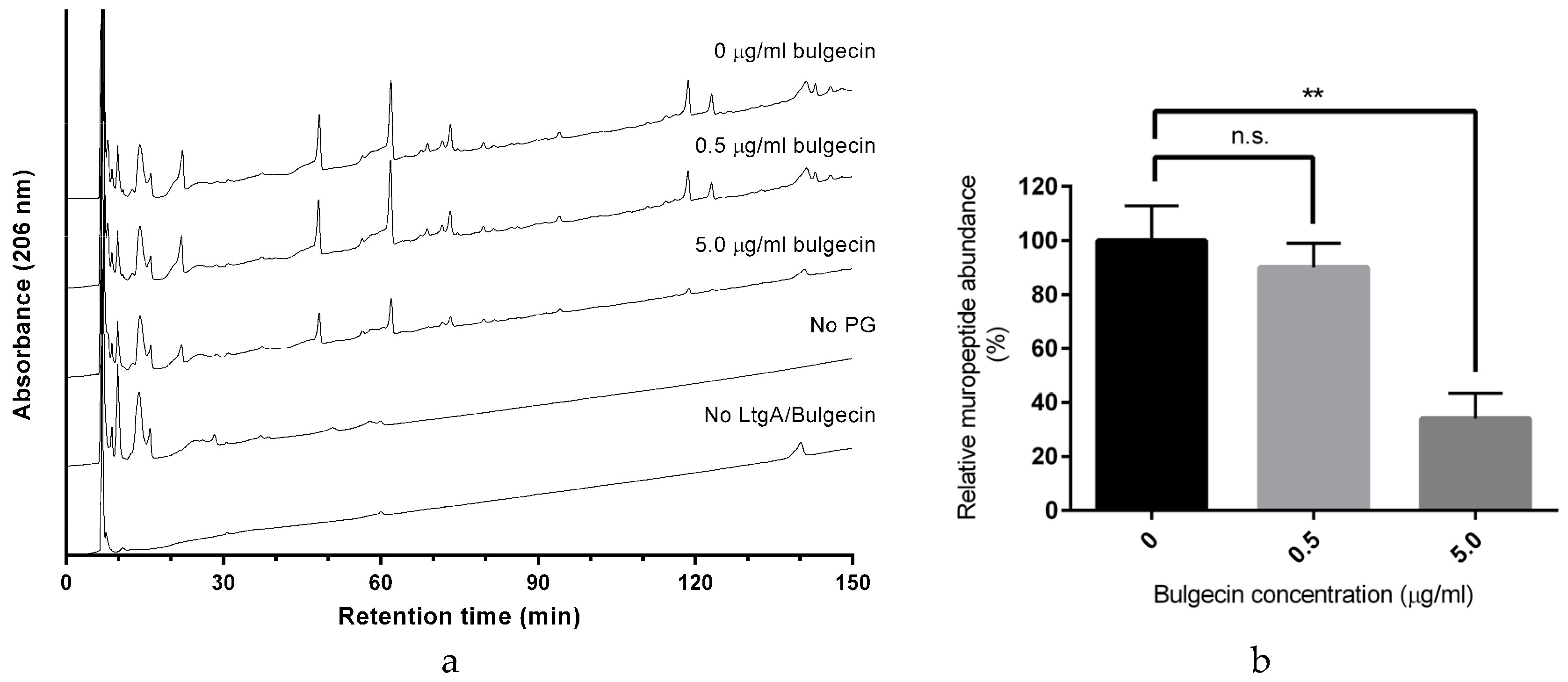
2.3. Bulgecin A Inhibits LtgA, Slt70, and H. pylori Slt
2.4. The Effects of Bulgecin A on Viability of Neisseria sp.
3. Methods and Materials
3.1. Protein Expression and Purification
3.2. Primer List
| ltgA Forward | cgggatccacactgccagccggcaagaccccggc |
| ltgA Reverse | cgaattctcagcgtgcaggaacaatgcccatacgc |
3.3. Analysis of Recombinant LtgA and LtgA-GST Activity by Reversed-Phase HPLC
3.4. The Effects of Bulgecin A on Viability of Neisseria Species
3.5. X-ray Crystallography
3.6. Docking Studies
3.7. Sequence Alignments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Medicin. Chem. 2014, 6, 25–64. [Google Scholar] [PubMed]
- Cho, H.; Uehara, T.; Bernhardt, T.G. Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell 2014, 159, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Artola-Recolons, C.; Carrasco-López, C.; Llarrull, L.I.; Kumarasiri, M.; Lastochkin, E.; Martínez de Ilarduya, I.; Meindl, K.; Usón, I.; Mobashery, S.; et al. High-resolution crystal structure of MltE, an outer membrane-anchored endolytic peptidoglycan lytic transglycosylase from Escherichia coli. Biochemistry 2011, 50, 2384–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bateman, A.; Bycroft, M. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 2000, 299, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Madoori, P.K.; Thunnissen, A.M. Purification, crystallization and preliminary X-ray diffraction analysis of the lytic transglycosylase MltF from Escherichia coli. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010, 66, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Lommatzsch, J.; Templin, M.F.; Kraft, A.R.; Vollmer, W.; Höltje, J.V. Outer membrane localization of murein hydrolases: MltA, a third lipoprotein lytic transglycosylase in Escherichia coli. J. Bacteriol. 1997, 179, 5465–5470. [Google Scholar] [CrossRef] [PubMed]
- Artola-Recolons, C.; Lee, M.; Bernardo-García, N.; Blázquez, B.; Hesek, D.; Bartual, S.G.; Mahasenan, K.V.; Lastochkin, E.; Pi, H.; Boggess, B.; et al. Structure and cell wall cleavage by modular lytic transglycosylase MltC of Escherichia coli. ACS Chem. Biol. 2014, 9, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Thunnissen, A.M.; Dijkstra, A.J.; Kalk, K.H.; Rozeboom, H.J.; Engel, H.; Keck, W.; Dijkstra, B.W. Doughnut-shaped structure of a bacterial muramidase revealed by X-ray crystallography. Nature 1994, 367, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Thunnissen, A.M.; Isaacs, N.W.; Dijkstra, B.W. The catalytic domain of a bacterial lytic transglycosylase defines a novel class of lysozymes. Proteins 1995, 22, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Cloud, K.A.; Dillard, J.P. A lytic transglycosylase of Neisseria gonorrhoeae is involved in peptidoglycan-derived cytotoxin production. Infect. Immun. 2002, 70, 2752–2757. [Google Scholar] [CrossRef] [PubMed]
- Cloud, K.A.; Dillard, J.P. Mutation of a single lytic transglycosylase causes aberrant septation and inhibits cell separation of Neisseria gonorrhoeae. J. Bacteriol. 2004, 186, 7811–7814. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, K.L.; Chan, J.M.; Lenz, J.D.; Hackett, K.T.; Dillard, J.P. Peptidoglycan fragment release from Neisseria meningitidis. Infect. Immun. 2013, 81, 3490–3498. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Travassos, L.H.; Hervé, M.; Blanot, D.; Boneca, I.G.; Philpott, D.J.; Sansonetti, P.J.; Mengin-Lecreulx, D. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 2003, 278, 41702–41708. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Boneca, I.G.; Carneiro, L.A.M.; Antignac, A.; Jéhanno, M.; Viala, J.; Tedin, K.; Taha, M.K.; Labigne, A.; Zaehringer, U.; et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 2003, 300, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Boneca, I.G.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003, 278, 8869–8872. [Google Scholar] [CrossRef] [PubMed]
- Viala, J.; Chaput, C.; Boneca, I.G.; Cardona, A.; Girardin, S.E.; Moran, A.P.; Athman, R.; Mémet, S.; Huerre, M.; Coyle, A.J. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 2004, 5, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Van Heijenoort, J. Peptidoglycan hydrolases of Escherichia coli. Microbiol. Mol. Biol. Rev. 2011, 75, 636–663. [Google Scholar] [CrossRef] [PubMed]
- Kohler, P.L.; Hamilton, H.L.; Cloud-Hansen, K.; Dillard, J.P. AtlA functions as a peptidoglycan lytic transglycosylase in the Neisseria gonorrhoeae type IV secretion system. J. Bacteriol. 2007, 189, 5421–5428. [Google Scholar] [CrossRef] [PubMed]
- Cloud-Hansen, K.A.; Hackett, K.T.; Garcia, D.L.; Dillard, J.P. Neisseria gonorrhoeae uses two lytic transglycosylases to produce cytotoxic peptidoglycan monomers. J. Bacteriol. 2008, 190, 5989–5994. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.A.; Hackett, K.T.; Dillard, J.P. The lytic transglycosylases of Neisseria gonorrhoeae. Microb. Drug. Resist. 2012, 18, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Schaub, R.E.; Chan, Y.A.; Lee, M.; Hesek, D.; Mobashery, S.; Dillard, J.P. Lytic transglycosylases LtgA and LtgD perform distinct roles in remodeling, recycling and releasing peptidoglycan in Neisseria gonorrhoeae. Mol. Microbiol. 2016, 102, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Santin, Y.G.; Cascales, E. Domestication of a housekeeping transglycosylase for assembly of a Type VI secretion system. EMBO Rep. 2017, 18, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Holtje, J.V. The architecture of the murein (peptidoglycan) in gram-negative bacteria: Vertical scaffold or horizontal layer(s)? J. Bacteriol. 2004, 186, 5978–5987. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Joris, B.; Charlier, P.; Foster, S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 2008, 32, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Blanot, D.; de Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W. Structural variation in the glycan strands of bacterial peptidoglycan. FEMS Microbiol. Rev. 2008, 32, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Bertsche, U. Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochim. Biophys. Acta 2008, 1778, 1714–1734. [Google Scholar] [CrossRef] [PubMed]
- Scheurwater, E.; Reid, C.W.; Clarke, A.J. Lytic transglycosylases: Bacterial space-making autolysins. Int. J. Biochem. Cell Biol. 2008, 40, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Van Asselt, E.J.; Thunnissen, A.M.; Dijkstra, B.W. High resolution crystal structures of the Escherichia coli lytic transglycosylase Slt70 and its complex with a peptidoglycan fragment. J. Mol. Biol. 1999, 291, 877–898. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.W.; Blackburn, N.T.; Legaree, B.A.; Auzanneau, F.I.; Clarke, A.J. Inhibition of membrane-bound lytic transglycosylase B by NAG-thiazoline. FEBS Lett. 2004, 574, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Van Asselt, E.J.; Dijkstra, B.W. Binding of calcium in the EF-hand of Escherichia coli lytic transglycosylase Slt35 is important for stability. FEBS Lett. 1999, 458, 429–435. [Google Scholar] [CrossRef]
- Templin, M.F.; Edwards, D.H.; Holtje, J.V. A murein hydrolase is the specific target of bulgecin in Escherichia coli. J. Biol. Chem. 1992, 267, 20039–20043. [Google Scholar] [PubMed]
- Bonis, M.; Williams, A.H.; Guadagnini, S.; Werts, C.; Boneca, I.G. The effect of bulgecin A on peptidoglycan metabolism and physiology of Helicobacter pylori. Microb. Drug. Resist. 2012, 18, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Thunnissen, A.M.; Rozeboom, H.J.; Kalk, K.H.; Dijkstra, B.W. Structure of the 70-kDa soluble lytic transglycosylase complexed with bulgecin A. Implications for the enzymatic mechanism. Biochemistry 1995, 34, 12729–12737. [Google Scholar] [CrossRef] [PubMed]
- Skalweit, M.J.; Li, M. Bulgecin A as a beta-lactam enhancer for carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumannii clinical isolates containing various resistance mechanisms. Drug. Des. Devel. Ther. 2016, 10, 3013–3020. [Google Scholar] [CrossRef] [PubMed]
- Antignac, A.; Kriz, P.; Tzanakaki, G.; Alonso, J.M.; Taha, M.K. Polymorphism of Neisseria meningitidis penA gene associated with reduced susceptibility to penicillin. J. Antimicrob. Chemother. 2001, 47, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Antignac, A.; Boneca, I.G.; Rousselle, J.C.; Namane, A.; Carlier, J.P.; Vazquez, J.; Fox, A.; Alonso, J.M.; Taha, M.K. Correlation between alterations of the penicillin-binding protein 2 and modifications of the peptidoglycan structure in Neisseria meningitidis with reduced susceptibility to penicillin G. J. Biol. Chem. 2003, 278, 31529–31535. [Google Scholar] [CrossRef] [PubMed]
- Belkacem, N.; Hong, E.; Antunes, A.; Terrade, A.; Deghmane, A.E.; Taha, M.K. Use of Animal models to support revising meningococcal breakpoints of beta-lactams. Antimicrob. Agents Chemother. 2016, 60, 4023–4027. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, B.W.; Thunnissen, A.M. “Holy” proteins. II: The soluble lytic transglycosylase. Curr. Opin. Struct. Biol. 1994, 4, 810–813. [Google Scholar] [CrossRef]
- Romeis, T.; Holtje, J.V. Specific interaction of penicillin-binding proteins 3 and 7/8 with soluble lytic transglycosylase in Escherichia coli. J. Biol. Chem. 1994, 269, 21603–21607. [Google Scholar] [PubMed]
- Legaree, B.A.; Clarke, A.J. Interaction of penicillin-binding protein 2 with soluble lytic transglycosylase B1 in Pseudomonas aeruginosa. J. Bacteriol. 2008, 190, 6922–6926. [Google Scholar] [CrossRef] [PubMed]
- Von Rechenberg, M.; Ursinus, A.; Holtje, J.V. Affinity chromatography as a means to study multienzyme complexes involved in murein synthesis. Microb. Drug. Resist. 1996, 2, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, R.; Veyrier, F.; Werts, C.; Boneca, I.G. Peptidoglycan and nod receptor. In Glycoscience: Biology and Medicine; Taniguchi, N., Endo, T., Hart, G.W., Seeberger, P.H., Wong, C.-H., Eds.; Springer: Tokyo, Japan, 2014; pp. 737–747. [Google Scholar]
- Kellogg, D.S., Jr.; Peacock, W.L., Jr; Deacon, W.E.; Brown, L.; Pirkle, D.I. Neisseria Gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 1963, 85, 1274–1279. [Google Scholar] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Collaborative, C.P. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 1994, 50, 760–763. [Google Scholar]
- Davis, I.W.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MOLPROBITY: Structure validation and all-atom contact analysis for nucleic acids and their complexes. Nucleic. Acids Res. 2004, 32, W615–W619. [Google Scholar] [CrossRef] [PubMed]


| Data Collection | LtgA-Bulgecin A |
|---|---|
| Ligand Added | Bulgecin A |
| Data collection | |
| Wavelength (Å) | 0.9795 |
| Resolution range (Å) | 45.84–1.78 (1.567–1.513) |
| Space group | P212121 |
| Unit-cell parameters | |
| a (Å) | 65.83 |
| b (Å) | 71.56 |
| c (Å) | 121.12 |
| σ (°), β (°), γ (°) | 90 |
| Total reflections | 108,346 |
| Unique reflections | 54,379 |
| Multiplicity | 2.0 (2.0) |
| Completeness (%) | 98.00 (84.00) |
| Mean I/σ(I) | 4.46 (0.67) |
| Wilson B factor (Å2) | 21.65 |
| Rmerge † | 0.1064 (1.13) |
| Refinement | |
| Rfactor ‡ | 0.2131 (0.3912) |
| Rfree * | 0.2570 (0.4084) |
| No. of atoms | 4920 |
| No. of waters | 417 |
| No. of protein residues | 577 |
| R.m.s.d., bonds (Å) | 0.007 |
| R.m.s.d., angles (°) | 0.91 |
| Ramachandran favored (%) | 99 |
| Ramachandran outliers (%) | 1.0 |
| B factors (Å2) | |
| Average | 27.91 |
| Macromolecules | 27.43 |
| Ligand | 35.00 |
| Solvent | 33.54 |
| All-atom clash score | 2.09 |
| N. gonorrhoeae Strain 24753 TEM-1 | ||||
| Antibiotics | Bulgecin A (µg/mL) | |||
| 0 | 19 | 38 | 75 | |
| Penicillin G | 4 | 4 | 4 | 3 |
| Amoxicillin | 16 | 12 | 16 | 12 |
| Cefotaxim | 0.003 | 0.002 | 0.002 | 0.002 |
| Chloramphenicol | 0.75 | 0.5 | 0.5 | 0.5 |
| N. gonorrhoeae Strain 24970 PenI | ||||
| Antibiotics | Bulgecin A (µg/mL) | |||
| 0 | 19 | 38 | 75 | |
| Penicillin G | 0.5 | 0.094 | 0.094 | 0.047 |
| Amoxicillin | 0.75 | 0.38 | 0.250 | 0.19 |
| Cefotaxim | 0.125 | 0.047 | 0.047 | 0.047 |
| Chloramphenicol | 1.5 | 1.5 | 1 | 1 |
| N. meningitidis Strain 28671 PenI | ||||
| Antibiotics | Bulgecin A (µg/mL) | |||
| 0 | 19 | 38 | 75 | |
| Penicillin G | 0.25 | 0.095 | 0.064 | 0.047 |
| Amoxicillin | 0.75 | 0.40 | 0.40 | 0.25 |
| Cefotaxim | 0.125 | 0.064 | 0.064 | 0.064 |
| Chloramphenicol | 0.5 | 0.5 | 0.4 | 0.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, A.H.; Wheeler, R.; Thiriau, C.; Haouz, A.; Taha, M.; Boneca, I.G. Bulgecin A: The Key to a Broad‐Spectrum Inhibitor That Targets Lytic Transglycosylases. Antibiotics 2017, 6, 8. https://doi.org/10.3390/antibiotics6010008
Williams AH, Wheeler R, Thiriau C, Haouz A, Taha M, Boneca IG. Bulgecin A: The Key to a Broad‐Spectrum Inhibitor That Targets Lytic Transglycosylases. Antibiotics. 2017; 6(1):8. https://doi.org/10.3390/antibiotics6010008
Chicago/Turabian StyleWilliams, Allison H., Richard Wheeler, Constance Thiriau, Ahmed Haouz, Muhamed‐Kheir Taha, and Ivo G. Boneca. 2017. "Bulgecin A: The Key to a Broad‐Spectrum Inhibitor That Targets Lytic Transglycosylases" Antibiotics 6, no. 1: 8. https://doi.org/10.3390/antibiotics6010008





