The Oligopeptide Permease Opp Mediates Illicit Transport of the Bacterial P-site Decoding Inhibitor GE81112 †
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Plasmids
4.2. Antimicrobial Activity
4.3. Selection of Antibiotic Resistant Mutants
4.4. Cell Free mRNA Translation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MIC | minimal inhibitory concentration |
| IC50 | concentration causing 50% inhibition |
References
- Fabbretti, A.; Gualerzi, C.O.; Brandi, L. How to cope with the quest for new antibiotics. FEBS Lett. 2011, 585, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Brandi, L.; Lazzaroni, A.; Fabbretti, A.; Cavalletti, L.; Abbondi, M.; Corti, E.; Ciciliato, I.; Gastaldo, L.; Marazzi, A.; Feroggio, M.; et al. Novel tetrapeptide inhibitors of bacterial protein synthesis produced by a Streptomyces sp. Biochemistry 2006, 45, 3692–3702. [Google Scholar] [CrossRef] [PubMed]
- Brandi, L.; Fabbretti, A.; La Teana, A.; Abbondi, M.; Losi, D.; Donadio, S.; Gualerzi, C.O. Specific, efficient, and selective inhibition of prokaryotic translation initiation by a novel peptide antibiotic. Proc. Natl. Acad. Sci. USA 2006, 103, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Binz, T.M.; Maffioli, S.I.; Sosio, M.; Donadio, S.; Muller, R. Insights into an unusual nonribosomal peptide synthetase biosynthesis: Identification and characterization of the GE81112 biosynthetic gene cluster. J. Biol. Chem. 2010, 285, 32710–32719. [Google Scholar] [CrossRef] [PubMed]
- Fabbretti, A.; Schedlbauer, A.; Brandi, L.; Kaminishi, T.; Giuliodori, A.M.; Garofalo, R.; Ochoa-Lizarralde, B.; Takemoto, C.; Yokoyama, S.; Connell, S.R.; et al. Inhibition of translation initiation complex formation by GE81112 unravels a 16S rRNA structural switch involved in P-site decoding. Proc. Natl. Acad. Sci. USA 2016, 113, E2286–E2295. [Google Scholar] [CrossRef] [PubMed]
- Fabbretti, A.; Brandi, L.; Petrelli, D.; Pon, C.L.; Castanedo, N.R.; Medina, R.; Gualerzi, C.O. The antibiotic Furvina(R) targets the P-site of 30S ribosomal subunits and inhibits translation initiation displaying start codon bias. Nucleic Acids Res. 2012, 40, 10366–10374. [Google Scholar] [CrossRef] [PubMed]
- Gualerzi, C.; Risuleo, G.; Pon, C.L. Initial rate kinetic analysis of the mechanism of initiation complex formation and the role of initiation factor IF-3. Biochemistry 1977, 16, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.; Krebes, K.; McNally, C.; Neshat, S. Multiple antibiotic resistance in Pseudomonas aeruginosa: Evidence for involvement of an efflux operon. J. Bacteriol. 1993, 175, 7363–7372. [Google Scholar] [PubMed]
- Li, X.-Z.; Nikaido, H.; Poole, K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1995, 39, 1948–1953. [Google Scholar] [CrossRef] [PubMed]
- Perego, M.; Higgins, C.F.; Pearce, S.R.; Gallagher, M.P.; Hoch, J.A. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol. Microbiol. 1991, 5, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Ferro-Luzzi Ames, G.; Young, J.D.; Tsuchiya, D.; Lecocq, J. Illicit transport: The oligopeptide permease. Proc. Natl. Acad. Sci. USA 1973, 70, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Lazazzera, B.A. The intracellular function of extracellular signalingpeptides. Peptides 2001, 22, 1519–1527. [Google Scholar] [CrossRef]
- Pearce, S.R.; Mimmack, M.L.; Gallagher, M.P.; Gileadi, U.; Hyde, S.C.; Higgins, C.F. Membrane topology of the integral membrane components, OppB and OppC, of the oligopeptide permease of Salmonella typhimurium. Mol. Microbiol. 1992, 6, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.; Warren, M.S.; Cusick, J.K.; Karkhoff-Schweizer, R.R.; Lomovskaya, O.; Schweizer, H.P. High-level pacidamycin resistance in Pseudomonas aeruginosa is mediated by an opp oligopeptide permease encoded by the opp-fabI operon. Antimicrob. Agents Chemother. 2013, 57, 5565–5571. [Google Scholar] [CrossRef] [PubMed]
- Barak, Z.; Gilvarg, C. Triornithìne-resistant strains of Escherichia coli. Isolation, definition and genetic studies. J. Biol. Chem. 1974, 249, 143–148. [Google Scholar] [PubMed]
- Barak, Z.; Sarid, S.; Katchalski, E. Inhibition of protein biosynthesis in Escherichia coli B by tri-l-ornithine. Eur. J. Biochem. 1973, 34, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.C.; Short, S.A. Genetic analysis of Escherichia coli oligopeptide transport mutants. J. Bacteriol. 1985, 161, 484–492. [Google Scholar] [PubMed]
- Andrews, J.C.; Blevins, T.C.; Short, S.A. Regulation of peptide transport in Escherichia coli: Induction of the trp-linked operon encoding the oligopeptide permease. J. Bacteriol. 1986, 165, 428–433. [Google Scholar] [PubMed]
- Lin, B.; Short, S.A.; Eskildsen, M.; Klempner, M.S.; Hu, L.T. Functional testing of putative oligopeptide permease (Opp) proteins of Borrelia burgdorferi: A complementation model in opp(−) Escherichia coli. Biochim. Biophys. Acta 2001, 1499, 222–231. [Google Scholar] [CrossRef]
- Vila-Sanjurjo, A.; Squires, C.L.; Dahlberg, A.E. Isolation of kasugamycin resistant mutants in the 16 S ribosomal RNA of Escherichia coli. J. Mol. Biol. 1999, 293, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schuwirth, B.S.; Day, J.M.; Hau, C.W.; Janssen, G.R.; Dahlberg, A.E.; Cate, J.H.; Vila-Sanjurjo, A. Structural analysis of kasugamycin inhibition of translation. Nat. Struct. Mol. Biol. 2006, 13, 879–886. [Google Scholar] [CrossRef] [PubMed]
- LeDeaux, J.R.; Solomon, J.M.; Grossman, A.D. Analysis of non-polar deletion mutations in the genes of the spo0K (opp) operon of Bacillus subtilis. FEMS Microbiol. Lett. 1997, 153, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.; Su, L.; Shyn, S.; Grossman, A.D. Isolation and characterization of mutants of the Bacillus subtilis oligopeptide permease with altered specificity of oligopeptide transport. J. Bacteriol. 2003, 185, 6425–6433. [Google Scholar] [CrossRef] [PubMed]
- Koide, A.; Perego, M.; Hoch, J.A. ScoC regulates peptide transport and sporulation initiation in Bacillus subtilis. J. Bacteriol. 1999, 181, 4114–4117. [Google Scholar] [PubMed]
- Gardan, R.; Besset, C.; Guillot, A.; Gitton, C.; Monnet, V. The oligopeptide transport system is essential for the development of natural competence in Streptococcus thermophilus strain LMD-9. J. Bacteriol. 2009, 191, 4647–4655. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, C.; Ishikawa, S.; Stephenson, S.; Ogasawara, N.; Perego, M. Synergistic regulation of competence development in Bacillus subtilis by two Rap-Phr systems. J. Bacteriol. 2005, 187, 4353–4361. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.A.; Podbielski, A.; Hedberg, P.J.; Dunny, G.M. Enterococcus faecalis pheromone binding protein, PrgZ, recruits a chromosomal oligopeptide permease system to import sex pheromone cCF10 for induction of conjugation. Proc. Natl. Acad. Sci. USA 1996, 93, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Kuan, G.; Dassa, E.; Saurin, W.; Hofnung, M.; Saier, M.H., Jr. Phylogenetic analyses of the ATP-binding constituents of bacterial extracytoplasmic receptor-dependent ABC-type nutrient uptake permeases. Res. Microbiol. 1995, 146, 271–278. [Google Scholar] [CrossRef]
- Coulter, S.N.; Schwan, W.R.; Ng, E.Y.; Langhorne, M.H.; Ritchie, H.D.; Westbrock-Wadman, S.; Hufnagle, W.O.; Folger, K.R.; Bayer, A.S.; Stover, C.K. Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol. Microbiol. 1998, 30, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.M.; Casjens, S.; Huang, W.M.; Sutton, G.G.; Clayton, R.; Lathigra, R.; White, O.; Ketchum, K.A.; Dodson, R.; Hickey, E.K.; et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 1997, 390, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Bono, J.L.; Tilly, K.; Stevenson, B.; Hogan, D.; Rosa, P. Oligopeptide permease in Borrelia burgdorferi: Putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology 1998, 144, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Medrano, M.S.; Ding, Y.; Wang, X.G.; Lu, P.; Coburn, J.; Hu, L.T. Regulators of expression of the oligopeptide permease A proteins of Borrelia burgdorferi. J. Bacteriol. 2007, 189, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Guyer, M.S.; Reed, R.R.; Steitz, J.A.; Low, K.B. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb. Symp. Quant. Biol. 1981, 45 Pt 1, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Cammack, K.A.; Wade, H.E. The sedimentation behaviour of ribonuclease-active and -inactive ribosomes from bacteria. Biochem. J. 1965, 96, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Kurylo, C.M.; Alexander, N.; Dass, R.A.; Parks, M.M.; Altman, R.A.; Vincent, C.T.; Mason, C.E.; Blanchard, S.C. Genome sequence and analysis of Escherichia coli MRE600, a colicinogenic, nonmotile strain that lacks RNase I and the type I methyltransferase, EcoKI. Genome Biol. Evol. 2016, 8, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.G.; Walker, D.C.; McInnes, R.R. E. E. coli host strains significantly affect the quality of small scale plasmid DNA preparations used for sequencing. Nucleic Acids Res. 1993, 21, 1677–1678. [Google Scholar] [CrossRef] [PubMed]
- Koide, A.; Hoch, J.A. Identification of a second oligopeptide transport system in Bacillus subtilis and determination of its role in sporulation. Mol. Microbiol. 1994, 13, 417–412. [Google Scholar] [CrossRef] [PubMed]
- Brandi, L.; Fabbretti, A.; Milon, P.; Carotti, M.; Pon, C.L.; Gualerzi, C.O. Methods for identifying compounds that specifically target translation. Meth. Enzymol. 2007, 431, 229–267. [Google Scholar] [PubMed]
- Brandi, L.; Dresios, J.; Gualerzi, C.O. Assays for the identification of inhibitors targeting specific translational steps. Methods Mol. Med. 2008, 142, 87–105. [Google Scholar] [PubMed]
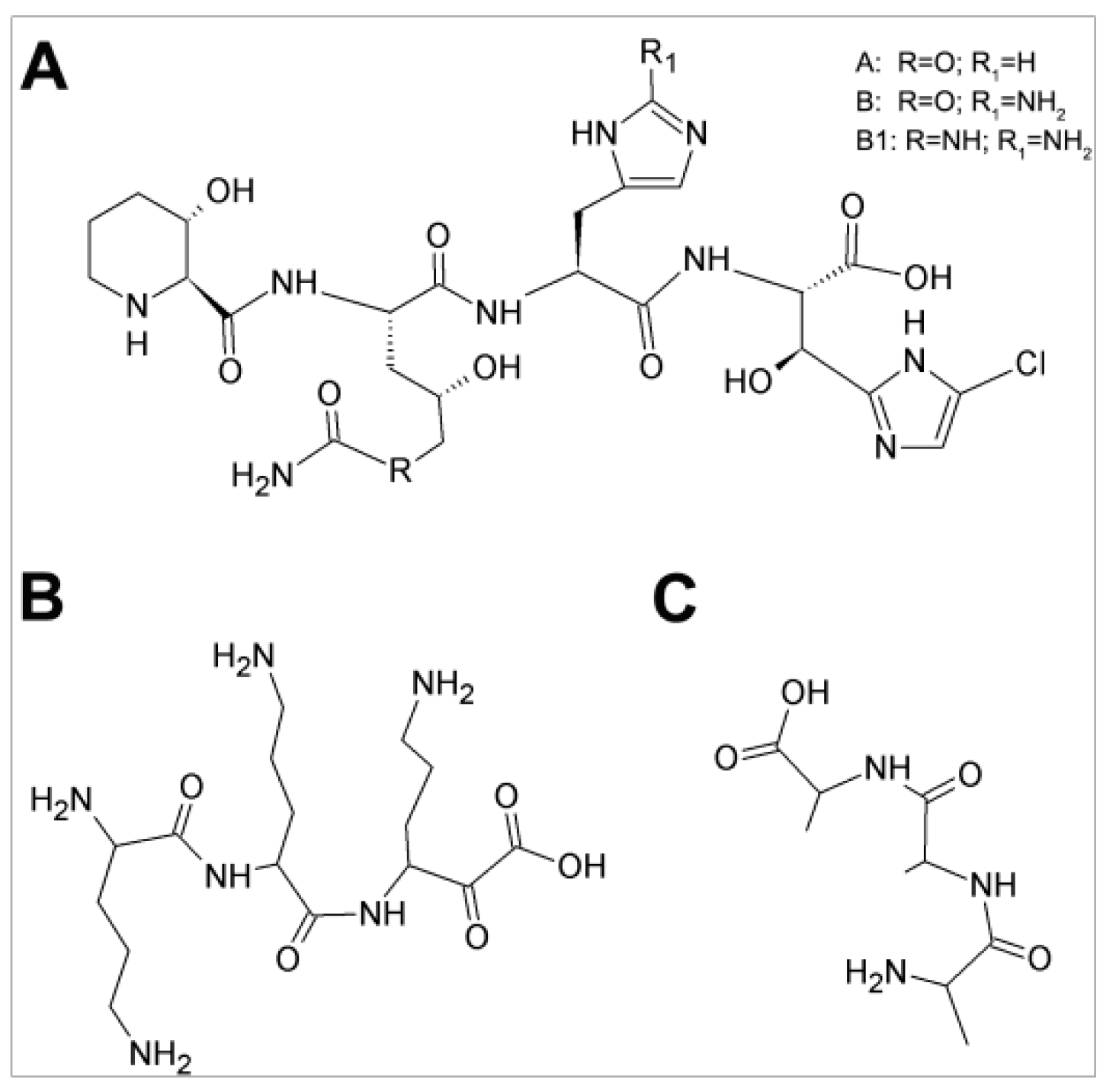
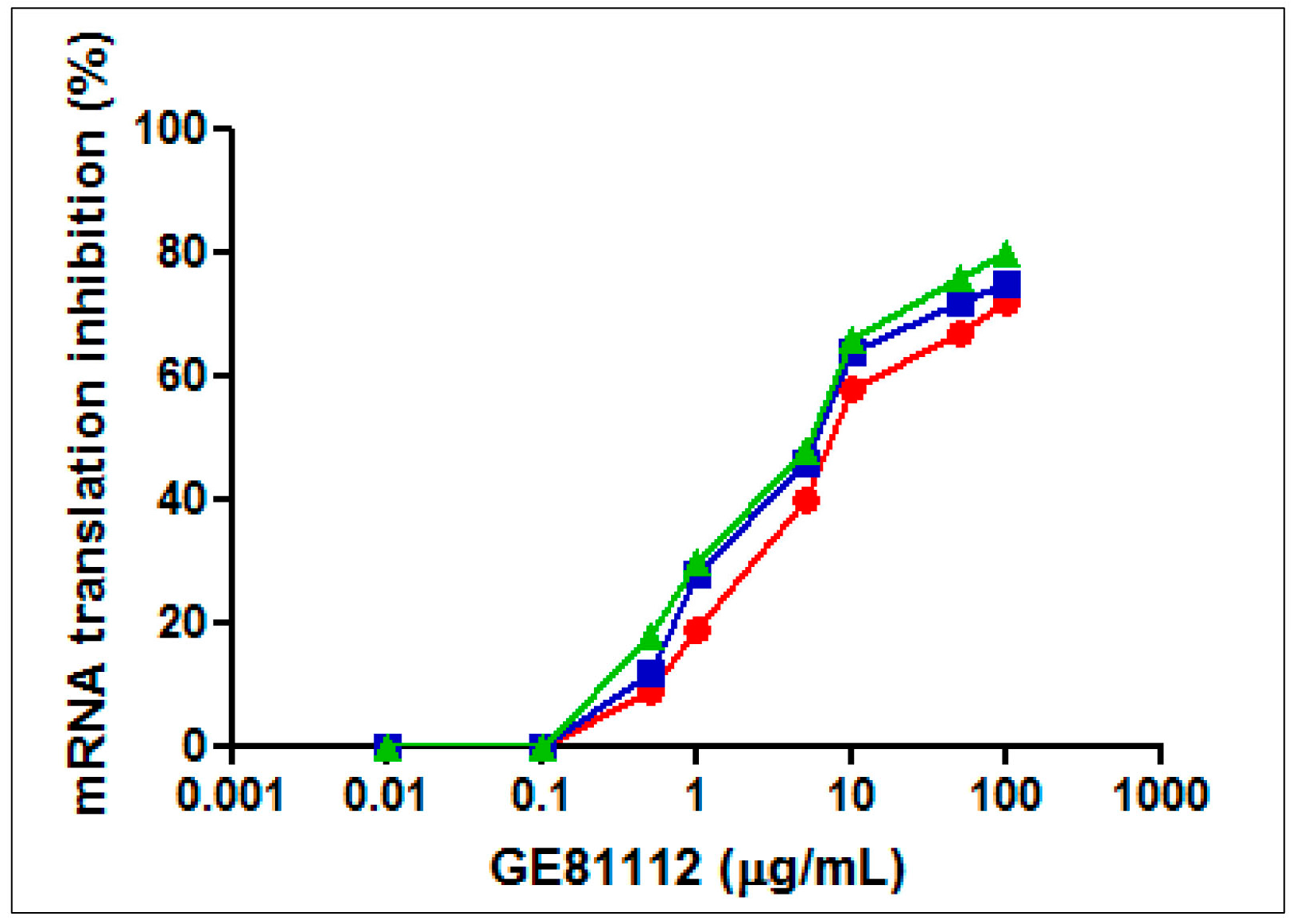
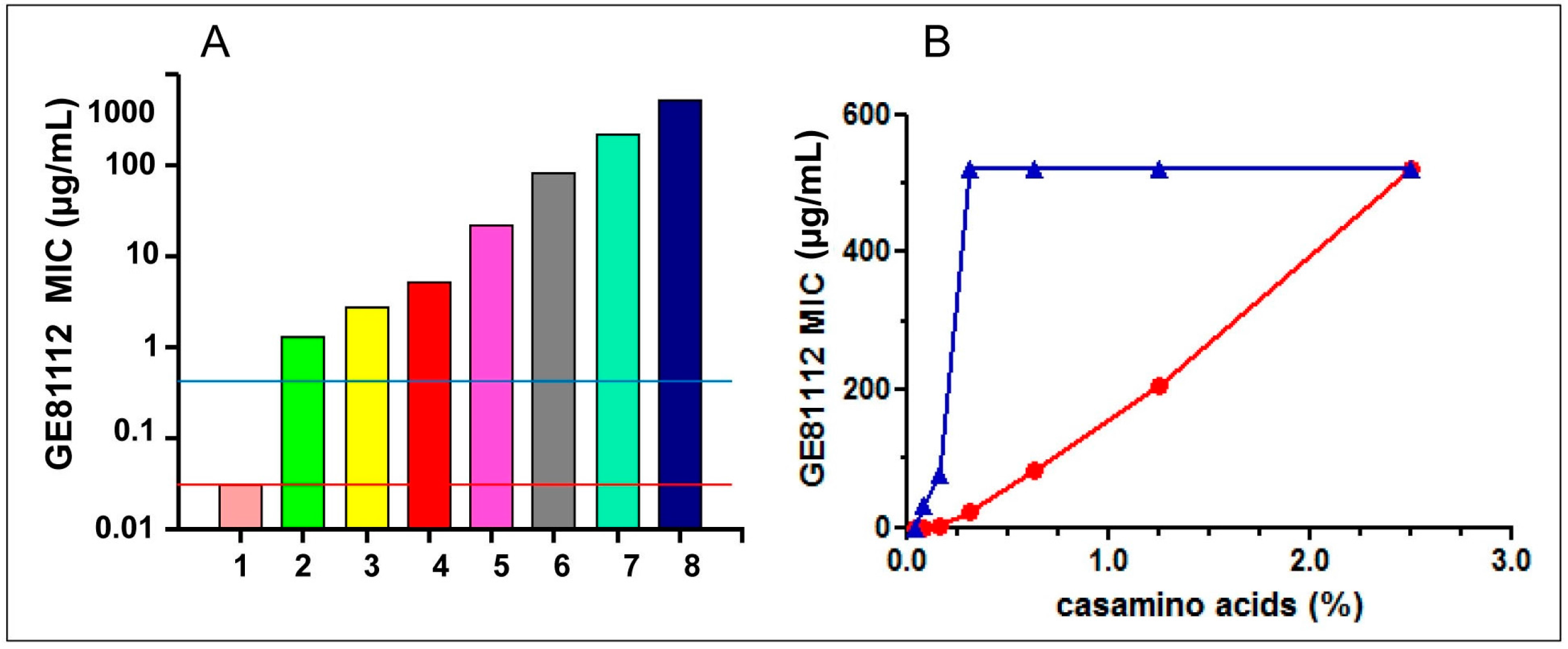

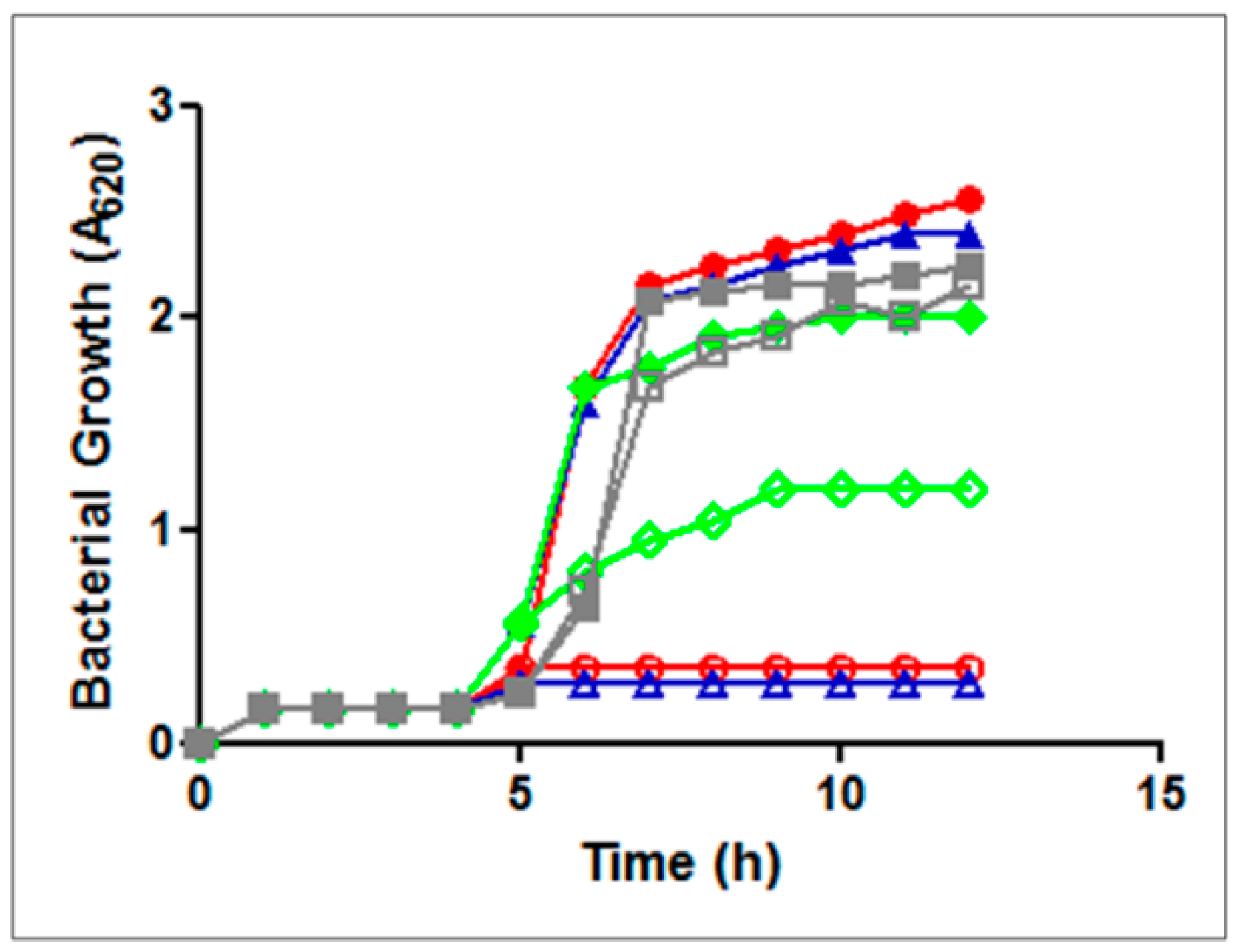
| Bacteria | GE81112 MIC (μg/mL) | |||
|---|---|---|---|---|
| Complete Media | Minimal Media | |||
| Rich | Chemically Defined | Inoculum 104 cfu/mL | Inoculum 106 cfu/mL | |
| Staphylococcus aureus Smith | >1024 b | |||
| Staphylococcus aureus L100 | >512 b | 2–4 | 1f | |
| Staphylococcus haemolyticus metR a | 2 b | |||
| Staphylococcus haemolyticus metS a | 8 b | |||
| Streptococcus pyogenes | >1024 | |||
| Streptococcus pneumoniae | 64 c | |||
| Enterococcus faecalis Van A a | 64 b | |||
| Bacillus subtilis ATCC6633 | >1024 d | 0.125 f | 4 f | |
| Moraxella catarrhalis a | 2 b | |||
| Haemophilus influenzae ATCC 19418 | 512 e | |||
| Escherichia coli MG1655 | >512 b | 2–4 | 0.062 g | 2 g |
| Escherichia coli MHB | 1024 b | |||
| Pseudomonas aeruginosa ATCC1156 a | >512 b | >512 g | ||
| Additions to Minimal Medium | GE81112 MIC (μg/mL) |
|---|---|
| None | 0.030 |
| BSA (2%) | 0.125 |
| FBS (30%) | 8 |
| BSA hydrolysate (2%) | 250 |
| Casein (2%) | 0.25 |
| Casein hydrolysate (2%) | 250 |
| E. coli Strain | Halo of Inhibition (mm) | |||
|---|---|---|---|---|
| GE81112 | Orn3 | |||
| 1 μg | 10 μg | 1 μg | 10 μg | |
| SS320 (wt) | 24 | 32 | 7 | 12 |
| SS5012 (ΔoppABCDF) | 0 | 0 | 0 | 0 |
| SS5012 + pB2 + pBΦ30 | 33 | 43 | 18 | 26 |
| SS5012 + pB2 (oppBCDF) | 0 | 0 | 0 | 0 |
| SS5012 + pBΦ30 (oppA) | 0 | 0 | 0 | 0 |
| SS320 (wt) + pB2 + pBΦ30 | 35 | 41 | 17 | 27 |
| E. coli Strain | Halo of Inhibition (mm) | |
|---|---|---|
| GE81112 (10 μg) | Orn3 (10 μg) | |
| MG1655 | 30 | 10 |
| DH5α | 0 | 0 |
| DH5α + pBΦ30 (oppA) | 32 | 11 |
| DH5α + pB2 (oppBCDF) | 27 | 10 |
| DH5α + pBΦ30 + pB2 | 37 | 14 |
| E. coli AVS6900916S rRNA Mutations | IC50 of mRNA Translation | |
|---|---|---|
| GE81112 (μg/mL) | Kasugamycin (μg/mL) | |
| wt | 2 | 12 |
| A794G | 18 | 780 |
| A794U | 15 | 384 |
| G926A | 32 | 390 |
| G926C | 28 | 192 |
| G926U | 35 | 750 |
| A1518U | 3 | 880 |
| Strain | Genotype | Reference |
|---|---|---|
| E. coli MG1655 | LAM−, rph-1 | [33] |
| E. coli MRE600 | rna | [34,35] |
| E. coli SS320 | F−, lacI22, lacZ, pro-48, met90, trpA, trpR, his-85, rpsL, azi-9, gyrA, λ−, P1s | [17] |
| E. coli SS5012 | Like SS320, but rna Δ(trp-tdk) | [17] |
| E. coli DH5α | Φ80d lacZΔM15, recA1, endA1, gyrA96, thi-1, hsdR17 (rk−, mk+), supE44, relA1, deoR, Δ(lacZYA-argF) U169 | [36] |
| B. subtilis ATCC6633 | ATCC | |
| B. subtilis JH642 | trpC2, phe-1, appA168 | [37] |
| B. subtilis JH12795 | trpC2, phe-1, ΔoppD, ::kan, appA168 | [37] |
| B. subtilis JH14115 | trpC2, phe-1, ::kan, (App+) | [37] |
| B. subtilis JH14116 | trpC2, phe-1, ΔoppD, ::kan, (App+) | [37] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maio, A.; Brandi, L.; Donadio, S.; Gualerzi, C.O. The Oligopeptide Permease Opp Mediates Illicit Transport of the Bacterial P-site Decoding Inhibitor GE81112. Antibiotics 2016, 5, 17. https://doi.org/10.3390/antibiotics5020017
Maio A, Brandi L, Donadio S, Gualerzi CO. The Oligopeptide Permease Opp Mediates Illicit Transport of the Bacterial P-site Decoding Inhibitor GE81112. Antibiotics. 2016; 5(2):17. https://doi.org/10.3390/antibiotics5020017
Chicago/Turabian StyleMaio, Alessandro, Letizia Brandi, Stefano Donadio, and Claudio O. Gualerzi. 2016. "The Oligopeptide Permease Opp Mediates Illicit Transport of the Bacterial P-site Decoding Inhibitor GE81112" Antibiotics 5, no. 2: 17. https://doi.org/10.3390/antibiotics5020017






