Cost Reduction of Inhaled Tobramycin by Use of Preservative-Free Intravenous Tobramycin Given via Inhalation
Abstract
:1. Introduction
| Preservatives | TOBI® | PFIT |
|---|---|---|
| No | No | |
| Packaging | 300 mg tobramycin plus 11.25 mg sodium chloride in 5 mL sterile water per ampoule | 1.2 g power vial |
| Reconstitution | None Required | Under sterile conditions, pharmacy-reconstituted with 30 mL of 0.9% sodium chloride to a concentration of 40 mg tobramycin per mL |
| Dosing | 300 mg (5 mL) inhaled twice daily | 300 mg (7.5 mL) inhaled twice daily, dispensed in an oral syringe with a “for inhalation” auxiliary label |
| Stability | Per labeled package or more than 28 days at room temperature (up to 25° Celsius) | Reconstituted vial has a 24 h stability at room temperature (up to 25° Celsius) and 96 h stability under refrigeration (2° to 8° Celsius). PFIT doses are labeled with a 24-h expiration a |
| Storage | May be stored in ADC | Due to 24-h expiration, limited ADC storage potential |
| Product Name | Manufacturer | AWP Per Dose | AWP Date a |
|---|---|---|---|
| TOBI® | Novartis® | $157.24 | April 2014 |
| Generic inhaled tobramycin | TEVA® | $128.77 | November 2013 |
| BETHKIS® | Cornerstone Therapeutics® | $121.61 | May 2014 |
| PFIT | X-Gen Pharmaceuticals® | $52.50 | June 2012 |
2. Results
2.1. Drug Usage Assessment
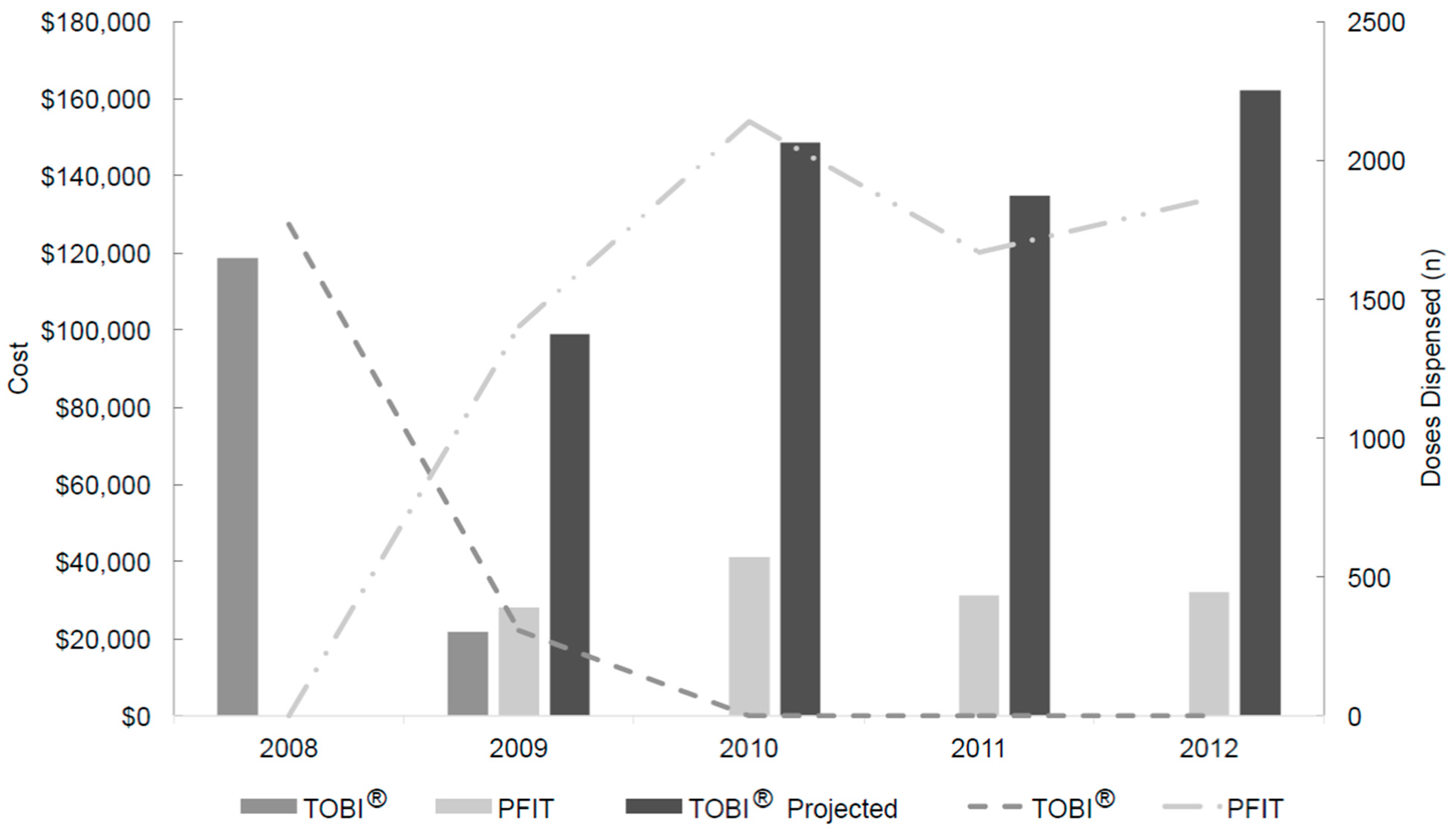
2.2. Workload Evaluation
2.3. Acquisition Drug Costs and Savings Determination
| Product | Production Cost Per Dose ($) a | Pre-Intervention Period (January 2008 to May 2009) | Post-Intervention Period (May 2009 to December 2012) | Production Cost ($) c | Cost Savings ($) | ||
|---|---|---|---|---|---|---|---|
| Doses Dispensed (n) | Drug Cost ($) | Doses Dispensed (n) | Drug Cost ($) b | ||||
| TOBI® | 0 | 2077 | 140,402 (actual) | 0 | 544,497 (projected) | 0 | - |
| PFIT | 5.28 | 0 | 0 (actual) | 7069 | 132,467 (actual) | 37,324 | 374,706 |
2.4. Safety Assessment
3. Discussion
4. Methods
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Dellit, T.H.; Owens, R.C.; McGowan, J.E.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious diseases society of America and the society for healthcare epidemiology of america guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.A.; Bauer, K.A.; Reed, E.E.; Stevenson, K.B.; Taylor, J.J.; West, J.E. Is the “low-hanging fruit” worth picking for antimicrobial stewardship programs? Clin. Infect. Dis. 2012, 55, 587–592. [Google Scholar] [CrossRef] [PubMed]
- TOBI. (Inhaled Tobramycin) Package Insert; Norvartis Pharmaceuticals Corporation: East Hanover, NJ, USA, 2009. [Google Scholar]
- Tobramycin for Injection Package Insert; X-Gen Pharmaceuticals: Northport, NY, USA, 2006.
- United States Pharmacopeia 27. In Chapter <797> Pharmaceutical Compounding-Sterile Preparations; US Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2004; pp. 2350–2370.
- Information For Healthcare Professionals: Colistimethate (Marketed as Coly-Mycin M and Generic Products). USA Food and Drug Administration. Available online: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124896.htm (accessed on 3 December 2015).
- Le, J.; Ashley, E.D.; Neuhauser, M.M.; Brown, J.; Gentry, C.; Klepser, M.E.; Marr, A.M.; Schiller, D.; Schwiesow, J.N.; Tice, S.; et al. Consensus summary of aerosolized antimicrobial agents: Application of guideline criteria. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2010, 30, 562–584. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, J.; Pepe, M.; Williams-Warren, J.; Vasiliev, M.; Montgomery, A.B.; Smith, A.L.; Ramsey, B.W.; Aerosolized Tobramycin Study Group. A comparison of peak sputum tobramycin concentration in patients with cystic fibrosis using jet and ultrasonic nebulizer systems. Chest 1997, 111, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, B.W.; Dorkin, H.L.; Eisenberg, J.D.; Gibson, R.L.; Harwood, I.R.; Kravitz, R.M.; Schidlow, D.V.; Wilmott, R.W.; Astley, S.J.; Mcburnie, M.A.; et al. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. NEJM 1993, 328, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Cooney, G.F.; Lum, B.L.; Tomaselli, M.; Fiel, S.B. Absolute bioavailability and absorption characteristics of aerosolized tobramycin in adults with cystic fibrosis. J. Clin. Pharmacol. 1994, 34, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Nikolaizik, W.H.; Jenni-Galović, V.; Schöni, M.H. Bronchial constriction after nebulized tobramycin preparations and saline in patients with cystic fibrosis. Eur. J. Pediatr. 1996, 155, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Touw, D.J.; Jacobs, F.A.; Brimicombe, R.W.; Heijerman, H.G.; Bakker, W.; Briemer, D.D. Pharmacokinetics of aerosolized tobramycin in adult patients with cystic fibrosis. Antimicrob. Agents Chemother. 1997, 41, 184–187. [Google Scholar] [PubMed]
- Nikolaizik, W.H.; Vietzke, D.; Ratjen, F. A pilot study to compare tobramycin 80 mg injectable preparation with 300 mg solution for inhalation in cystic fibrosis patients. Can. Respir. J. 2008, 15, 259–262. [Google Scholar] [PubMed]
- Ramsey, B.W.; Pepe, M.S.; Quan, J.M.; Otto, K.L.; Montgomery, A.B.; Williams-Warren, J.; Vasiljev-K, M.; Borowitz, D.; Bowman, C.M.; Marshall, B.C.; et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. NEJM 1999, 340, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ramagopal, M.; Lands, L.C. Inhaled tobramycin and bronchial hyperactivity in cystic fibrosis. Pediatr. Pulmonol. 2000, 29, 366–370. [Google Scholar] [CrossRef]
- Gibson, R.L.; Emerson, J.; Mayer-Hamblett, N.; Burns, J.L.; McNamara, S.; Accurso, F.J.; Konstan, M.W.; Chatfield, B.A.; Retsch-Bogart, G.; Waltz, D.A.; et al. Duration of treatment effect after tobramycin solution for inhalation in young children with cystic fibrosis. Pediatr. Pulmonol. 2007, 42, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Hodson, M.E.; Gallagher, C.G.; Govan, J.R. A randomised clinical trial of nebulized tobramycin or colistin in cystic fibrosis. Eur. Respir. J. 2002, 20, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Nikolaizik, W.H.; Trociewicz, K.; Ratjen, F. Bronchial reactions to the inhalation of high-dose tobramycin in cystic fibrosis. Eur. Respir. J. 2002, 20, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Alothman, G.A.; Alsaadi, M.M.; Ho, B.L.; Ho, S.L.; Dupuis, A.; Corey, M.; Coates, A.L. Evaluation of bronchial constriction in children with cystic fibrosis after inhaling two different preparations of tobramycin. Chest 2002, 122, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Drobnic, M.E.; Suñé, P.; Montoro, J.B.; Ferrer, A.; Orriols, R. Inhaled tobramycin in non-cystic fibrosis patients with bronchiectasis and chronic bronchial infection with Pseudomonas aeruginosa. Ann. Pharmacother. 2005, 39, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B.; Kruse, J.A.; Counts, G.W.; Russell, J.A.; Christou, N.V.; Sands, M.L. Double-blind study of endotracheal tobramycin in the treatment of gram-negative bacterial pneumonia. Antimicrob. Agents Chemother. 1990, 34, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Medi-Span® Price Rx® Select; Wolters Kluwer Health: Indianapolis, IN, USA, 2013; Available online: https://pricerx.medispan.com (accessed on 5 November 2014).
- United States Department of Labor, Bureau of Labor Statistics. Occupational Employment and Wages, May 2013: 29-2052 Pharmacy Technicians. Available online: http://www.bls.gov/oes/current/oes292052.htm (accessed on 29 July 2014).
- United States Department of Labor, Bureau of Labor Statistics. Occupational Employment and Wages, May 2013: 29-1051 Pharmacists. Available online: http://www.bls.gov/oes/current/oes291051.htm (accessed on 29 July 2014).
- John, J.F., Jr.; Fishman, N.O. Programmatic role of the infectious diseases physician in controlling antimicrobial costs in the hospital. Clin. Infect. Dis. 1997, 24, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Li, E.; Doloresco, F.; Matusiak, L.; Hunkler, R.J.; Shah, N.D.; Vermeulen, L.C.; Schumock, G.T. Projecting future drug expenditures in U.S. nonfederal hospitals and clinics-2013. Am. J. Health Syst. Pharm. 2013, 70, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Get Smart for Healthcare: Impact of Antibiotic Stewardship Program Interventions on Cost. Available online: http://www.cdc.gov/getsmart/healthcare/support-efforts/asp-int-costs.html (accessed on 21 September 2015).
- Döring, G.; Conway, S.P.; Heijerman, H.G.; Hodson, M.E.; Høiby, N.; Smyth, A.; Touw, D.J. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: A European consensus. Eur. Respir. J. 2000, 16, 749–767. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Labor. Bureau of Labor Statistics Consumer Price Index. Available online: http://www.bls.gov/data/inflation_calculator.htm (accessed on 21 September 2015).
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–345. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gauthier, T.P.; Wasko, J.; Unger, N.R.; Abbo, L.M.; Fernandez, M.; Aragon, L. Cost Reduction of Inhaled Tobramycin by Use of Preservative-Free Intravenous Tobramycin Given via Inhalation. Antibiotics 2016, 5, 2. https://doi.org/10.3390/antibiotics5010002
Gauthier TP, Wasko J, Unger NR, Abbo LM, Fernandez M, Aragon L. Cost Reduction of Inhaled Tobramycin by Use of Preservative-Free Intravenous Tobramycin Given via Inhalation. Antibiotics. 2016; 5(1):2. https://doi.org/10.3390/antibiotics5010002
Chicago/Turabian StyleGauthier, Timothy P., Justin Wasko, Nathan R. Unger, Lilian M. Abbo, Margaret Fernandez, and Laura Aragon. 2016. "Cost Reduction of Inhaled Tobramycin by Use of Preservative-Free Intravenous Tobramycin Given via Inhalation" Antibiotics 5, no. 1: 2. https://doi.org/10.3390/antibiotics5010002
APA StyleGauthier, T. P., Wasko, J., Unger, N. R., Abbo, L. M., Fernandez, M., & Aragon, L. (2016). Cost Reduction of Inhaled Tobramycin by Use of Preservative-Free Intravenous Tobramycin Given via Inhalation. Antibiotics, 5(1), 2. https://doi.org/10.3390/antibiotics5010002





