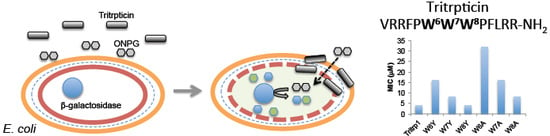
Position-Dependent Influence of the Three Trp Residues on the Membrane Activity of the Antimicrobial Peptide, Tritrpticin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Peptide Design
| Peptide | Sequence | MIC (µM) | MBC (µM) |
|---|---|---|---|
| Tritrp1 | VRRFPWWWPFLRR-NH2 | 4 | 4 |
| Trp-to-Tyr analogs | |||
| W6Y | VRRFPYWWPFLRR-NH2 | 16 | 16 |
| W7Y | VRRFPWYWPFLRR-NH2 | 8 | 8 |
| W8Y | VRRFPWWYPFLRR-NH2 | 4 | 4 |
| W67Y | VRRFPYYWPFLRR-NH2 | 32 | 32 |
| W78Y | VRRFPWYYPFLRR-NH2 | 16 | 16 |
| W68Y | VRRFPYWYPFLRR-NH2 | 16 | 16 |
| Y-Tritrp | VRRFPYYYPFLRR-NH2 | 16–32 | 16–32 |
| Trp-to-Ala analogs | |||
| W6A | VRRFPAWWPFLRR-NH2 | 32–64 | 32–64 |
| W7A | VRRFPWAWPFLRR-NH2 | 16 | 16 |
| W8A | VRRFPWWAPFLRR-NH2 | 8 | 8 |
| W67A | VRRFPAAWPFLRR-NH2 | 64–128 | 64–128 |
| W78A | VRRFPWAAPFLRR-NH2 | 64–128 | 64–128 |
| W68A | VRRFPAWAPFLRR-NH2 | 64–128 | 64–128 |
| A-Tritrp | VRRFPAAAPFLRR-NH2 | >128 | >128 |
2.2. Antibacterial Activity
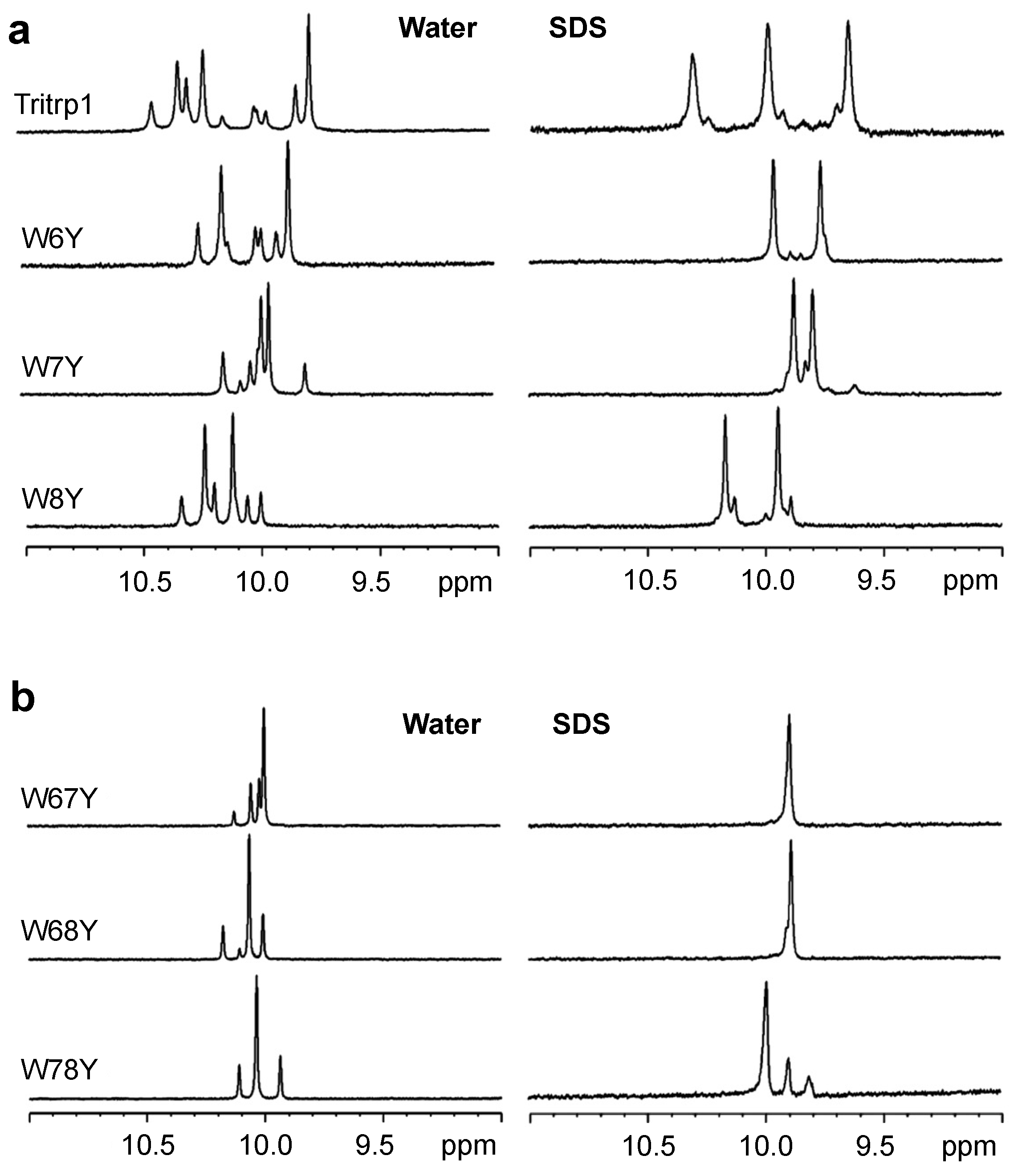
2.3. 1H NMR Spectroscopy

2.4. Tryptophan Fluorescence Spectroscopy
| Peptide | λmax | Blue Shift (nm) | |
|---|---|---|---|
| Buffer | ePC:ePG | ePC:Chol | |
| Tritrp1 | 351 | 14 | 2 |
| W6Y | 353 | 18 | 1 |
| W7Y | 354 | 17 | −1 |
| W8Y | 354 | 18 | 0 |
| W67Y | 354 | 22 | 1 |
| W78Y | 353 | 18 | 0 |
| W68Y | 353 | 23 | 2 |
| W6A | 353 | 19 | 0 |
| W7A | 356 | 16 | 0 |
| W8A | 355 | 20 | 1 |
| W67A | 355 | 17 | 0 |
| W78A | 354 | 18 | 0 |
| W68A | 356 | 16 | −2 |

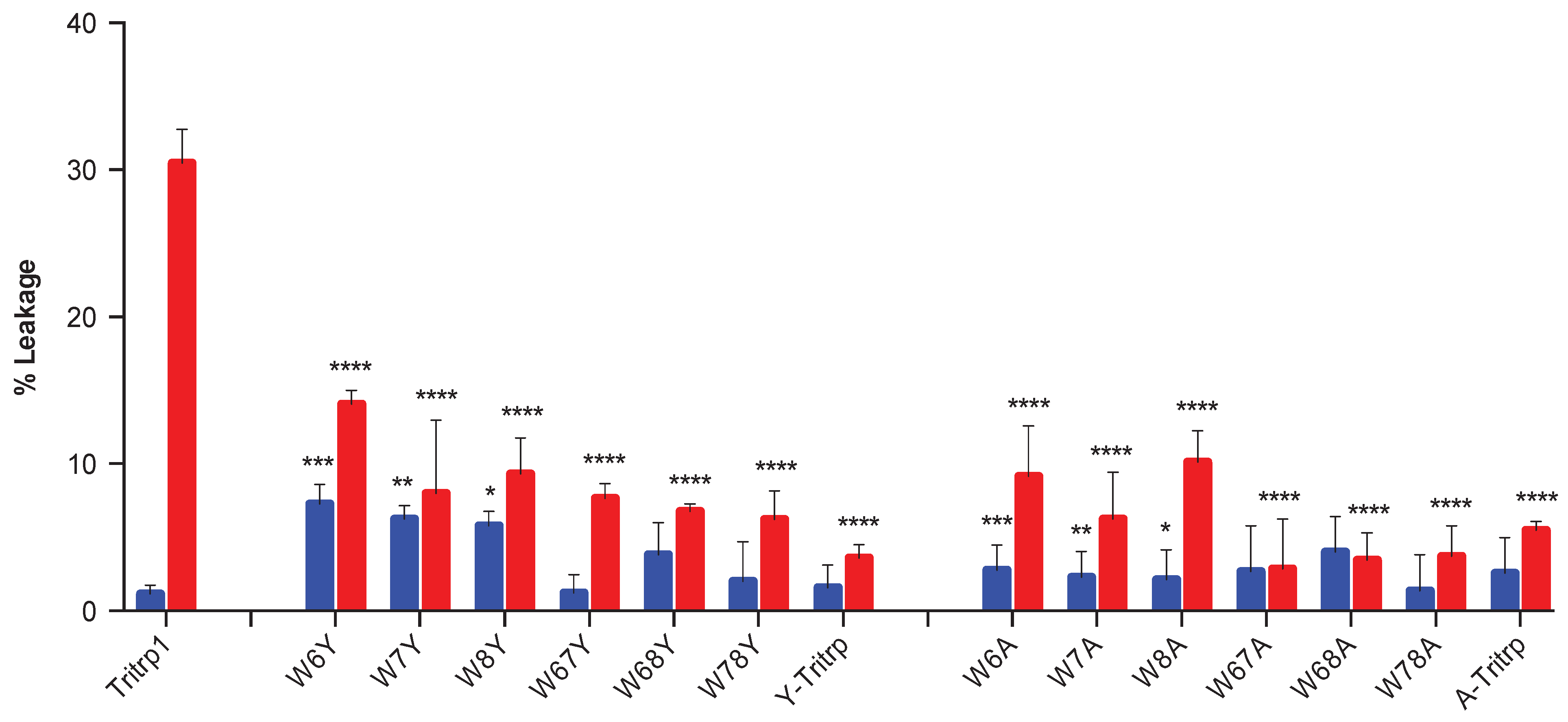
2.5. Calcein Leakage from LUVs



2.6. E. coli Inner Membrane Permeabilization
 ), 1/2 MIC (
), 1/2 MIC (  ), 1/4 MIC (
), 1/4 MIC (  ), 1/8 MIC (
), 1/8 MIC (  ), 1/16 MIC (
), 1/16 MIC (  ), 1/32 MIC (
), 1/32 MIC (  ) and 0 µM (
) and 0 µM (  ). The MIC values for each peptide are derived from Table 1. For peptides with a range of MICs, the higher concentration was used. The results are the average of three independent experiments, and the standard deviation (SD) for two selected peptide concentrations are depicted in Figure 10.
). The MIC values for each peptide are derived from Table 1. For peptides with a range of MICs, the higher concentration was used. The results are the average of three independent experiments, and the standard deviation (SD) for two selected peptide concentrations are depicted in Figure 10.
 ), 1/2 MIC (
), 1/2 MIC (  ), 1/4 MIC (
), 1/4 MIC (  ), 1/8 MIC (
), 1/8 MIC (  ), 1/16 MIC (
), 1/16 MIC (  ), 1/32 MIC (
), 1/32 MIC (  ) and 0 µM (
) and 0 µM (  ). The MIC values for each peptide are derived from Table 1. For peptides with a range of MICs, the higher concentration was used. The results are the average of three independent experiments, and the standard deviation (SD) for two selected peptide concentrations are depicted in Figure 10.
). The MIC values for each peptide are derived from Table 1. For peptides with a range of MICs, the higher concentration was used. The results are the average of three independent experiments, and the standard deviation (SD) for two selected peptide concentrations are depicted in Figure 10.
 ), 1/2 MIC (
), 1/2 MIC (  ), 1/4 MIC (
), 1/4 MIC (  ), 1/8 MIC (
), 1/8 MIC (  ), 1/16 MIC (
), 1/16 MIC (  ), 1/32 MIC (
), 1/32 MIC (  ) and 0 µM (
) and 0 µM (  ). The MIC values for each peptide are derived from Table 1. For peptides with a range of MICs, the higher concentration was used. For A-Tritrp, the highest peptide concentration selected was 128 µM. The results are the average of three independent experiments, and the standard deviation (SD) for two selected peptide concentrations are depicted in Figure 10.
). The MIC values for each peptide are derived from Table 1. For peptides with a range of MICs, the higher concentration was used. For A-Tritrp, the highest peptide concentration selected was 128 µM. The results are the average of three independent experiments, and the standard deviation (SD) for two selected peptide concentrations are depicted in Figure 10.
 ), 1/2 MIC (
), 1/2 MIC (  ), 1/4 MIC (
), 1/4 MIC (  ), 1/8 MIC (
), 1/8 MIC (  ), 1/16 MIC (
), 1/16 MIC (  ), 1/32 MIC (
), 1/32 MIC (  ) and 0 µM (
) and 0 µM (  ). The MIC values for each peptide are derived from Table 1. For peptides with a range of MICs, the higher concentration was used. For A-Tritrp, the highest peptide concentration selected was 128 µM. The results are the average of three independent experiments, and the standard deviation (SD) for two selected peptide concentrations are depicted in Figure 10.
). The MIC values for each peptide are derived from Table 1. For peptides with a range of MICs, the higher concentration was used. For A-Tritrp, the highest peptide concentration selected was 128 µM. The results are the average of three independent experiments, and the standard deviation (SD) for two selected peptide concentrations are depicted in Figure 10.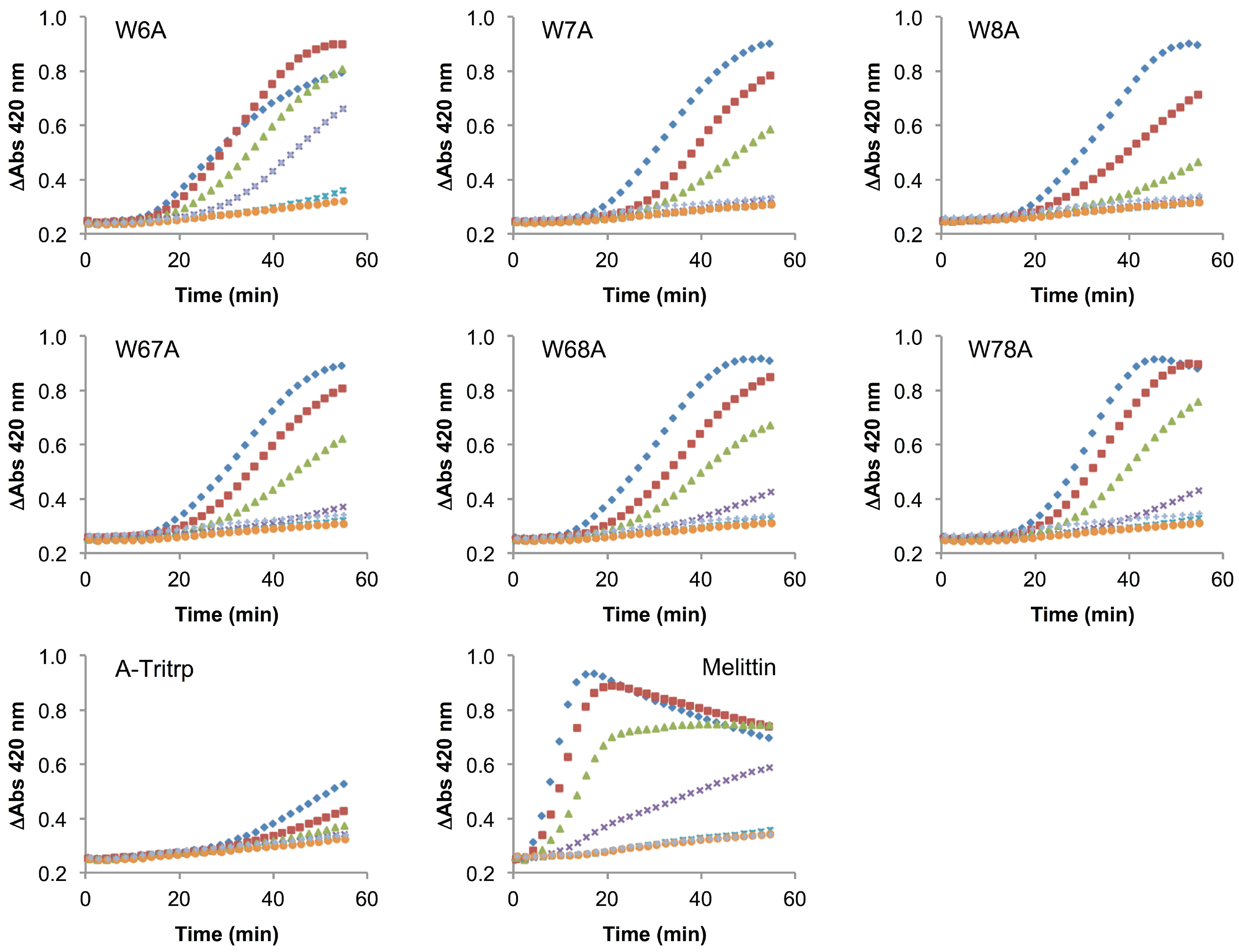

2.7. QSAR Analysis
3. Experimental Section
3.1. Materials, Peptides and Bacterial Strains
3.2. Antibacterial Activity
3.3. 1H Nuclear Magnetic Resonance (NMR) Spectroscopy
3.4. Large Unilamellar Vesicles (LUVs) Preparation
3.5. Tryptophan Fluorescence and Acrylamide Quenching
3.6. Calcein Leakage
3.7. E. coli Inner Membrane Permeabilization
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization antimicrobial resistance global report on surveillance 2014. Available online: http://www.who.int/drugresistance/en/ (accessed on 3 September 2014).
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Steckbeck, J.D.; Deslouches, B.; Montelaro, R.C. Antimicrobial peptides: New drugs for bad bugs? Expert Opin. Biol. Ther. 2014, 14, 11–14. [Google Scholar]
- Hancock, R.E.W.; Lehrer, R. Cationic peptides: A new source of antibiotics. Trends Biotechnol. 1998, 16, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Vogel, H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1999, 1462, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hamill, P.; Hancock, R.E.W. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Steinstraesser, L.; Kraneburg, U.; Jacobsen, F.; Al-Benna, S. Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology 2011, 216, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Hancock, R.E.W. Cationic host defence peptides: Innate immune regulatory peptides as a novel approach for treating infections. Cell. Mol. Life Sci. 2007, 64, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.T.Y.; Gellatly, S.L.; Hancock, R.E.W. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. 2011, 68, 2161–2176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Lu, H.; Li, G.; Huang, Q. LAMP: A database linking antimicrobial peptides. PLoS One 2013, 8, e66557. [Google Scholar] [CrossRef] [PubMed]
- Waghu, F.H.; Gopi, L.; Barai, R.S.; Ramteke, P.; Nizami, B.; Idicula-Thomas, S. CAMP: Collection of sequences and structures of antimicrobial peptides. Nucleic Acids Res. 2014, 42, D1154–D1158. [Google Scholar] [CrossRef] [PubMed]
- Lawyer, C.; Pai, S.; Watabe, M.; Borgia, P.; Mashimo, T.; Eagleton, L.; Watabe, K. Antimicrobial activity of a 13 amino acid tryptophan-rich peptide derived from a putative porcine precursor protein of a novel family of antibacterial peptides. FEBS Lett. 1996, 390, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.I.; Prenner, E.J.; Vogel, H.J. Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biochim. Biophys. Acta 2006, 1758, 1184–1202. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; de Boer, L.; Zaat, S.A.J.; Vogel, H.J. Investigating the cationic side chains of the antimicrobial peptide tritrpticin: Hydrogen bonding properties govern its membrane-disruptive activities. Biochim. Biophys. Acta 2011, 1808, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Mitsuyasu, K.; Soeda, Y.; Hidaka, M.; Ito, Y.; Matsubara, K.; Shindo, M.; Uchida, Y.; Aoyagi, H. Structure-activity relationship of indolicidin, a Trp-rich antibacterial peptide. J. Pept. Sci. 2010, 16, 171–177. [Google Scholar] [PubMed]
- Strøm, M.B.; Rekdal, O.; Svendsen, J.S. Antibacterial activity of 15-residue lactoferricin derivatives. J. Pept. Res. 2000, 56, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Strøm, M.B.; Rekdal, O.; Svendsen, J.S. Antimicrobial activity of short arginine- and tryptophan-rich peptides. J. Pept. Sci. 2002, 8, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Shin, S.Y.; Kim, Y.C.; Kim, Y.; Hahm, K.S.; Kim, J. Il Conformation-dependent antibiotic activity of tritrpticin, a cathelicidin-derived antimicrobial peptide. Biochem. Biophys. Res. Commun. 2002, 296, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Schibli, D.J.; Nguyen, L.T.; Kernaghan, S.D.; Rekdal, Ø.; Vogel, H.J. Structure-function analysis of tritrpticin analogs: Potential relationships between antimicrobial activities, model membrane interactions, and their micelle-bound NMR structures. Biophys. J. 2006, 91, 4413–4426. [Google Scholar] [CrossRef]
- Yang, S.T.; Shin, S.Y.; Hahm, K.S.; Kim, J.I. Different modes in antibiotic action of tritrpticin analogs, cathelicidin-derived Trp-rich and Pro/Arg-rich peptides. Biochim. Biophys. Acta 2006, 1758, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Jensen, K.V; Nguyen, L.T.; Storey, D.G.; Vogel, H.J. Hydroxy-tryptophan containing derivatives of tritrpticin: Modification of antimicrobial activity and membrane interactions. Biochim. Biophys. Acta 2014. [Google Scholar] [CrossRef]
- Selsted, M.E.; Novotny, M.J.; Morris, W.L.; Tang, Y.; Smith, W.; Cullor, J.S. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J. Biol. Chem. 1992, 267, 4292–4295. [Google Scholar] [PubMed]
- Yau, W.M.; Wimley, W.C.; Gawrisch, K.; White, S.H. The preference of tryptophan for membrane interfaces. Biochemistry 1998, 37, 14713–14718. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C.; White, S.H. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat. Struct. Biol. 1996, 3, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Staubitz, P.; Peschel, A.; Nieuwenhuizen, W.F.; Otto, M.; Jung, G.; Jack, R.W.; Gotz, F. Structure-function relationships in the tryptophan-rich, antimicrobial peptide indolicidin. J. Pept. Sci. 2001, 564, 552–564. [Google Scholar] [CrossRef]
- Dathe, M.; Nikolenko, H.; Klose, J.; Bienert, M. Cyclization increases the antimicrobial activity and selectivity of arginine- and tryptophan-containing hexapeptides. Biochemistry 2004, 43, 9140–9150. [Google Scholar] [CrossRef] [PubMed]
- Strøm, M.B.; Haug, B.E.; Rekdal, O.; Skar, M.L; Stensen, W.; Svendsen, J.S. Important structural features of 15-residue lactoferricin derivatives and methods for improvement of antimicrobial activity. Biochem. Cell. Biol. 2002, 80, 65–74. [Google Scholar] [CrossRef]
- Pellegrini, A.; Thomas, U.; Bramaz, N.; Klauser, S.; Hunziker, P.; von Fellenberg, R. Identification and isolation of a bactericidal domain in chicken egg white lysozyme. J. Appl. Microbiol. 1997, 82, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.A.; McDaniel, R.V.; McIntosh, T.J. Interaction of benzene with micelles and bilayers. J. Phys. Chem. 1982, 86, 1449–1456. [Google Scholar] [CrossRef]
- Eilers, M.; Patel, A.B.; Liu, W.; Smith, S.O. Comparison of helix interactions in membrane and soluble alpha-bundle proteins. Biophys. J. 2002, 82, 2720–2736. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.M.; Vogel, H.J. Structure-function relationships of antimicrobial peptides. Biochem. Cell Biol. 1998, 76, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Schibli, D.J.; Hwang, P.M.; Vogel, H.J. Structure of the antimicrobial peptide tritrpticin bound to micelles: A distinct membrane-bound peptide fold. Biochemistry 1999, 38, 16749–16755. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Hunter, H.N.; Matsuzaki, K.; Vogel, H.J. Solution NMR studies of amphibian antimicrobial peptides: Linking structure to function? Biochim. Biophys. Acta 2009, 1788, 1639–1655. [Google Scholar] [CrossRef]
- Rozek, A.; Friedrich, C.L.; Hancock, R.E. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemistry 2000, 39, 15765–15774. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, E.; Ulrich, A.S. NMR methods for studying membrane-active antimicrobial peptides. Concepts Magn. Reson. Part A 2004, 23A, 89–120. [Google Scholar] [CrossRef]
- Henry, B.G.D.; Sykes, B.D. Methods to study membrane protein structure in solution. Methods Enzymol. 1994, 239, 515–535. [Google Scholar] [PubMed]
- Warschawski, D.E.; Arnold, A.A.; Beaugrand, M.; Gravel, A.; Chartrand, É.; Marcotte, I. Choosing membrane mimetics for NMR structural studies of transmembrane proteins. Biochim. Biophys. Acta 2011, 1808, 1957–1974. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, L.W.; da Silva, A., Jr.; Leite, A.; Valente, A.P.; Almeida, F.C. NMR structure of PW2 bound to SDS micelles. A tryptophan-rich anticoccidial peptide selected from phage display libraries. J. Biol. Chem. 2002, 277, 36351–36356. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J. Principles of Fluorescence Spectroscopy, 2nd ed.; Lakowicz, J., Ed.; Kluwer Academic/Plenum: New York, NY, USA, 1999; p. 533. [Google Scholar]
- Lohner, K.; Sevcsik, E.; Pabst, G. Liposome-based biomembrane mimetic systems: Implications for lipid-peptide interactions. Adv. Planar Lipid Bilayers Liposomes 2008, 6, 103–137. [Google Scholar]
- Eftink, M.R.; Ghiron, C.A. Fluorescence quenching studies with proteins. Anal. Biochem. 1981, 114, 199–227. [Google Scholar] [CrossRef] [PubMed]
- Eftink, M.R.; Ghiron, C.A. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry 1976, 15, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Salay, L.C.; Procopio, J.; Oliveira, E.; Nakaie, C.R.; Schreier, S. Ion channel-like activity of the antimicrobial peptide tritrpticin in planar lipid bilayers. FEBS Lett. 2004, 565, 171–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haney, E.F.; Nguyen, L.T.; Schibli, D.J.; Vogel, H.J. Design of a novel tryptophan-rich membrane-active antimicrobial peptide from the membrane-proximal region of the HIV glycoprotein, gp41. Beilstein J. Org. Chem. 2012, 8, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Brender, J.R.; McHenry, A.J.; Ramamoorthy, A. Does cholesterol play a role in the bacterial selectivity of antimicrobial peptides? Front. Immunol. 2012, 3, e195. [Google Scholar]
- Epand, R.F.; Pollard, J.E.; Wright, J.O.; Savage, P.B.; Epand, R.M. Depolarization, bacterial membrane composition, and the antimicrobial action of ceragenins. Antimicrob. Agents Chemother. 2010, 54, 3708–3713. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, C.E. The actions of melittin on membranes. Biochim. Biophys. Acta 1990, 1031, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Raghuraman, H.; Chattopadhyay, A. Melittin: A membrane-active peptide with diverse functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef] [PubMed]
- Rekdal, Ø.; Haug, B.E.; Kalaaji, M.; Hunter, H.N.; Lindin, I.; Israelsson, I.; Solstad, T.; Yang, N.; Brandl, M.; Mantzilas, D.; Vogel, H.J. Relative spatial positions of tryptophan and cationic residues in helical membrane-active peptides determine their cytotoxicity. J. Biol. Chem. 2012, 287, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Lejon, T.; Strøm, M.B.; Svendsen, J.S. Antibiotic activity of pentadecapeptides modelled from amino acid descriptors. J. Pept. Sci. 2001, 7, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lejon, T.; Svendsen, J.S.; Haug, B.E. Simple parameterization of non-proteinogenic amino acids for QSAR of antibacterial peptides. J. Pept. Sci. 2002, 8, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Lejon, T.; Stiberg, T.; Strøm, M.B.; Svendsen, J.S. Prediction of antibiotic activity and synthesis of new pentadecapeptides based on lactoferricins. J. Pept. Sci. 2004, 10, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Svendsen, J.S.; Vogel, H.J. Comparison of NMR structures and model-membrane interactions of 15-residue antimicrobial peptides derived from bovine lactoferricin. Biochem. Cell Biol. 2006, 84, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Demcoe, A.R.; Vogel, H.J. Conformation of a bactericidal domain of Puroindoline a: Structure and mechanism of action of a 13-residue antimicrobial peptide. J. Bacteriol. 2003, 185, 4938–4947. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, N.; Nagaraj, R. Interaction of antimicrobial peptides with biological and model membranes: Structural and charge requirements for activity. Biochim. Biophys. Acta 1999, 1462, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Petersen, A.P.; Lau, C.K.; Jing, W.; Storey, D.G.; Vogel, H.J. Mechanism of action of puroindoline derived tryptophan-rich antimicrobial peptides. Biochim. Biophys. Acta 2013, 1828, 1802–1813. [Google Scholar] [CrossRef] [PubMed]
- Falla, T.J.; Karunaratne, D.N.; Hancock, R.E.W. Mode of action of the antimicrobial peptide indolicidin. J. Biol. Chem. 1996, 271, 19298–19303. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Neufeld, E.F.; Ginsberg, V. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1966, 8, 115–118. [Google Scholar]
- GraphPad Prism 6.0, GraphPad Software Inc.: La Jolla, CA, USA, 2014.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, M.; Nguyen, L.T.; Kuczynski, A.M.; Lejon, T.; Vogel, H.J. Position-Dependent Influence of the Three Trp Residues on the Membrane Activity of the Antimicrobial Peptide, Tritrpticin. Antibiotics 2014, 3, 595-616. https://doi.org/10.3390/antibiotics3040595
Arias M, Nguyen LT, Kuczynski AM, Lejon T, Vogel HJ. Position-Dependent Influence of the Three Trp Residues on the Membrane Activity of the Antimicrobial Peptide, Tritrpticin. Antibiotics. 2014; 3(4):595-616. https://doi.org/10.3390/antibiotics3040595
Chicago/Turabian StyleArias, Mauricio, Leonard T. Nguyen, Andrea M. Kuczynski, Tore Lejon, and Hans J. Vogel. 2014. "Position-Dependent Influence of the Three Trp Residues on the Membrane Activity of the Antimicrobial Peptide, Tritrpticin" Antibiotics 3, no. 4: 595-616. https://doi.org/10.3390/antibiotics3040595