Anti-Adhesion Activity of A2-type Proanthocyanidins (a Cranberry Major Component) on Uropathogenic E. coli and P. mirabilis Strains
Abstract
:1. Introduction
2. Results and Discussion
2.1. Adhesion Assay
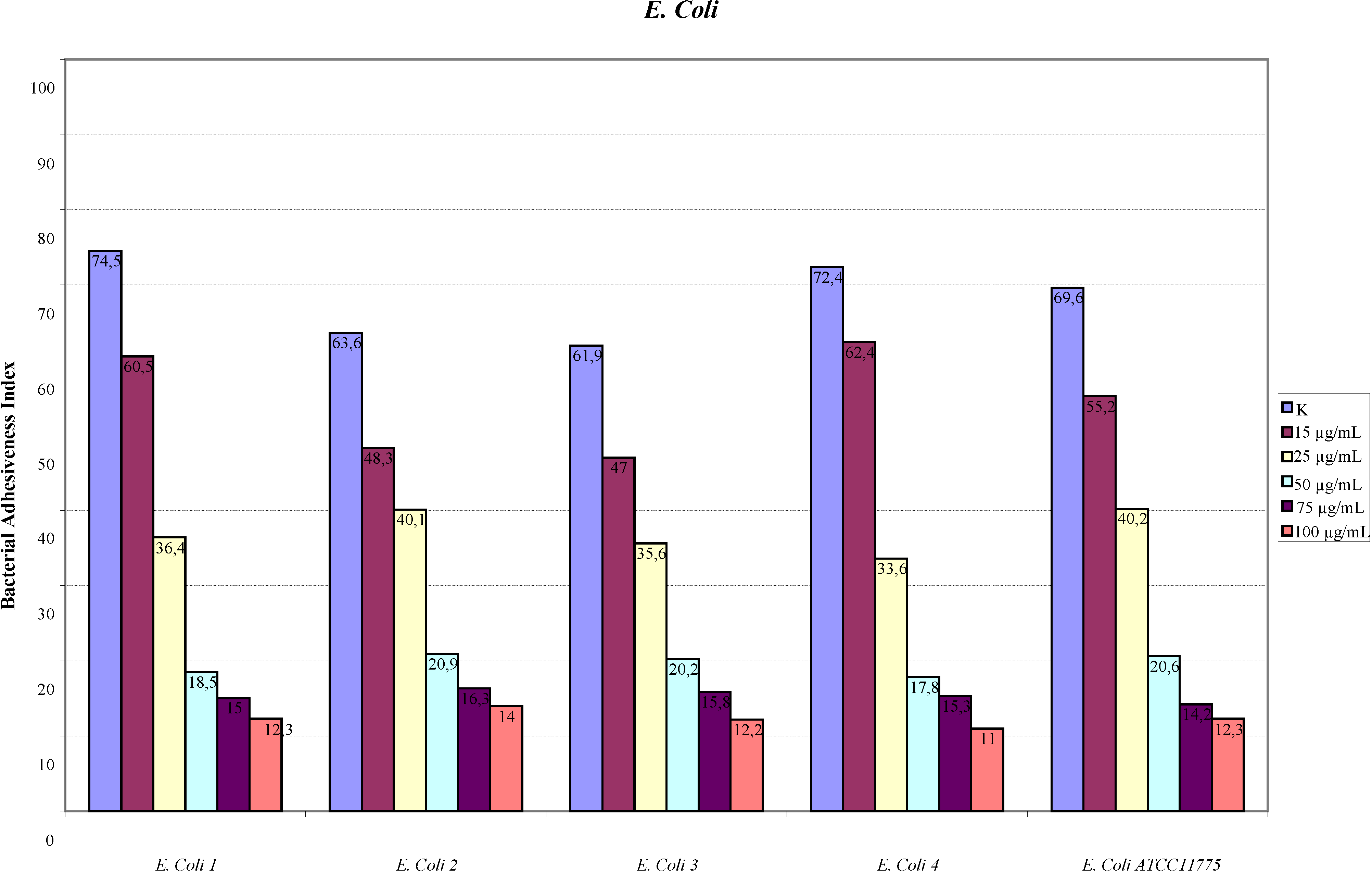
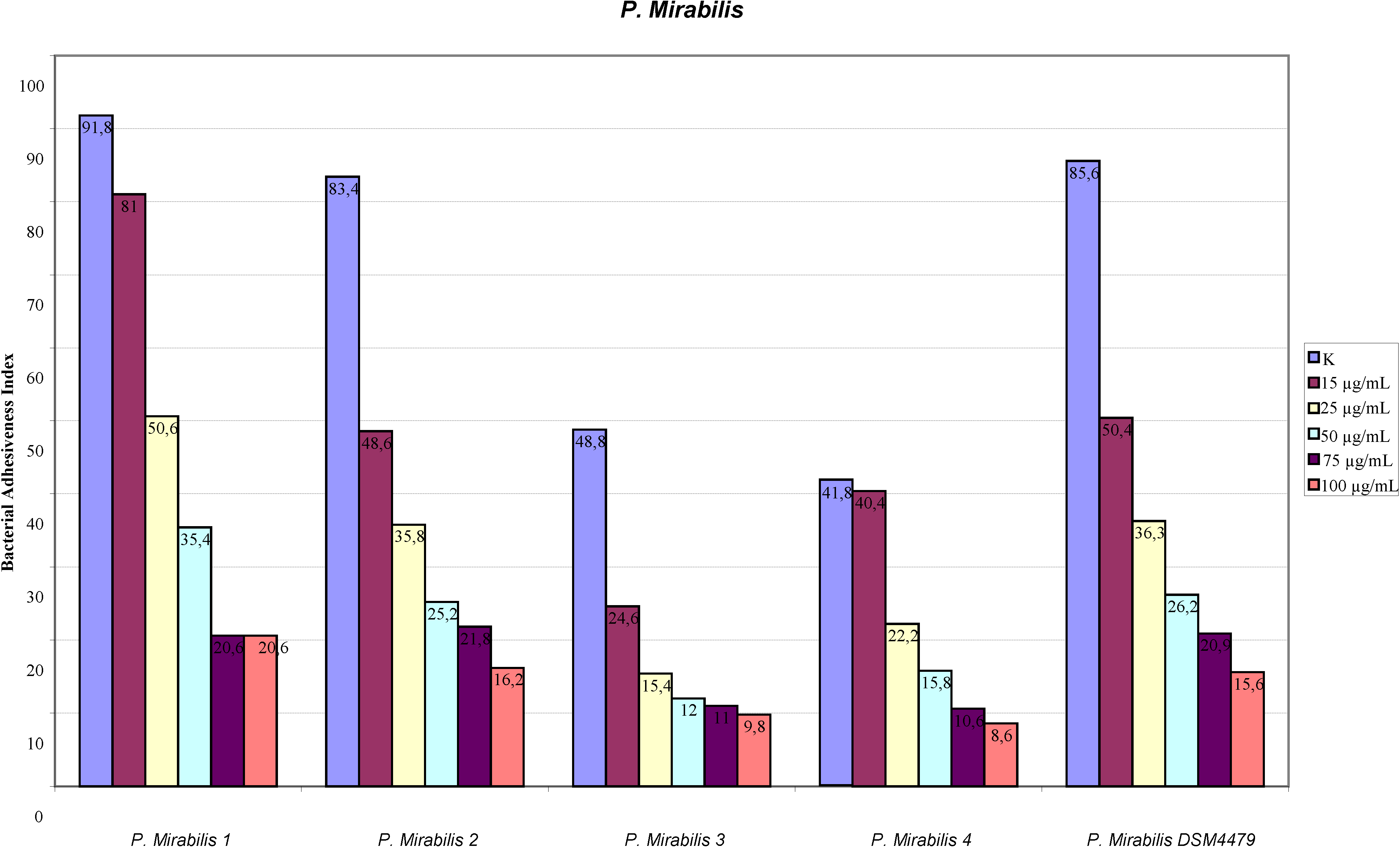
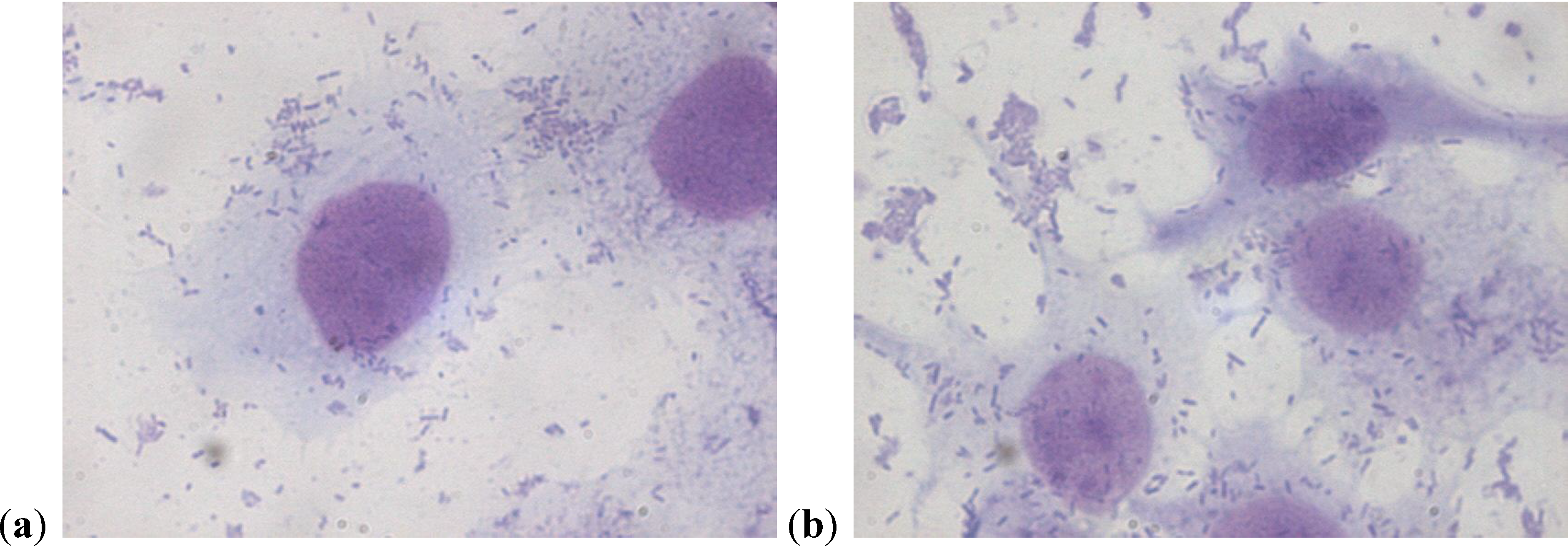

2.2. Scanning Electron Microscopy
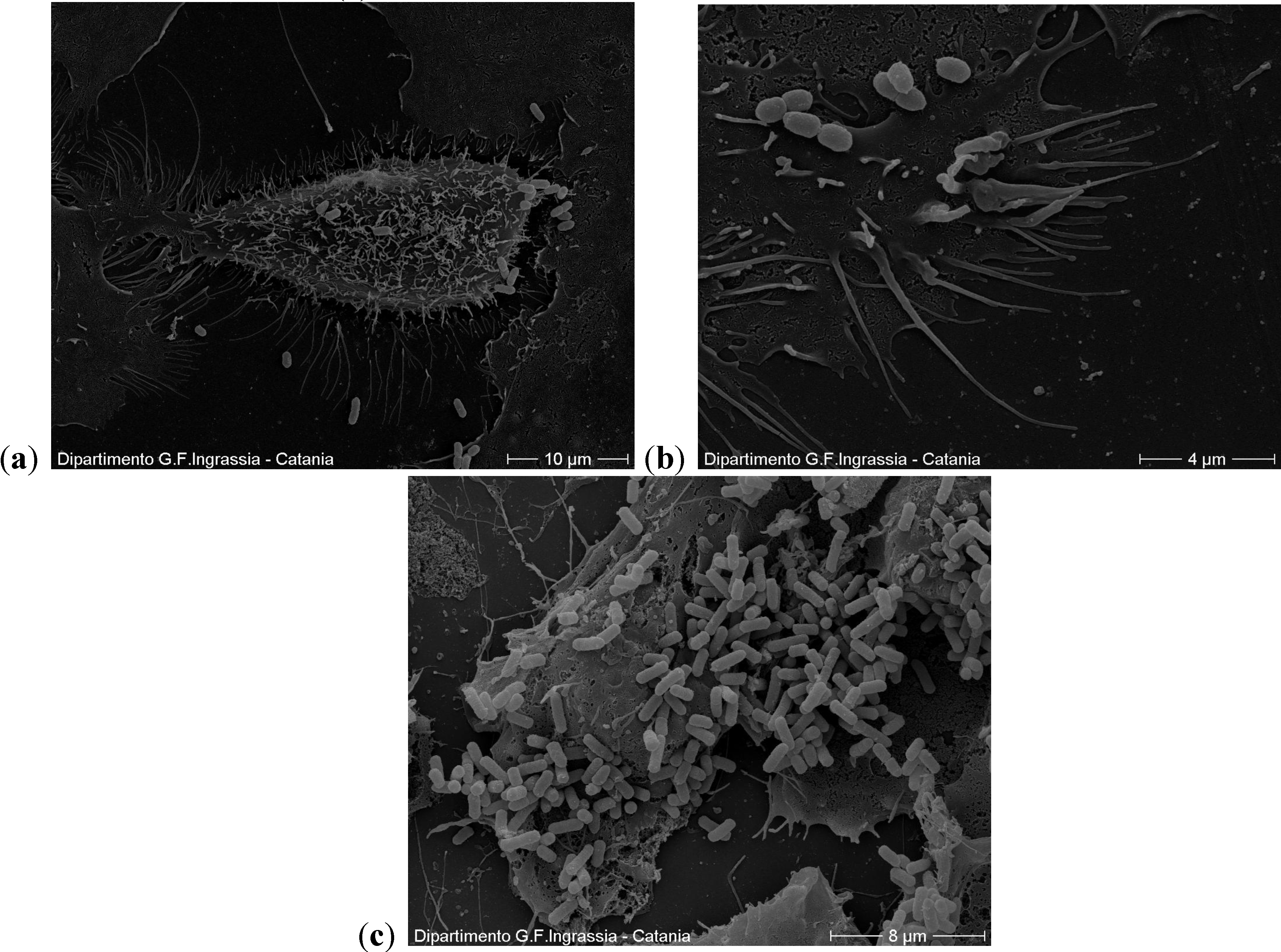
2.3. Motility Test
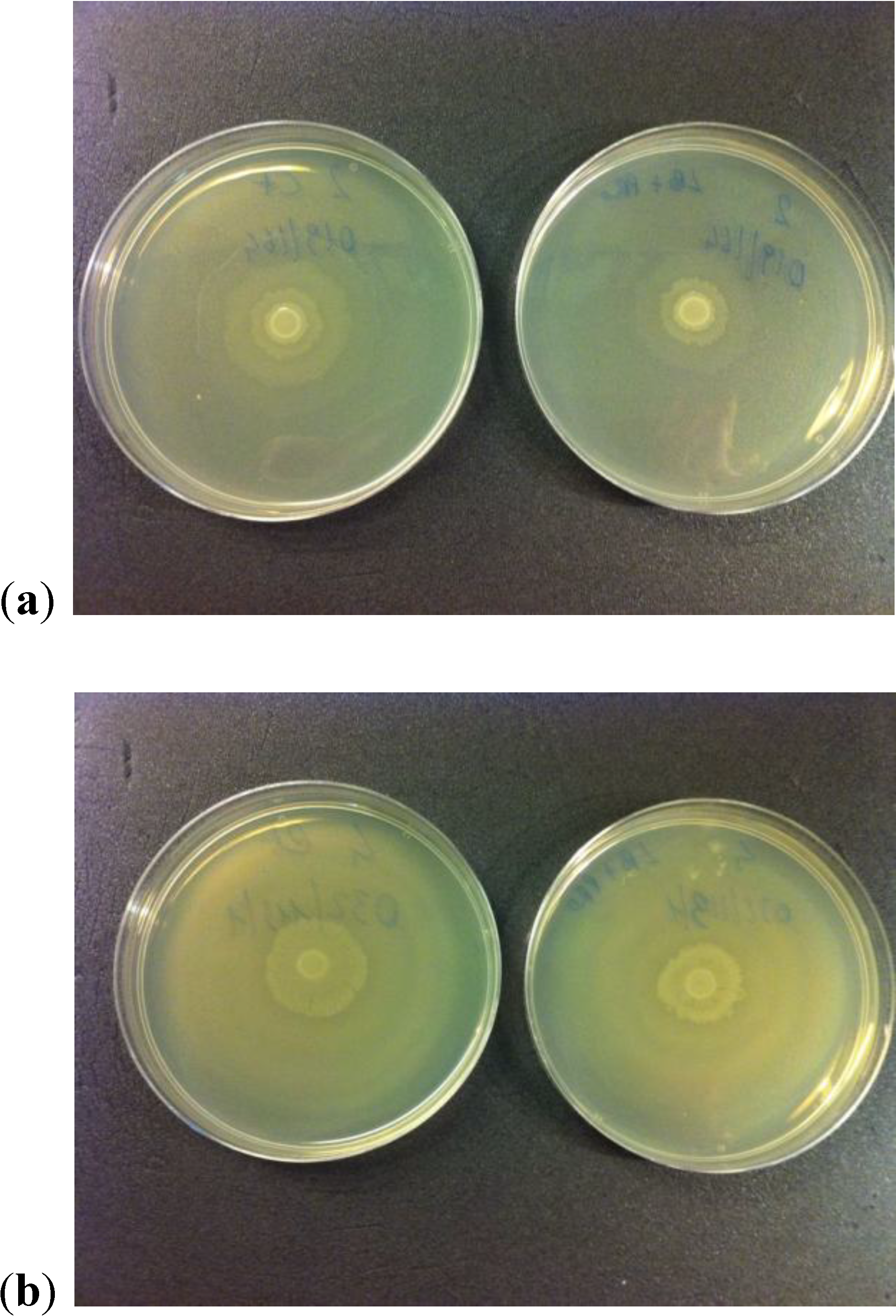
2.4. Production of Urease
2.5. Discussion
3. Experimental
3.1. Bacterial Strains
3.2. Adhesion Assay
3.3. Scanning Electron Microscopy
3.4. Motility Test
3.5. Production of Urease
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic cost. Am. J. Med. 2002, 113, 5–13. [Google Scholar] [CrossRef]
- Foxman, B. Recurring urinary tract infection: incidence and risk factors. Am. J. Public Health 1990, 80, 331–333. [Google Scholar] [CrossRef]
- Foxman, B.; Gillespie, B.; Koopman, J.; Zhang, L.; Palin, K.; Tallman, P.; Marsch, J.V.; Spear, S.; Sobel, J.D.; Marty, M.J.; et al. Risk factors for second urinary tract infection among college women. Am. J. Epidemiol. 2000, 151, 1194–1205. [Google Scholar] [CrossRef]
- Stamm, W.E. Estrogens and urinary tract infection. J. Infect. Dis. 2007, 195, 623–624. [Google Scholar] [CrossRef]
- Grabe, M.; Bjerklund-Johansen, T.E.; Botto, H.; Çek, M.; Naber, K.G.; Pickard, R.S.; Tenke, P.; Wagenlehner, F.; Wullt, B. Guidelines on Urological Infections. European Association of Urology 2013. Available online: http://www.uroweb.org/gls/pdf/18_Urological%20infections_LR.pdf (accessed on 28 March 2014).
- Lichtenberger, P.; Hooton, T.M. Antimicrobial prophylaxis in women with recurrent urinary tract infections. Int. J. Antimicrob. Agents 2011, 38, 36–41. [Google Scholar] [CrossRef]
- Rozalski, A.; Sidorczyk, Z.; Kotelko, K. Potential virulence factors of Proteus bacilli. Microbiol. Mol. Biol. Rev. 1997, 61, 65–98. [Google Scholar]
- Zunino, P.; Sosa, V.; Schlapp, G.; Allen, A.G.; Preston, A.; Maskell, D.J. Mannose-resistant Proteus-like and P. mirabilis fimbriae have specific and additive roles in P. mirabilis urinary tract infections. FEMS Immunol. Med. Microbiol. 2007, 51, 125–133. [Google Scholar] [CrossRef]
- Zunino, P.; Sosa, V.; Allen, A.G.; Preston, A.; Schlapp, G.; Maskell, D.J. Proteus mirabilis fimbriae (PMF) are important for both bladder and kidney colonization in mice. Microbiology 2003, 149, 3231–3237. [Google Scholar] [CrossRef]
- Johnson, D.E.; Russell, R.G.; Lockatell, C.V.; Zulty, J.C.; Warren, J.W.; Mobley, H.L. Contribution of Proteus mirabilis urease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect. Immun. 1993, 61, 2748–2754. [Google Scholar]
- Rocha, S.P.; Pelayo, J.S.; Elias, W.P. Fimbriae of uropathogenic Proteus mirabilis. FEMS Immunol. Med. Microbiol. 2007, 51, 1–7. [Google Scholar] [CrossRef]
- Sareneva, T.; Holthofer, H.; Korhonen, T.K. Tissue-binding affinity of Proteus mirabilis fimbriae in the human urinary tract. Infect. Immun. 1990, 58, 3330–3336. [Google Scholar]
- Tempera, G.; Corsello, S.; Genovese, C; Caruso, F.E.; Nicolosi, D. Inhibitory activity of cranberry extract on the bacterial adhesiveness in the urine of women: An ex-vivo study. Int. J. Immunopathol. Pharmacol. 2010, 23, 611–618. [Google Scholar]
- Wang, C.H.; Fong, C.C.; Chen, N.C.; Liu, S.S.; You, P.H.; Wu, T.Y.; Chen, W.T.; Lee, C.C.; Chen, S.C. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: A systematic review and meta-analysis of randomized controlled trials. Arch. Intern. Med. 2012, 172, 988–996. [Google Scholar]
- Nowack, R.; Schmitt, W. Cranberry juice for prophylaxis of urinary tract infections—Conclusionsfrom clinical experience and research. Phytomedicine 2008, 15, 653–667. [Google Scholar] [CrossRef]
- Howell, A.B. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol. Nutr. Food Res. 2007, 51, 732–737. [Google Scholar] [CrossRef]
- Raz, R.B.; Chazan, B.; Dan, M. Cranberry juice and urinary tract infection. Clin. Infect. Dis. 2004, 38, 1413–1419. [Google Scholar] [CrossRef]
- Jepson, R.G.; Craig, J.C. Cranberries for preventing urinary tract infections. Cochrane Database Syst. Rev. 2008, 1, CD001321. [Google Scholar]
- Ermel, G.; Georgeault, S.; Inisan, C.; Besnard, M. Inhibition of adhesion of uropathogenic Escherichia coli bacteria to uroepithelial cells by extracts from cranberry. J. Med. Food 2012, 15, 126–134. [Google Scholar] [CrossRef]
- Manos, J.; Belas, R. The genera Proteus, Providencia, and Morganella. In The Prokaryotes, 3rd ed.; Dworkin, S.F.M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 6, pp. 245–269. [Google Scholar]
- McCall, J.; Hidalgo, G.; Asadishad, B.; Tufenkji, N. Cranberry impairs selected behaviors essential for virulence in Proteus mirabilis HI4320. Can. J. Microbiol. 2013, 59, 430–436. [Google Scholar] [CrossRef]
- Griffith, D.P.; Musher, D.M.; Itin, C. Urease. The primary cause of infection-induced urinary stones. Invest. Urol. 1976, 13, 346–350. [Google Scholar]
- Mobley, H.L.T.; Warren, J.M. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J. Clin. Microbiol. 1987, 25, 2216–2217. [Google Scholar]
- Di Martino, P.; Agniel, R.; David, K.; Templer, C.; Gaillard, J.L.; Denys, P.; Botto, H. Reduction of Escherichia coli adherence to uroepithelial bladder cells after consumption of cranberry juice: A double-blind randomized placebo-controlled cross-over trial. World J. Urol. 2006, 24, 21–27. [Google Scholar]
- Nicolosi, D.; Scalia, M.; Nicolosi, V.M.; Pignatello, R. Encapsulation in fusogenic liposomesbroadens the spectrum of action of vancomycin against Gram-negative bacteria. Int. J. Antimicrob. Agents 2010, 35, 553–558. [Google Scholar] [CrossRef]
- Muñoz‐Criado, S.; Muñoz‐Bellido, J.L.; Alonso‐Manzanares, M.A.; Gutiérrez‐Zufiaurre, M.N.; García‐Rodríguez, J.A. Psychotropic drugs inhibit swarming in Proteus spp. and related genera. Clin. Microbiol. Infect. 1998, 4, 447–449. [Google Scholar] [CrossRef]
- Konieczna, I.; Żarnowiec, P.; Kwinkowski, M.; Kolesińska, B.; Frączyk, J.; Kamiński, Z.; Kaca, W. Bacterial urease and its role in long-lasting human diseases. Curr. Protein Pept. Sci. 2012, 13, 789–806. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. A-Type Proanthocyanidin Trimers from Cranberry that Inhibit Adherence of Uropathogenic P-Fimbriated Escherichia coli. J. Nat. Prod. 2000, 63, 1225–1228. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nicolosi, D.; Tempera, G.; Genovese, C.; Furneri, P.M. Anti-Adhesion Activity of A2-type Proanthocyanidins (a Cranberry Major Component) on Uropathogenic E. coli and P. mirabilis Strains. Antibiotics 2014, 3, 143-154. https://doi.org/10.3390/antibiotics3020143
Nicolosi D, Tempera G, Genovese C, Furneri PM. Anti-Adhesion Activity of A2-type Proanthocyanidins (a Cranberry Major Component) on Uropathogenic E. coli and P. mirabilis Strains. Antibiotics. 2014; 3(2):143-154. https://doi.org/10.3390/antibiotics3020143
Chicago/Turabian StyleNicolosi, Daria, Gianna Tempera, Carlo Genovese, and Pio M. Furneri. 2014. "Anti-Adhesion Activity of A2-type Proanthocyanidins (a Cranberry Major Component) on Uropathogenic E. coli and P. mirabilis Strains" Antibiotics 3, no. 2: 143-154. https://doi.org/10.3390/antibiotics3020143
APA StyleNicolosi, D., Tempera, G., Genovese, C., & Furneri, P. M. (2014). Anti-Adhesion Activity of A2-type Proanthocyanidins (a Cranberry Major Component) on Uropathogenic E. coli and P. mirabilis Strains. Antibiotics, 3(2), 143-154. https://doi.org/10.3390/antibiotics3020143





