Designing Safer and Greener Antibiotics
Abstract
:1. Introduction
- (1)
- The effect on the environment due to the synthetic route selected (number of steps, toxicity of reagents, and treatment of waste). i.e., a shorter, cleaner and greener synthesis is preferred.
- (2)
- Inclusion of impact on the environment studies and lifecycle assessment as part of the drug development process. This should preferably avoid the selection of compounds expected to have a major adverse effect on the environment.
- (3)
- A comprehensive evaluation of the toxicity/biodegradation and possible persistence problem of the antibiotic in the environment. This review will focus on the second factor, with the first and third points included for context rather than a review.
2. Antibiotics
3. Penicillin: The First Drug Casualty to Bacterial Resistance
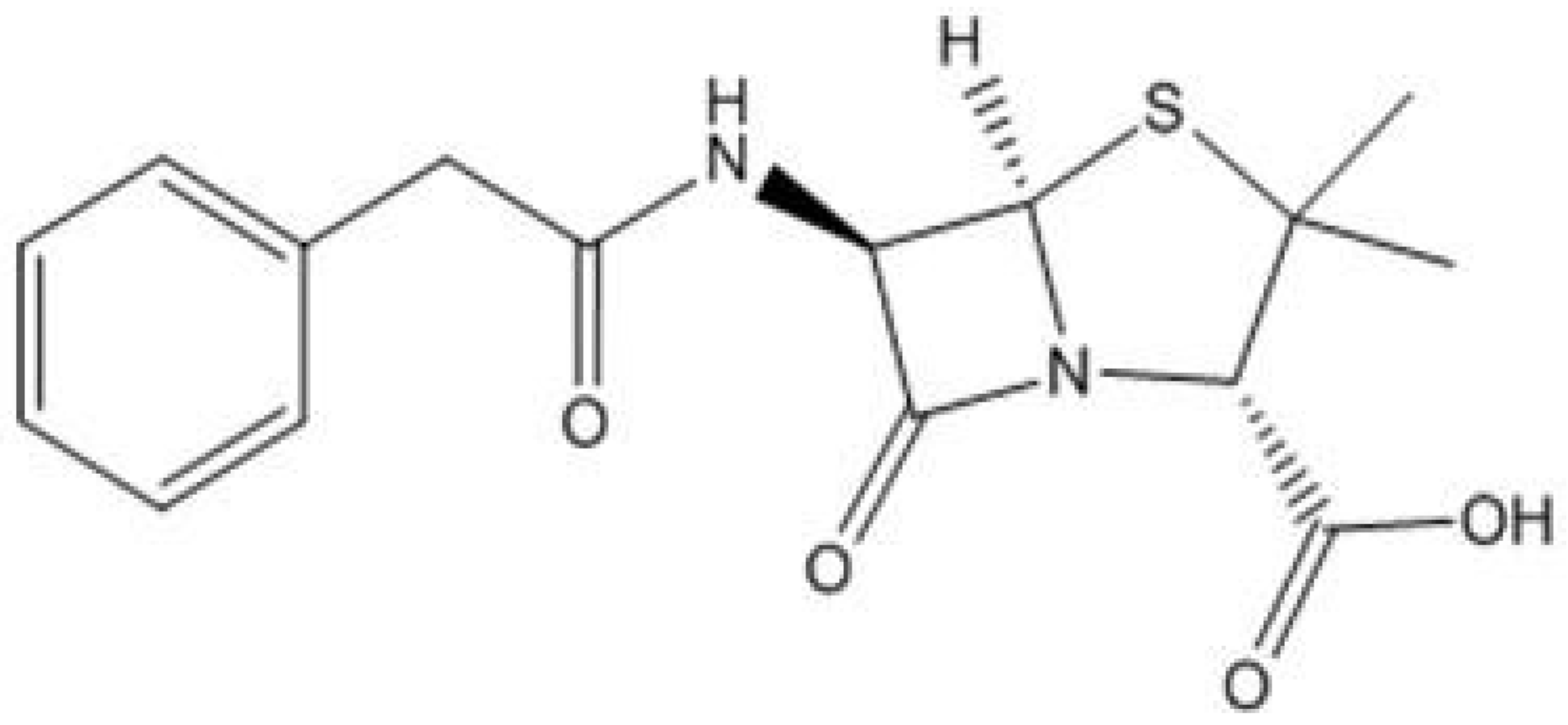
4. Resistance in the Environment
| Sampling Site | Log (CFU/g or 100 mL) | ||||||
| Total counts | Gram-negative | Gram-positive | Pseudomonads | E. coli | Enterobacter | Enterococci | |
| Dairy farm soil | 8.15 | 8.02 | 7.54 | 5.18 | 2.47 | 4.8 | 3.08 |
| Dairy farm cow manure | 6.6 | 5.62 | 6.55 | 2.88 | 2.5 | 3.45 | 1.22 |
| Dairy canal water | 5.48 | 5.31 | 4.99 | 2.2 | 1.75 | 2.01 | 1.1 |
| Residential garden soil | 6.3 | 5.9 | 6.08 | 3.92 | 2.4 | 3.55 | 2.35 |
| Lake by hospital | 5.9 | 5.77 | 5.32 | 3.5 | 2.35 | 2.88 | 1.99 |
| Public park canal water | 4.7 | 4.6 | 4.05 | 1.8 | 0.8 | 1.27 | 2.01 |
| Residential estate lake | 6.51 | 6.48 | 5.32 | 2.1 | 2.02 | 3.5 | 1.04 |
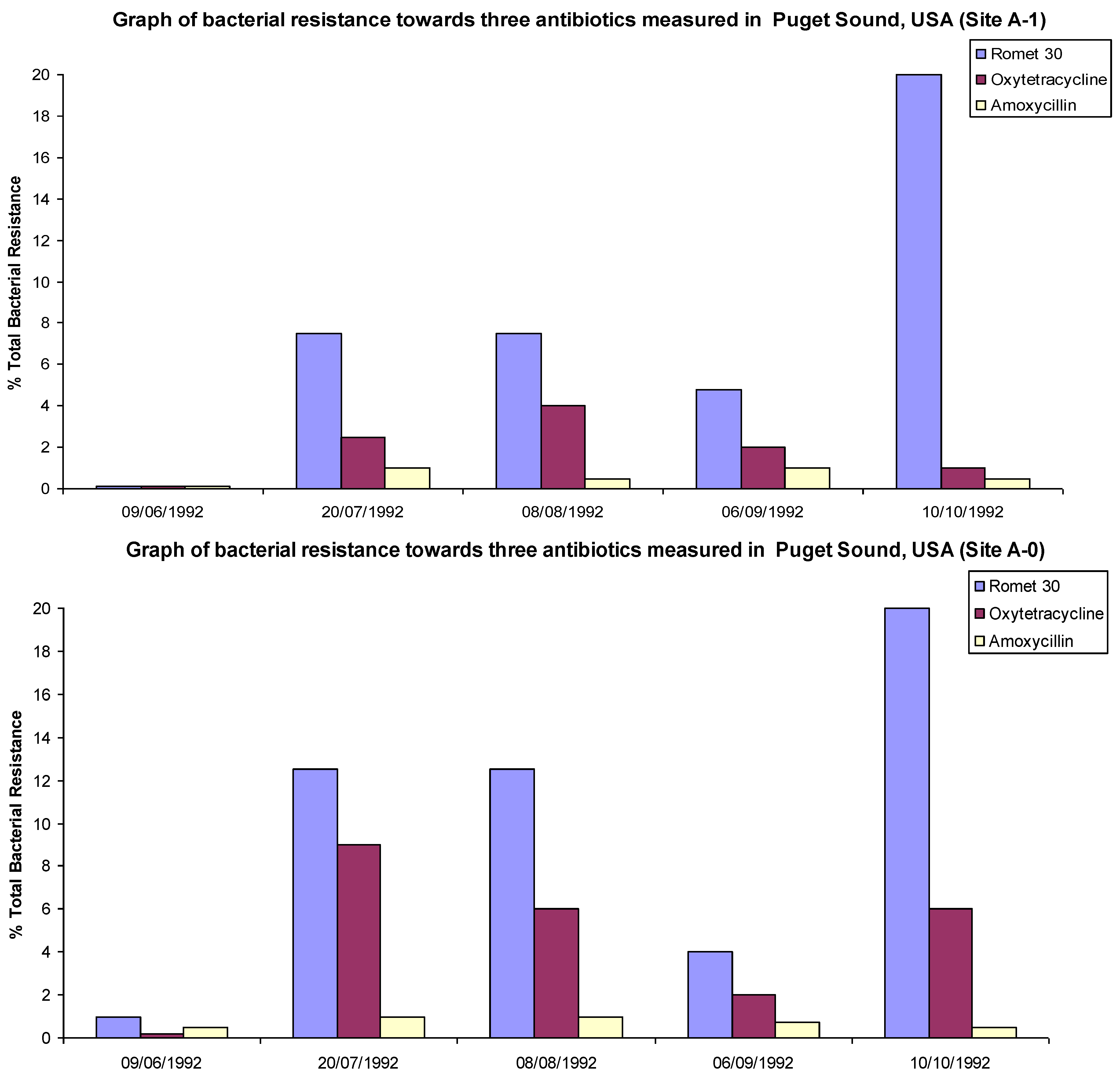
“A variety of antibiotics and their metabolites at sub-inhibitory level concentrations are suspected to expand resistance genes in the environment. However, knowledge is limited on the causal correlation of trace antibiotics or their metabolites with resistance proliferation”.[15]
5. Damage to Ecosystems
6. Drug Development—Benign by Design
7. Green Chemistry Principles
- PreventionIt is better to prevent waste than treating waste after it is produced [22];
- Atom EconomySynthetic methods should be employed so as to maximize incorporation of all reagents used, in the final product [23];
- Less Hazardous Chemical SynthesesWhen possible reagents and synthetic methods less toxic to human health and the environment should be used [24];
- Designing Safer ChemicalsChemicals should be designed, fit for purpose, with minimum toxicity to humans and the environment [25];
- Safer Solvents and AuxiliariesThe use of auxiliary substances such as solvents, drying agents etc. should be reduced when possible [26];
- Design for Energy EfficiencyThe energy required to perform a chemical process should be kept to a minimum, e.g., temperature and pressure conditions of reactions [27];
- Use of Renewable FeedstocksRaw materials should be from a renewable source if possible [28];
- Reduce Derivatives
- CatalysisEmploying catalysts reduces waste and energy requirements and should be used when possible and appropriate, i.e., selectivity [30].
- Design for DegradationSynthetic molecules should be designed to breakdown in the environment after use to avoid chronic build-up effects. Eventual fate in the environment must be considered [31].
- Real-time Analysis for Pollution PreventionReal-time process monitoring to control and prevent the production of potentially hazardous materials should be employed [32].
- Inherently Safer Chemistry for Accident PreventionSafer reagents, procedures and processes should be employed to reduce the change of accidents and exposure of chemicals to the environment and people [33].
8. Green Research Processes
9. Life Cycle Assessment
- (1)
- Goal, Scope and Definition of a Study;
- (2)
- The Life Cycle Inventory—A Comprehensive and Exhaustive Analysis of all Interactions of a Product with the Environment;
- (3)
- Life Cycle Impact Assessment—Analyzing the Data Gathered in Phase 2 to Assess the Overall Impact of the Object of the Study;
- (4)
- Interpretation of Results—Interpreting Consequences of Data Gathered from Phase 2 and 3, Making an Informed Conclusion and Proposing Suggested Courses of Action.
10. ADMET—Absorption, Distribution, Metabolism, Excretion, Toxicity
11. Green Chemistry—Pharmaceutical Successes
“...eliminates the high-pressure hydrogenation, all metals (rhodium and iron), and the wasteful chiral purification step. The benefits of the new process include a 56 percent improvement in productivity with the existing equipment, a 10–13 percent overall increase in yield, and a 19 percent reduction in overall waste generation”.[50]
“...improve the sustainability of the paclitaxel supply, allows year-round harvest, and eliminates solid biomass waste. Compared to the semisynthesis from 10-DAB, the PCF process has no chemical transformations, thereby eliminating six intermediates. During its first five years, the PCF process will eliminate an estimated 71,000 pounds of hazardous chemicals and other materials. In addition, the PCF process eliminates 10 solvents and 6 drying steps, saving a considerable amount of energy. BMS is now manufacturing paclitaxel using only plant cell cultures”.[51]
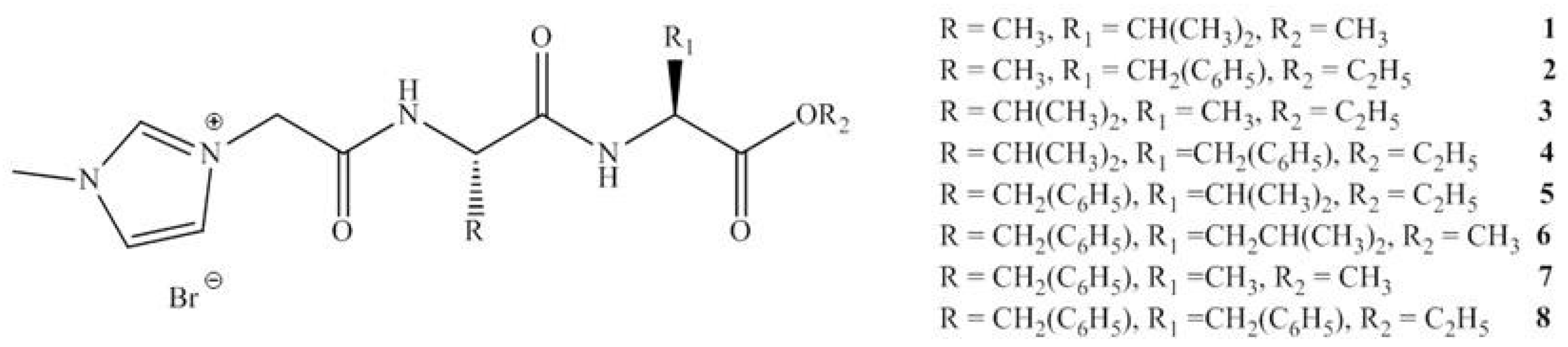
12. Ionic Liquids (ILs) as Green API’s

13. Toxicity

| Organism | Time (h) | IL | |||
|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | ||
| S. aureus (ATCC 6538) | 24, 48 | 500, 1000 | >2000, >2000 | >2000, >2000 | 1000, 1000 |
| Methicillin-res.S.a. (HK5996/08) | 24, 48 | 125, 500 | >2000, >2000 | >2000, >2000 | 2000, 2000 |
| S. epidermidis (HK6966/08) | 24, 48 | 500, >2000 | 2000, >2000 | 1000, >2000 | 2000, >2000 |
| Enterococcus sp. (HK14365/08) | 24, 48 | 2000, >2000 | >2000, >2000 | >2000, >2000 | 2000, 2000 |
| E. coli (ATCC 8739) | 24, 48 | >2000, >2000 | >2000, >2000 | >2000, >2000 | >2000, >2000 |
| K. pneumoniae (HK11750/08) | 24, 48 | >2000, >2000 | >2000, >2000 | >2000, >2000 | >2000, >2000 |
| K. pneumoniae-ESBL (HK14368/08) | 24, 48 | >2000, >2000 | >2000, >2000 | >2000, >2000 | >2000, >2000 |
| P. aeruginosa (ATCC 9027) | 24, 48 | >2000, >2000 | >2000, >2000 | >2000, >2000 | >2000, >2000 |
14. Biodegradation
| CO2 Headspace Test | % Biodegradation | ||||
|---|---|---|---|---|---|
| Compound | 0 day | 7 day | 15 day | 21 day | 28 day |
| SDS | 0 | 81 | 85 | 90 | 92 |
 12 12 | 0 | 45 | 54 | 56 | 59 |
 13 13 | 0 | 54 | 59 | 59 | 59 |
 14 14 | 0 | 51 | 58 | 61 | 65 |
 15 15 | 0 | 26 | 30 | 29 | 29 |
| CO2 Headspace Test | % Biodegradation | ||||
|---|---|---|---|---|---|
| Compound | 0 day | 6 day | 13 day | 20 day | 28 day |
| SDS | 0 | 67 | 91 | 91 | 87 |
 6 6 | 0 | 42 | 65 | 68 | 64 |
| CO2 Headspace Test | % Biodegradation | ||||
|---|---|---|---|---|---|
| Compound | 0 day | 7 day | 15 day | 21 day | 28 day |
| SDS | 0 | 78 | 89 | 91 | 94 |
 11 11 | 0 | 16 | 59 | 61 | 61 |
15. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bell, F.G.; Genske, D.D.; Hytiris, N.; Lindsay, P. A survey of contaminated ground with illustrative case histories. Land Degrad. Dev. 2000, 11, 419–437. [Google Scholar] [CrossRef]
- Kummerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Demain, A.L. Antibiotics: Natural products essential to human health. Med. Res. Rev. 2009, 29, 821–842. [Google Scholar] [CrossRef]
- Milla, S.; Depiereux, S.; Kestemont, P. The effects of estrogenic and androgenic endocrine disruptors on the immune system of fish: A review. Ecotoxicology 2011, 20, 305–319. [Google Scholar] [CrossRef]
- Lanzky, P.F.; Halling-Sorensen, B. The toxic effect of the antibiotic metronidazole on aquatic organisms. Chemosphere 1997, 35, 2553–2561. [Google Scholar] [CrossRef]
- Smyth, E.C.M.; Hannan, M.; McMahon, S.; Philbin, M. Meticillin-resistant Staphylococcus aureus (Mrsa) in Ireland: Addressing the issues. Available online: http://www.pfizer.ie/UserFiles/File/news_releases/MRSA_Report_FINAL.pdf (acessed on 27 November 2012).
- Harbottle, H.; Thakur, S.; Zhao, S.; White, D.G. Genetics of antimicrobial resistance. Anim. Biotechnol. 2006, 17, 111–124. [Google Scholar] [CrossRef]
- Schnabel, E.L.; Jones, A.L. Distribution of tetracycline resistance genes and transposons among phylloplane bacteria in Michigan apple orchards. Appl. Environ. Microbiol. 1999, 65, 4898–4907. [Google Scholar]
- Esiobu, N.; Armenta, L.; Ike, J. Antibiotic resistance in soil and water environments. Int. J. Environ. Health Res. 2002, 12, 133–144. [Google Scholar] [CrossRef]
- Herwig, R.P.; Gray, J.P.; Weston, D.P. Antibacterial resistant bacteria in surficial sediments near salmon net-cage farms in Puget Sound, Washington. Aquaculture 1997, 149, 263–283. [Google Scholar] [CrossRef]
- Halling-Sorensen, B.; Nielsen, S.N.; Lanzky, P.F.; Ingerslev, F.; Lutzhoft, H.C.H.; Jorgensen, S.E. Occurrence, fate and effects of pharmaceutical substances in the environment—A review. Chemosphere 1998, 36, 357–394. [Google Scholar] [CrossRef]
- Li, D.; Yang, M.; Hu, J.; Zhang, Y.; Chang, H.; Jin, F. Determination of penicillin G and its degradation products in a penicillin production wastewater treatment plant and the receiving river. Water Res. 2008, 42, 307–317. [Google Scholar] [CrossRef]
- Summers, A.O. Genetic linkage and horizontal gene transfer, the roots of the antibiotic multi-resistance problem. Anim. Biotechnol. 2006, 17, 125–135. [Google Scholar] [CrossRef]
- Olsen, J.E. Antibiotic resistance: Genetic mechanisms and mobility. Acta Vet. Scand. Suppl. 1999, 92, 15–22. [Google Scholar]
- Fan, C.A.; He, J.Z. Proliferation of antibiotic resistance genes in microbial consortia of sequencing batch reactors (SBRs) upon exposure to trace erythromycin or erythromycin-H2O. Water Res. 2011, 45, 3098–3106. [Google Scholar] [CrossRef]
- Kotzerke, A.; Sharma, S.; Schauss, K.; Heuer, H.; Thiele-Bruhn, S.; Smalla, K.; Wilke, B.M.; Schloter, M. Alterations in soil microbial activity and N-transformation processes due to sulfadiazine loads in pig-manure. Environ. Pollut. 2008, 153, 315–322. [Google Scholar] [CrossRef]
- Costanzo, S.D.; Murby, J.; Bates, J. Ecosystem response to antibiotics entering the aquatic environment. Mar. Pollut. Bull. 2005, 51, 218–223. [Google Scholar] [CrossRef]
- Lai, H.T.; Hou, J.H.; Su, C.I.; Chen, C.L. Effects of chloramphenicol, florfenicol, and thiamphenicol on growth of algae Chlorella pyrenoidosa, Isochrysis galbana, and Tetraselmis chui. Ecotox. Environ. Safe. 2009, 72, 329–334. [Google Scholar] [CrossRef]
- Lutzhoft, H.C.H.; Halling-Sorensen, B.; Jorgensen, S.E. Algal toxicity of antibacterial agents applied in Danish fish farming. Arch. Environ. Contam. Toxicol. 1999, 36, 1–6. [Google Scholar] [CrossRef]
- Kummerer, K. Handbook of Green Chemistry Volume 9: Designing Safer Chemicals, 1st ed.; Wiley-VCH: Hoboken, NJ, USA, 2012; pp. 251–272. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; p. 3. [Google Scholar]
- Sheldon, R.A. Atom utilisation, E factors and the catalytic solution. CR Acad. Sci. 2000, 3, 541–551. [Google Scholar]
- Trost, B.M. The atom economy—A search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar]
- Ali, A.R.; Ghosh, H.; Patel, B.K. A greener synthetic protocol for the preparation of carbodiimide. Tetrahedron Lett. 2010, 51, 1019–1021. [Google Scholar] [CrossRef]
- Jungnickel, C.; Stock, F.; Brandsch, T.; Ranke, J. Risk assessment of biocides in roof paint. Environ. Sci. Pollut. Res. 2008, 15, 258–265. [Google Scholar] [CrossRef]
- DeSimone, J.M. Practical approaches to green solvents. Science 2002, 297, 799–803. [Google Scholar] [CrossRef]
- Roberts, B.A.; Strauss, C.R. Toward rapid, “green”, predictable microwave-assisted synthesis. Acc. Chem. Res. 2005, 38, 653–661. [Google Scholar] [CrossRef]
- Infante, M.R.; Perez, L.; Moran, M.C.; Pons, R.; Mitjans, M.; Vinardell, M.P.; Garcia, M.T.; Pinazo, A. Biocompatible surfactants from renewable hydrophiles. Eur. J. Lipid Sci. Technol. 2010, 112, 110–121. [Google Scholar] [CrossRef]
- Dunn, P.J. The importance of green chemistry in process research and development. Chem. Soc. Rev. 2012, 41, 1452–1461. [Google Scholar] [CrossRef]
- Anastas, P.T.; Kirchhoff, M.M.; Williamson, T.C. Catalysis as a foundational pillar of green chemistry. Appl. Catal. A 2001, 221, 3–13. [Google Scholar] [CrossRef]
- Jungnickel, C.; Mrozik, W.; Markiewicz, M.; Luczak, J. Fate of ionic liquids in soils and sediments. Curr. Org. Chem. 2011, 15, 1928–1945. [Google Scholar] [CrossRef]
- Wang, J. Real-time electrochemical monitoring: Toward green analytical chemistry. Acc. Chem. Res. 2002, 35, 811–816. [Google Scholar] [CrossRef]
- Duspara, P.A.; Islam, M.S.; Lough, A.J.; Batey, R.A. Synthesis and Reactivity of N-Alkyl Carbamoylimidazoles: Development of N-Methyl Carbamoylimidazole as a Methyl Isocyanate Equivalent. J. Org. Chem. 2012, 77, 10362–10368. [Google Scholar] [CrossRef]
- Andraos, J. On using tree analysis to quantify the material, input energy, and cost throughput efficiencies of simple and complex synthesis plans and networks: Towards a blueprint for quantitative total synthesis and green chemistry. Org. Process Res. Dev. 2006, 10, 212–240. [Google Scholar] [CrossRef]
- Andraos, J. Green Chemistry Metrics: Measuring and Monitoring Sustainable Processes; Blackwell-Wiley: Oxford, UK, 2008; pp. 69–200. [Google Scholar]
- Andraos, J. Global green chemistry metrics analysis algorithm and spreadsheets: Evaluation of the material efficiency performances of synthesis plans for oseltamivir phosphate (Tamiflu) as a test case. Org. Process Res. Dev. 2009, 13, 161–185. [Google Scholar] [CrossRef]
- Andraos, J. A database tool for process chemists and chemical engineers to gauge the material and synthetic efficiencies of synthesis plans to industrially important targets. Pure Appl. Chem. 2011, 83, 1361–1378. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on solvent use in the pharmaceutical industry. Org. Process Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Curzons, A.; Constable, D.C.; Cunningham, V. Cradle-to-gate life cycle inventory and assessment of pharmaceutical compounds. Int. J. LCA 2004, 9, 114–121. [Google Scholar] [CrossRef]
- Han van de Waterbeemd, E.G. ADMET in silico modelling: towards prediction paradise? Nat. Rev. Drug Discov. 2003, 2, 192–204. [Google Scholar] [CrossRef]
- Cho, C.W.; Preiss, U.; Jungnickel, C.; Stolte, S.; Arning, J.; Ranke, J.; Klamt, A.; Krossing, I.; Thoming, J. Ionic liquids: Predictions of physicochemical properties with experimental and/or DFT-calculated LFER parameters to understand molecular interactions in solution. J. Phys. Chem. B 2011, 115, 6040–6050. [Google Scholar] [CrossRef]
- Kümmerer, K.; Hempel, M. Green and Sustainable Pharmacy; Springer: Berlin Heidelberg, Germany, 2010; p. 313. [Google Scholar]
- META. Available online: http://www.multicase.com/products/prod05.htm (accessed on 9 August 2013).
- BIOWIN. Available online: http://www.epa.gov/oppt/exposure/pubs/episuitedl.htm (accessed on 9 August 2013).
- CATABOL. Available online: http://oasis-lmc.org/?section=software&swid=1 (accessed on 9 August 2013).
- TOPKAT. Available online: http://accelrys.com/products/discovery-studio/predictive-toxicology.html (accessed on 9 August 2013).
- Marchetti, S.; Schellens, J.H.M. The impact of FDA and EMEA guidelines on drug development in relation to Phase 0 trials. Br. J. Cancer 2007, 97, 577–581. [Google Scholar] [CrossRef]
- Schellens, J.H.M. Phase 0 (zero) clinical trials: More than zero benefit? Eur. J. Cancer 2009, 45, 728–729. [Google Scholar] [CrossRef]
- Kummar, S.R.L.; Kinders, R.; Parchment, R.E.; Gutierrez, M.E.; Murgo, A.J.; Ji, J.; Mroczkowski, B.; Pickeral, O.K.; Simpson, M.; Hollingshead, M.; et al. Phase 0 clinical trials: Conceptions and misconceptions. Cancer J. 2008, 14, 133–137. [Google Scholar] [CrossRef]
- Green Chemistry. Available online: http://www.epa.gov/opptintr/greenchemistry/pubs/pgcc/winners/grca10.html (accessed on 24 October 2012).
- Green Chemistry. Available online: http://www.epa.gov/opptintr/greenchemistry/pubs/pgcc/winners/gspa04.html (accessed on 24 October 2012).
- Coleman, D.; Gathergood, N. Biodegradation studies of ionic liquids. Chem. Soc. Rev. 2010, 39, 600–637. [Google Scholar] [CrossRef]
- Morrissey, S.; Pegot, B.; Coleman, D.; Garcia, M.T.; Ferguson, D.; Quilty, B.; Gathergood, N. Biodegradable, non-bactericidal oxygen-functionalised imidazolium esters: A step towards ‘greener’ ionic liquids. Green Chem. 2009, 11, 475–483. [Google Scholar] [CrossRef]
- Bouquillon, S.; Courant, T.; Dean, D.; Gathergood, N.; Morrissey, S.; Pegot, B.; Scammells, P.J.; Singer, R.D. Biodegradable ionic liquids: Selected synthetic applications. Aust. J. Chem. 2007, 60, 843–847. [Google Scholar] [CrossRef]
- Hough, W.L.; Rogers, R.D. Ionic liquids then and now: From solvents to materials to active pharmaceutical ingredients. Bull. Chem. Soc. Jpn. 2007, 80, 2262–2269. [Google Scholar] [CrossRef]
- Choi, S.Y.; Rodriguez, H.; Mirjafari, A.; Gilpin, D.F.; McGrath, S.; Malcolm, K.R.; Tunney, M.M.; Rogers, R.D.; McNally, T. Dual functional ionic liquids as plasticisers and antimicrobial agents for medical polymers. Green Chem. 2011, 13, 1527–1535. [Google Scholar] [CrossRef]
- Carson, L.; Chau, P.K.W.; Earle, M.J.; Gilea, M.A.; Gilmore, B.F.; Gorman, S.P.; McCann, M.T.; Seddon, K.R. Antibiofilm activities of 1-alkyl-3-methylimidazolium chloride ionic liquids. Green Chem. 2009, 11, 492–497. [Google Scholar] [CrossRef]
- Craythorne, S.J.; Anderson, K.; Lorenzini, F.; McCausland, C.; Smith, E.F.; Licence, P.; Marr, A.C.; Marr, P.C. The co-entrapment of a homogeneous catalyst and an ionic liquid by a sol-gel method: Recyclable ionogel hydrogenation catalysts. Chem.-Eur. J. 2009, 15, 7094–7100. [Google Scholar] [CrossRef]
- Hough, W.L.; Smiglak, M.; Rodriguez, H.; Swatloski, R.P.; Spear, S.K.; Daly, D.T.; Pernak, J.; Grisel, J.E.; Carliss, R.D.; Soutullo, M.D.; et al. The third evolution of ionic liquids: Active pharmaceutical ingredients. New J. Chem. 2007, 31, 1429–1436. [Google Scholar] [CrossRef]
- Pinazo, A.; Lozano, N.; Perez, L.; Moran, M.C.; Infante, M.R.; Pons, R. Arginine diacyl-glycerolipid conjugates as multifunctional biocompatible surfactants. CR Chim. 2011, 14, 726–735. [Google Scholar] [CrossRef]
- Gathergood, N.; Scammells, P.J.; Garcia, M.T. Biodegradable ionic liquids: Part III. The first readily biodegradable ionic liquids. Green Chem. 2006, 8, 156–160. [Google Scholar] [CrossRef]
- Garcia, M.T.; Gathergood, N.; Scammells, P.J. Biodegradable ionic liquids: Part II. Effect of the anion and toxicology. Green Chem. 2005, 7, 9–14. [Google Scholar] [CrossRef]
- Gathergood, N.; Garcia, M.T.; Scammells, P.J. Biodegradable ionic liquids: Part I. Concept, preliminary targets and evaluation. Green Chem. 2004, 6, 166–175. [Google Scholar] [CrossRef]
- Coleman, D.; Spulak, M.; Garcia, M.T.; Gathergood, N. Antimicrobial toxicity studies of ionic liquids leading to a ‘hit’ MRSA selective antibacterial imidazolium salt. Green Chem. 2012, 14, 1350–1356. [Google Scholar] [CrossRef]
- Castillo, J.A.; Clapes, P.; Infante, M.R.; Comas, J.; Manresa, A. Comparative study of the antimicrobial activity of bis(N-alpha-caproyl-L-arginine)-1,3-propanediamine dihydrochloride and chlorhexidine dihydrochloride against Staphylococcus aureus and Escherichia coli. J. Antimicrob. Chemother. 2006, 57, 691–698. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jordan, A.; Gathergood, N. Designing Safer and Greener Antibiotics. Antibiotics 2013, 2, 419-438. https://doi.org/10.3390/antibiotics2030419
Jordan A, Gathergood N. Designing Safer and Greener Antibiotics. Antibiotics. 2013; 2(3):419-438. https://doi.org/10.3390/antibiotics2030419
Chicago/Turabian StyleJordan, Andrew, and Nicholas Gathergood. 2013. "Designing Safer and Greener Antibiotics" Antibiotics 2, no. 3: 419-438. https://doi.org/10.3390/antibiotics2030419
APA StyleJordan, A., & Gathergood, N. (2013). Designing Safer and Greener Antibiotics. Antibiotics, 2(3), 419-438. https://doi.org/10.3390/antibiotics2030419




