Efficacy and Safety of Procalcitonin-Guided Antibiotic Therapy in Lower Respiratory Tract Infections
Abstract
:1. Introduction
2. Methods

3. Results
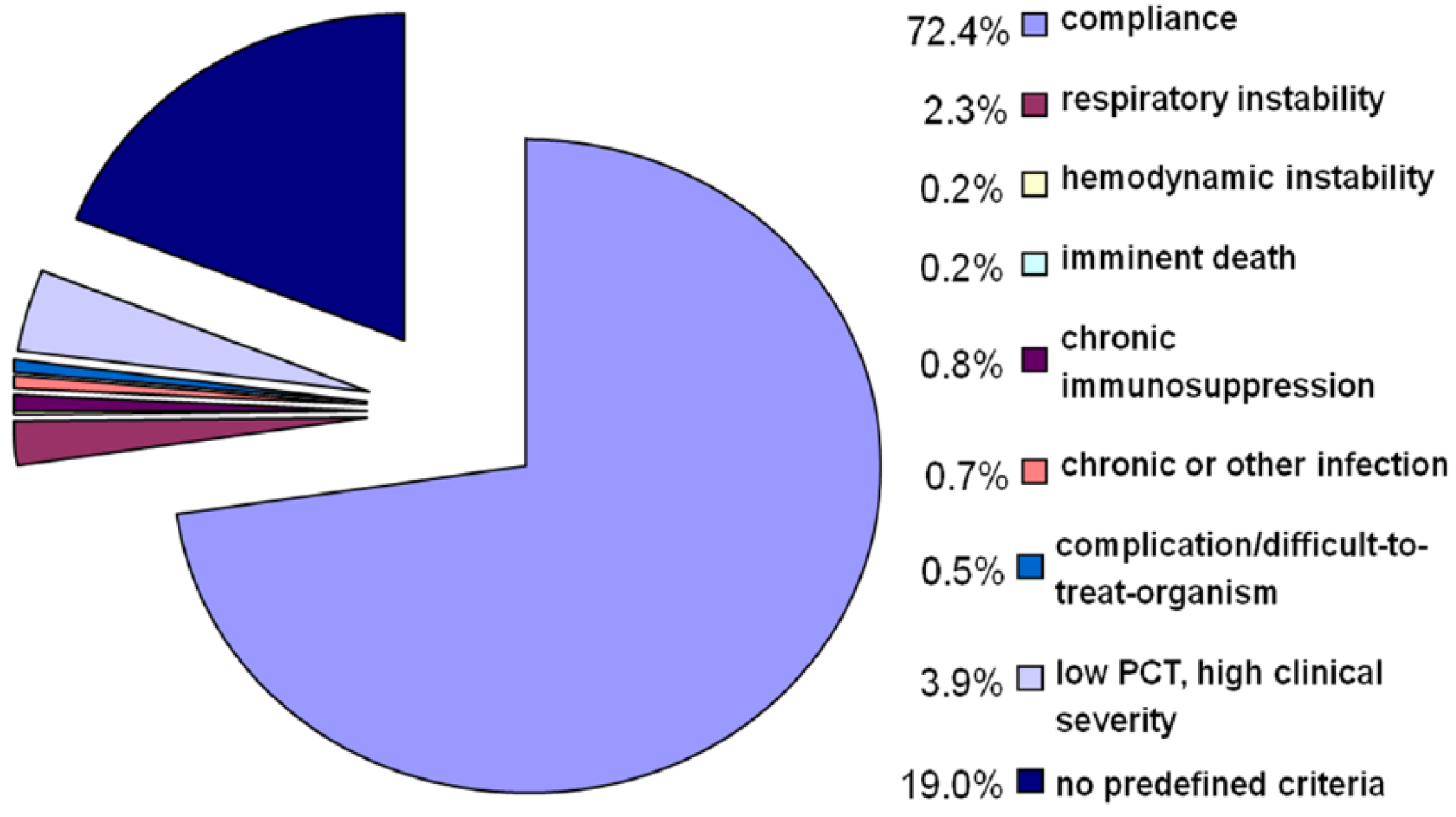
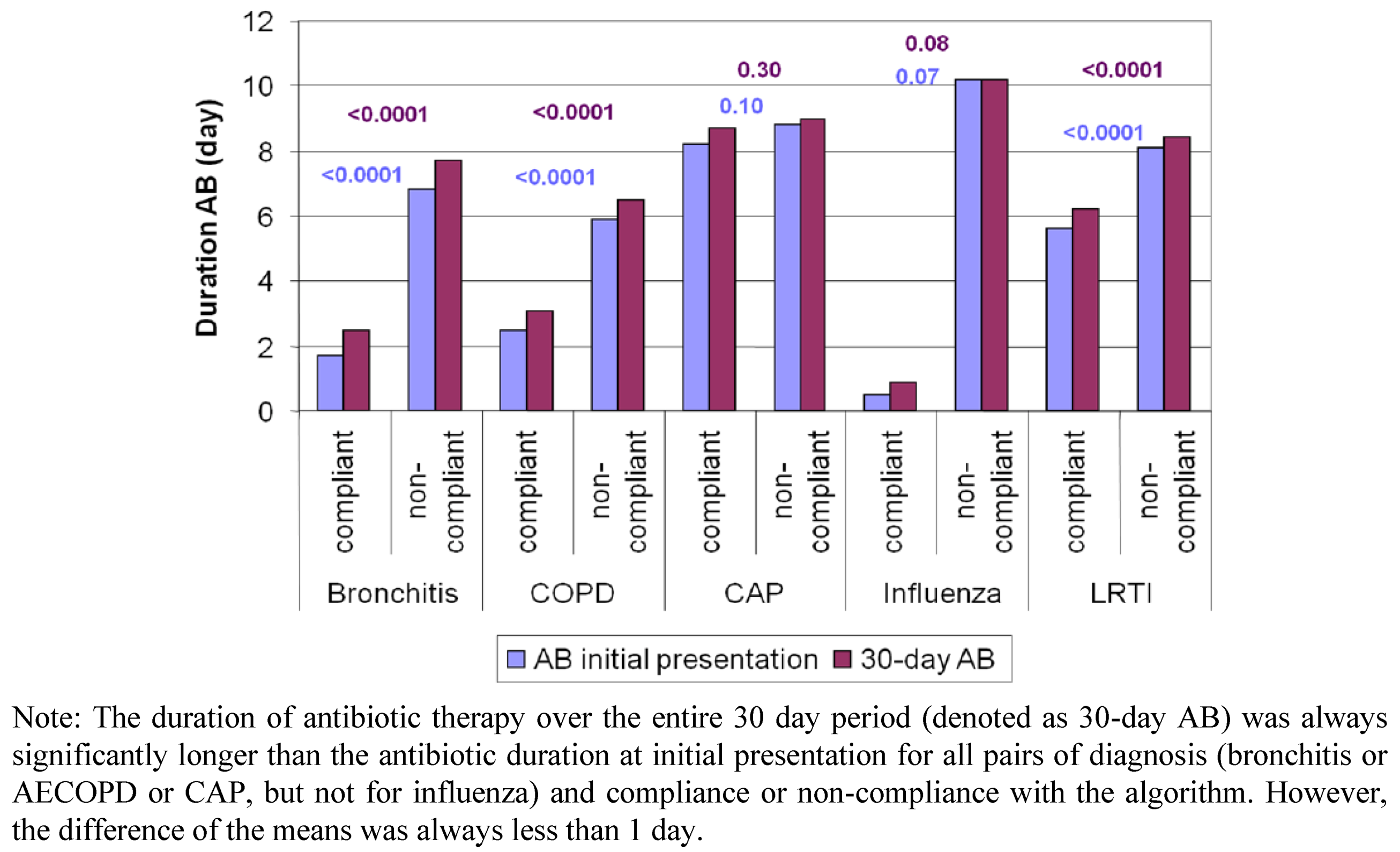
| Cox proportional hazard ratio | |||
|---|---|---|---|
| 30-day antibiotic duration | HR | 95% CI | P |
| Compliance | 1.27 | 1.13–1.43 | <0.0001 |
| CAP (vs. bronchitis) | 0.53 | 0.45–0.62 | <0.0001 |
| France (vs. Switzerland) | 0.66 | 0.55–0.79 | <0.0001 |
| Hospital treatment | 0.62 | 0.52–0.75 | <0.0001 |
| Algorithm naive | 0.86 | 0.76–0.97 | 0.015 |
| Renal insufficiency | 0.82 | 0.72–0.93 | 0.003 |
| 30 day complications | OR | 95% CI | P |
|---|---|---|---|
| CURB65 | 1.35 | 1.07–1.72 | 0.01 |
| CAP (vs. bronchitis) | 6.81 | 2.97–15.61 | <0.0001 |
| Multilobar pneumonia | 5.25 | 1.62–17.02 | 0.006 |
| History of stroke | 0.25 | 0.08–0.77 | 0.015 |
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Briel, M.; Schuetz, P.; Mueller, B.; Young, J.; Schild, U.; Nusbaumer, C.; Periat, P.; Bucher, H.C.; Christ-Crain, M. Procalcitonin-guided antibiotic use vs. a standard approach for acute respiratory tract infections in primary care. Arch. Intern. Med. 2008, 168, 2000–2007; discussion 2007–2008. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Jaccard-Stolz, D.; Bingisser, R.; Gencay, M.M.; Huber, P.R.; Tamm, M.; Muller, B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: Cluster-randomised, single-blinded intervention trial. Lancet 2004, 363, 600–607. [Google Scholar]
- Christ-Crain, M.; Stolz, D.; Bingisser, R.; Muller, C.; Miedinger, D.; Huber, P.R.; Zimmerli, W.; Harbarth, S.; Tamm, M.; Muller, B. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: A randomized trial. Am. J. Respir. Crit. Care Med. 2006, 174, 84–93. [Google Scholar] [CrossRef]
- Schuetz, P.; Christ-Crain, M.; Thomann, R.; Falconnier, C.; Wolbers, M.; Widmer, I.; Neidert, S.; Fricker, T.; Blum, C.; Schild, U.; et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The prohosp randomized controlled trial. JAMA 2009, 302, 1059–1066. [Google Scholar] [CrossRef]
- Stolz, D.; Christ-Crain, M.; Bingisser, R.; Leuppi, J.; Miedinger, D.; Muller, C.; Huber, P.; Muller, B.; Tamm, M. Antibiotic treatment of exacerbations of copd: A randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007, 131, 9–19. [Google Scholar] [CrossRef]
- Kristoffersen, K.B.; Sogaard, O.S.; Wejse, C.; Black, F.T.; Greve, T.; Tarp, B.; Storgaard, M.; Sodemann, M. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission—A randomized trial. Clin. Microbiol. Infect. 2009, 15, 481–487. [Google Scholar] [CrossRef]
- Burkhardt, O.; Ewig, S.; Haagen, U.; Giersdorf, S.; Hartmann, O.; Wegscheider, K.; Hummers-Pradier, E.; Welte, T. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur. Respir. J. 2010, 36, 601–607. [Google Scholar] [CrossRef]
- Schuetz, P.; Batschwaroff, M.; Dusemund, F.; Albrich, W.; Burgi, U.; Maurer, M.; Brutsche, M.; Huber, A.R.; Muller, B. Effectiveness of a procalcitonin algorithm to guide antibiotic therapy in respiratory tract infections outside of study conditions: A post-study survey. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 269–277. [Google Scholar] [CrossRef]
- Albrich, W.C.; Dusemund, F.; Bucher, B.; Meyer, S.; Thomann, R.; Kuhn, F.; Bassetti, S.; Sprenger, M.; Bachli, E.; Sigrist, T.; et al. Effectiveness and safety of procalcitonin-guided antibiotic therapy in lower respiratory tract infections in "real life": An international, multicenter poststudy survey (proreal). Arch. Intern. Med. 2012, 172, 715–722. [Google Scholar] [CrossRef]
- Woodhead, M.; Blasi, F.; Ewig, S.; Huchon, G.; Ieven, M.; Ortqvist, A.; Schaberg, T.; Torres, A.; van der Heijden, G.; Verheij, T.J. Guidelines for the management of adult lower respiratory tract infections. Eur. Respir. J. 2005, 26, 1138–1180. [Google Scholar] [CrossRef]
- Schuetz, P.; Albrich, W.; Christ-Crain, M.; Chastre, J.; Mueller, B. Procalcitonin for guidance of antibiotic therapy. Expert Rev. Anti Infect. Ther. 2010, 8, 575–587. [Google Scholar] [CrossRef]
- Yealy, D.M.; Auble, T.E.; Stone, R.A.; Lave, J.R.; Meehan, T.P.; Graff, L.G.; Fine, J.M.; Obrosky, D.S.; Mor, M.K.; Whittle, J.; et al. Effect of increasing the intensity of implementing pneumonia guidelines: A randomized, controlled trial. Ann. Intern. Med. 2005, 143, 881–894. [Google Scholar]
- Aujesky, D.; McCausland, J.B.; Whittle, J.; Obrosky, D.S.; Yealy, D.M.; Fine, M.J. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin. Infect. Dis. 2009, 49, e100–e108. [Google Scholar] [CrossRef]
- Harbarth, S.; Albrich, W.; Goldmann, D.A.; Huebner, J. Control of multiply resistant cocci: Do international comparisons help? Lancet Infect. Dis. 2001, 1, 251–261. [Google Scholar] [CrossRef]
- Filippini, M.; Masiero, G.; Moschetti, K. Socioeconomic determinants of regional differences in outpatient antibiotic consumption: Evidence from switzerland. Health Policy 2006, 78, 77–92. [Google Scholar] [CrossRef]
- Albrich, W.C.; Monnet, D.L.; Harbarth, S. Antibiotic selection pressure and resistance in streptococcus pneumoniae and streptococcus pyogenes. Emerg. Infect. Dis. 2004, 10, 514–517. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Muller, B. Procalcitonin in bacterial infections—Hype, hope, more or less? Swiss Me. Wkly. 2005, 135, 451–460. [Google Scholar]
- Muller, F.; Christ-Crain, M.; Bregenzer, T.; Krause, M.; Zimmerli, W.; Mueller, B.; Schuetz, P.; Pro, H.S.G. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: A prospective cohort trial. Chest 2010, 138, 121–129. [Google Scholar]
- Kruger, S.; Ewig, S.; Papassotiriou, J.; Kunde, J.; Marre, R.; von Baum, H.; Suttor, N.; Welte, T. Inflammatory parameters predict etiologic patterns but do not allow for individual prediction of etiology in patients with cap: Results from the german competence network capnetz. Respir. Res. 2009, 10. [Google Scholar] [CrossRef] [Green Version]
- Kruger, S.; Ewig, S.; Kunde, J.; Hartmann, O.; Marre, R.; Suttorp, N.; Welte, T. Assessment of inflammatory markers in patients with community-acquired pneumonia—Influence of antimicrobial pre-treatment results from the german competence network capnetz. Clin. Chim. Acta 2010, 411, 1929–1934. [Google Scholar] [CrossRef]
- Uzzan, B.; Cohen, R.; Nicolas, P.; Cucherat, M.; Perret, G.Y. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: A systematic review and meta-analysis. Crit. Care Med. 2006, 34, 1996–2003. [Google Scholar] [CrossRef]
- Hunziker, S.; Hugle, T.; Schuchardt, K.; Groeschl, I.; Schuetz, P.; Mueller, B.; Dick, W.; Eriksson, U.; Trampuz, A. The value of serum procalcitonin level for differentiation of infectious from noninfectious causes of fever after orthopaedic surgery. J. Bone Joint Surg. Am. 2010, 92, 138–148. [Google Scholar] [CrossRef]
- Sponholz, C.; Sakr, Y.; Reinhart, K.; Brunkhorst, F. Diagnostic value and prognostic implications of serum procalcitonin after cardiac surgery: A systematic review of the literature. Crit. Care 2006, 10. [Google Scholar] [CrossRef]
- Schuetz, P.; Chiappa, V.; Briel, M.; Greenwald, J.L. Procalcitonin algorithms for antibiotic therapy decisions: A systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch. Intern. Med. 2011, 171, 1322–1331. [Google Scholar] [CrossRef]
- Schuetz, P.; Muller, B.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; Tubach, F.; et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst. Rev. 2012, 9, CD007498. [Google Scholar]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum procalcitonin and c-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Drozdov, D.; Dusemund, F.; Müller, B.; Albrich, W.C. Efficacy and Safety of Procalcitonin-Guided Antibiotic Therapy in Lower Respiratory Tract Infections. Antibiotics 2013, 2, 1-10. https://doi.org/10.3390/antibiotics2010001
Drozdov D, Dusemund F, Müller B, Albrich WC. Efficacy and Safety of Procalcitonin-Guided Antibiotic Therapy in Lower Respiratory Tract Infections. Antibiotics. 2013; 2(1):1-10. https://doi.org/10.3390/antibiotics2010001
Chicago/Turabian StyleDrozdov, Daniel, Frank Dusemund, Beat Müller, and Werner C. Albrich. 2013. "Efficacy and Safety of Procalcitonin-Guided Antibiotic Therapy in Lower Respiratory Tract Infections" Antibiotics 2, no. 1: 1-10. https://doi.org/10.3390/antibiotics2010001




