Study of Immobilization Procedure on Silver Nanolayers and Detection of Estrone with Diverged Beam Surface Plasmon Resonance (SPR) Imaging
Abstract
:1. Introduction
| Reference | Concentration [mM] | Incubation [h] | Solvent |
|---|---|---|---|
| [4] | 10 | 24 | Ethanol |
| [5] | 10 | 20–24 | Ethanol |
| [6] | 5 | 2 | Ethanol |
| [7] | 2 | 12 | Ethanol |
| [8] | 1 | 18 | Ethanol |
| [9,10,11] | 1 | 24 | Ethanol |
| [12,13] | 150 | 12 | Glycerol/Ethanol |
| [14] | 10 | 24 | Ethanol |
| [15] | 1 | 12 | Ethanol |
| [16] | 20 | 16 | Ethanol |
| [17] | 5 | 2 | Ethanol |
| [18] | 10 | 12 | Ethanol |
| [19] | 10 | 24 | Ethanol |
| [20] | 1 | 4 | Ethanol |
2. Experimental Section
2.1. Materials
2.2. Preparation of Silver Samples
2.3. Experimental Set-Up
2.3.1. Refractometer Based on SPR for Testing of Immobilization Quality



2.3.2. Diverged Beam SPR Imaging Set-Up
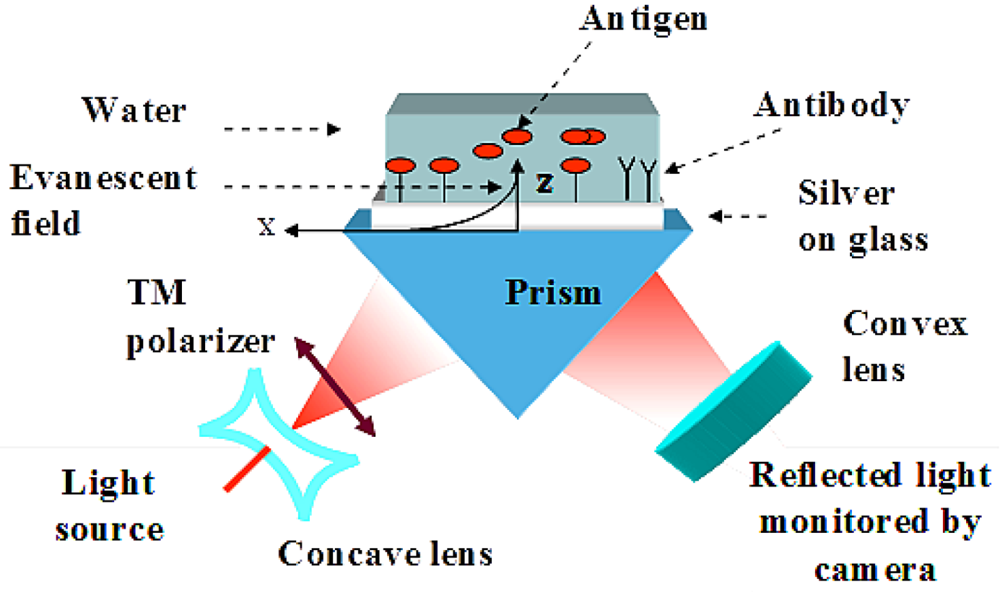
2.4. Immobilization Procedure Based on Thiol Self Assembly Monolayer
2.4.1. Protocol Based on Ethanol as a Solvent
 of 11-MUA of 11-MUA | |||
| Test | SPR signal in air | SPR signal in DI | |
| Incubation time (h) | |||
| 4 | v | silver was destroyed | |
| 12 | v | - | |
| 24 | v | - | |
| 48 | v | - | |
 of 11-MUA of 11-MUA | |||
| Test | air in signal SPR | SPR signal in DI | |
| Incubation time (h) | |||
| 4 | v | v | |
| 12 | v | v | |
| 24 | v | v | |
| 48 | v | v | |
 of 11-MUA + (NHS and EDC) of 11-MUA + (NHS and EDC) | |||
| Test | air in signal SPR | SPR signal in DI | |
| Incubation time (h) | |||
| 4 | silver was destroyed | - | |
| 12 | silver was damaged partially | - | |
| 24 | silver was damaged partially | - | |
| 48 | silver was damaged partially | - | |
 of 11-MUA + (NHS and EDC) of 11-MUA + (NHS and EDC) | |||
| Test | air in signal SPR | SPR signal in DI | |
| Incubation time (h) | |||
| 4 | silver was damaged partially | - | |
| 12 | v | v | |
| 24 | v | v | |
| 48 | v | v | |
 of 11-MUA + (NHS and EDC) + HRP of 11-MUA + (NHS and EDC) + HRP | |||
| Test | Luminiscence signal | air in signal SPR | SPR signal in DI |
| Incubation time (h) | |||
| 4 | silver was destroyed | - | - |
| 12 | silver was destroyed | - | - |
| 24 | silver was destroyed | - | - |
| 48 | silver was destroyed | - | - |
 of 11-MUA + (NHS and EDC) + HRP of 11-MUA + (NHS and EDC) + HRP | |||
| Test | Luminiscence signal | air in signal SPR | SPR signal in DI |
| Incubation time (h) | |||
| 4 | silver was damaged partially | ||
| 12 | v | v | v |
| 24 | v | v | v |
| 48 | v | v | v |

2.4.2. Optimization of the Protocol with DCC and DMSO as a Solvent
3. Results and Discussion
3.1. Specific Sensing of Estrone with DBSPRI Sensor


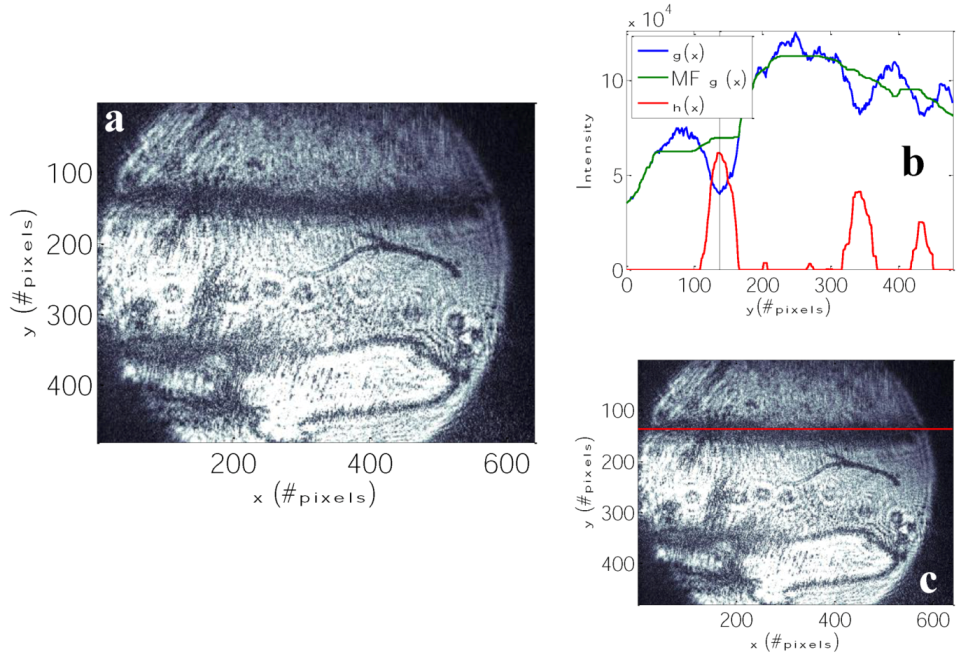
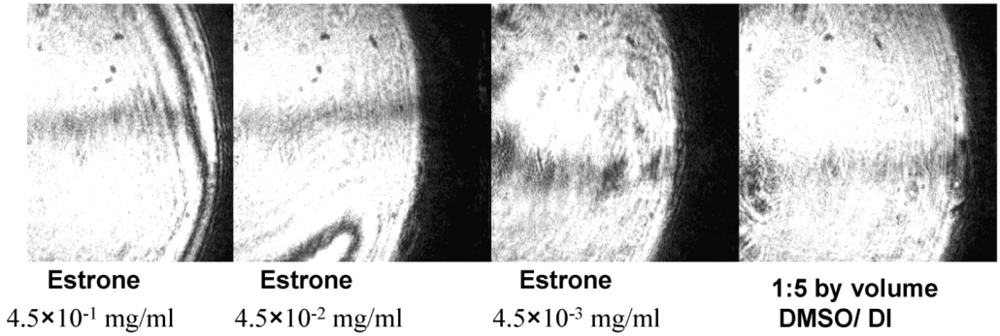
4. Conclusions
Acknowledgments
References
- Homola, J.; Piliarik, M. Surface Plasmon Resonance Based Sensors; Homola, J., Ed.; Springer: Berlin, Germany, 2006; Volume 4, pp. 45–67. [Google Scholar]
- Shalabney, A.; Abdulhalim, I. Sensitivity-enhancement methods for surface plasmon sensors. Laser Photon. Rev. 2011, 5, 571–606. [Google Scholar] [CrossRef]
- Abdulhalim, I.; Zourob, M.; Lakhtakia, A. Surface plasmon resonance for biosensing: A mini-review. Electromagnetics 2008, 28, 214–242. [Google Scholar] [CrossRef]
- Lee, J.W.; Sim, S.J.; Cho, S.M.; Lee, J. Characterization of a self-assembled monolayer of thiol on a gold surface and the fabrication of a biosensor chip based on surface plasmon resonance for detecting anti-gad antibody. Biosens. Bioelectron. 2005, 20, 1422–1427. [Google Scholar] [CrossRef]
- Kim, M.-G.; Shin, Y.-B.; Jung, J.-M.; Ro, H.-S.; Chung, B.H. Enhanced sensitivity of surface plasmon resonance (SPR) immunoassays using a peroxidase-catalyzed precipitation reaction and its application to a protein microarray. J. Immunol. Meth. 2005, 297, 125–132. [Google Scholar] [CrossRef]
- Chung, J.W.; Kim, S.D.; Bernhardt, R.; Pyun, J.C. Application of SPR biosensor for medical diagnostics of human hepatitis B virus (hHBV). Sens. Actuator. B Chem. 2005, 111–112, 416–422. [Google Scholar]
- Vijayendran, R.A.; Motsegood, K.M.; Beebe, D.J.; Leckband, D.E. Evaluation of a three-dimensional micromixer in a surface-based biosensor. Langmuir 2002, 19, 1824–1828. [Google Scholar]
- Frey, B.L.; Jordan, C.E.; Kornguth, S.; Corn, R.M. Control of the specific adsorption of proteins onto gold surfaces with poly(l-lysine) monolayers. Anal. Chem. 1995, 67, 4452–4457. [Google Scholar] [CrossRef]
- Brogan, K.L.; Schoenfisch, M.H. Influence of antibody immobilization strategy on molecular recognition force microscopy measurements. Langmuir 2005, 21, 3054–3060. [Google Scholar] [CrossRef]
- Haes, A.J.; van Duyne, R.P. A nanoscale optical biosensor: Sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. J. Am. Chem. Soc. 2002, 124, 10596–10604. [Google Scholar] [CrossRef]
- Riboh, J.C.; Haes, A.J.; McFarland, A.D.; Ranjit Yonzon, C.; van Duyne, R.P. A nanoscale optical biosensor: Real-time immunoassay in physiological buffer enabled by improved nanoparticle adhesion. J. Phys. Chem. B 2003, 107, 1772–1780. [Google Scholar] [CrossRef]
- Oh, B.-K.; Kim, Y.-K.; Lee, W.; Bae, Y.M.; Lee, W.H.; Choi, J.-W. Immunosensor for detection of legionella pneumophila using surface plasmon resonance. Biosens. Bioelectron. 2003, 18, 605–611. [Google Scholar] [CrossRef]
- Oh, B.-K.; Kim, Y.-K.; Park, K.W.; Lee, W.H.; Choi, J.-W. Surface plasmon resonance immunosensor for the detection of salmonella typhimurium. Biosens. Bioelectron. 2004, 19, 1497–1504. [Google Scholar] [CrossRef]
- Kang, C.D.; Lee, S.W.; Park, T.H.; Sim, S.J. Performance enhancement of real-time detection of protozoan parasite, cryptosporidium oocyst by a modified surface plasmon resonance (SPR) biosensor. Enzym. Microb. Tech. 2006, 39, 387–390. [Google Scholar] [CrossRef]
- Lee, H.J.; Nedelkov, D.; Corn, R.M. Surface plasmon resonance imaging measurements of antibody arrays for the multiplexed detection of low molecular weight protein biomarkers. Anal. Chem. 2006, 78, 6504–6510. [Google Scholar] [CrossRef]
- Veiseh, M.; Zareie, M.H.; Zhang, M. Highly selective protein patterning on gold-silicon substrates for biosensor applications. Langmuir 2002, 18, 6671–6678. [Google Scholar] [CrossRef]
- Chung, J.W.; Bernhardt, R.; Pyun, J.C. Sequential analysis of multiple analytes using a surface plasmon resonance (SPR) biosensor. J. Immunol. Meth. 2006, 311, 178–188. [Google Scholar] [CrossRef]
- Stigter, E.C.A.; Jong, G.J.D.; van Bennekom, W.P. An improved coating for the isolation and quantitation of interferon-γ in spiked plasma using surface plasmon resonance (SPR). Biosens. Bioelectron. 2005, 21, 474–482. [Google Scholar] [CrossRef]
- Patel, N.; Davies, M.C.; Hartshorne, M.; Heaton, R.J.; Roberts, C.J.; Tendler, S.J.B.; Williams, P.M. Immobilization of protein molecules onto homogeneous and mixed carboxylate-terminated self-assembled monolayers. Langmuir 1997, 13, 6485–6490. [Google Scholar] [CrossRef]
- Kausaite, A.; van Dijk, M.; Castrop, J.; Ramanaviciene, A.; Baltrus, J.P.; Acaite, J.; Ramanavicius, A. Surface plasmon resonance label-free monitoring of antibody antigen interactions in real time. Biochem. Mol. Biol. Educ. 2007, 35, 57–63. [Google Scholar] [CrossRef]
- Karabchevsky, A.; Auslender, M.; Abdulhalim, I. Dual surface plasmon excitation with thin metallic nanoslits. J. Nanophoton. 2011, 5, 051821–051829. [Google Scholar] [CrossRef]
- Karabchevsky, A.; Krasnykov, O.; Abdulhalim, I.; Hadad, B.; Goldner, A.; Auslender, M.; Hava, S. Metal grating on a substrate nanostructure for sensor applications. Photonics Nanostruct. 2009, 7, 170–175. [Google Scholar] [CrossRef]
- Karabchevsky, A.; Krasnykov, O.; Auslender, M.; Hadad, B.; Goldner, A.; Abdulhalim, I. Theoretical and experimental investigation of enhanced transmission through periodic metal nanoslits for sensing in water environment. Plasmonics 2009, 4, 281–292. [Google Scholar] [CrossRef]
- Karabchevsky, A.; Karabchevsky, S.; Abdulhalim, I. Fast surface plasmon resonance imaging sensor using radon transform. Sens. Actuator. B Chem. 2011, 155, 361–365. [Google Scholar] [CrossRef]
- Karabchevsky, A.; Khare, C.; Rauschenbach, B.; Abdulhalim, I. Microspot sensing based on surface-enhanced fluorescence from nanosculptured thin films. J. Nanophoton. 2012, 6, 1–12. [Google Scholar]
- Sesay, A.M.; Cullen, D.C. Detection of hormone mimics in water using a miniturised SPR sensor. Environ. Monit. Assess. 2001, 70, 83–92. [Google Scholar] [CrossRef]
- Geisler, J.; Berntsen, H.; Lønning, P.E. A novel HPLC-RIA method for the simultaneous detection of estrone, estradiol and estrone sulphate levels in breast cancer tissue. J. Steroid Biochem. Mol. Biol. 2000, 72, 259–264. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, K.R.; Chung, B.C. Determination of estrone and 17β-estradiol in human hair by gas chromatography-mass spectrometry. Analyst 2000, 125, 711–714. [Google Scholar] [CrossRef]
- Xiao, X.Y.; McCalley, D.V.; McEvoy, J. Analysis of estrogens in river water and effluents using solid-phase extraction and gas chromatography-negative chemical ionisation mass spectrometry of the pentafluorobenzoyl derivatives. J. Chrom. A 2001, 923, 195–204. [Google Scholar] [CrossRef]
- Kurauchi, K.; Nakaguchi, Y.; Tsutsumi, M.; Hori, H.; Kurihara, R.; Hashimoto, S.; Ohnuma, R.; Yamamoto, Y.; Matsuoka, S.; Kawai, S. In vivo visual reporter system for detection of estrogen -like substances by transgenic medaka. Environ. Sci. Technol. 2005, 39, 2762–2768. [Google Scholar] [CrossRef]
- Raether, H. Surface Plasmons on Smooth and Rough Surfaces and on Gratings; Springer-Verlag: Berlin, Germay, 1988. [Google Scholar]
- Karabchevsky, A.; Karabchevsky, S.; Abdulhalim, I. Nanoprecision algorithm for surface plasmon resonance determination from images with low contrast for improved sensor resolution. J. Nanophoton. 2011, 5, 1–12. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Karabchevsky, A.; Tsapovsky, L.; Marks, R.S.; Abdulhalim, I. Study of Immobilization Procedure on Silver Nanolayers and Detection of Estrone with Diverged Beam Surface Plasmon Resonance (SPR) Imaging. Biosensors 2013, 3, 157-170. https://doi.org/10.3390/bios3010157
Karabchevsky A, Tsapovsky L, Marks RS, Abdulhalim I. Study of Immobilization Procedure on Silver Nanolayers and Detection of Estrone with Diverged Beam Surface Plasmon Resonance (SPR) Imaging. Biosensors. 2013; 3(1):157-170. https://doi.org/10.3390/bios3010157
Chicago/Turabian StyleKarabchevsky, Alina, Lev Tsapovsky, Robert S. Marks, and Ibrahim Abdulhalim. 2013. "Study of Immobilization Procedure on Silver Nanolayers and Detection of Estrone with Diverged Beam Surface Plasmon Resonance (SPR) Imaging" Biosensors 3, no. 1: 157-170. https://doi.org/10.3390/bios3010157






