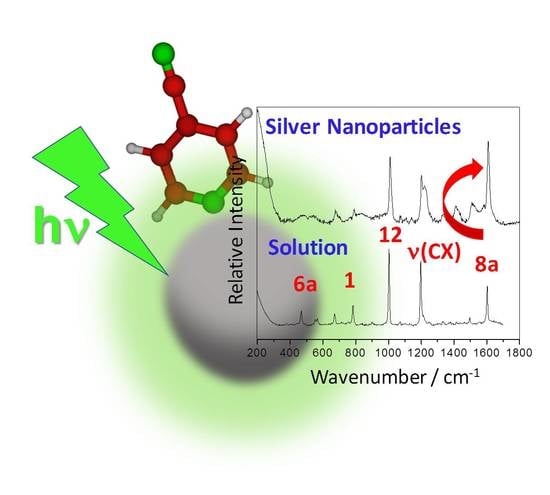
A DFT Approach to the Surface-Enhanced Raman Scattering of 4-Cyanopyridine Adsorbed on Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.2. Computational Details
3. Results and Discussion
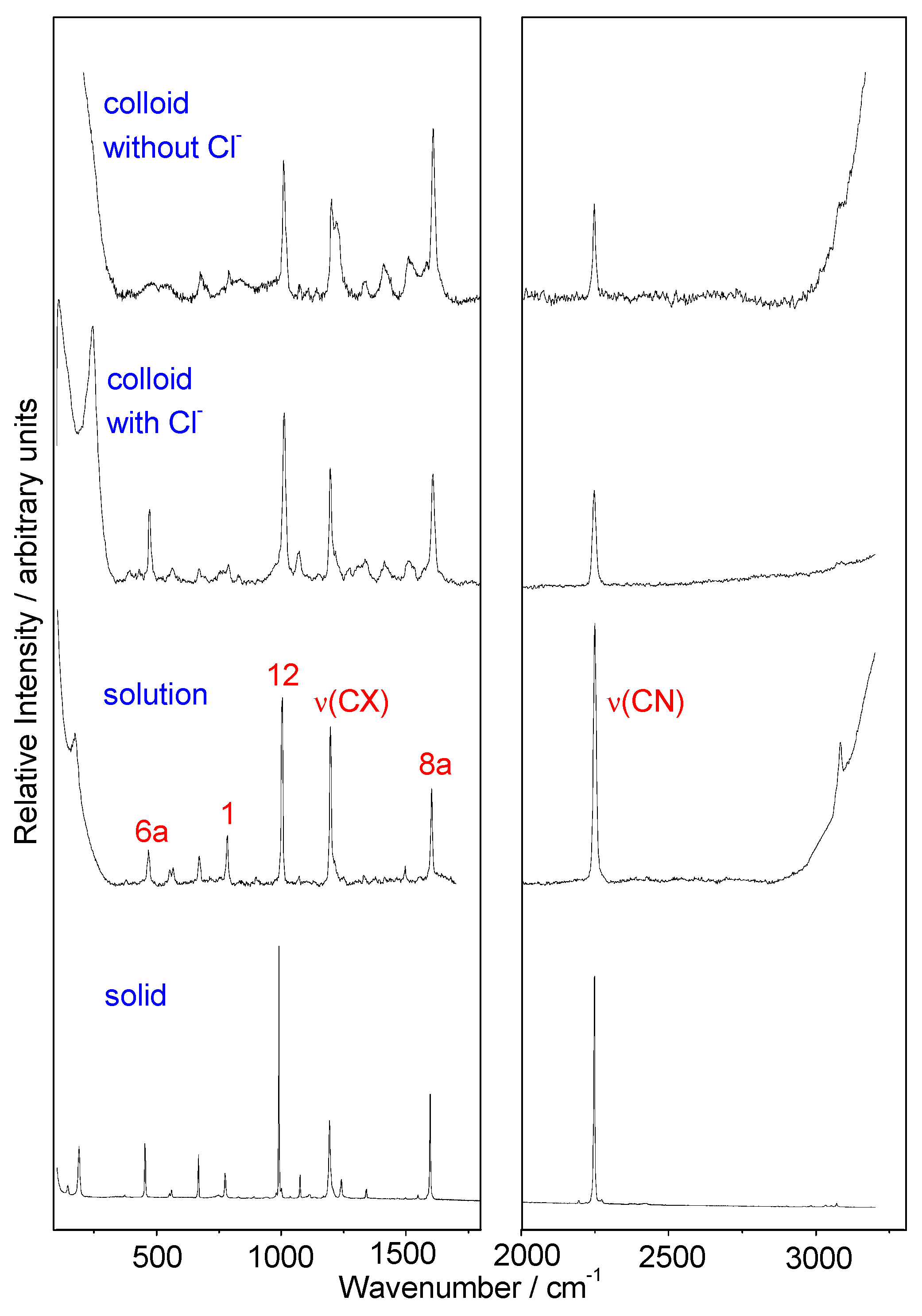
3.1. Raman and SERS Spectra of 4CNPy. Vibrational Assignment
3.2. DFT Calculations. SERS Wavenumber Shifts
3.3. DFT Calculations. Raman Intensities of Isolated 4CNPy or Bonded to Silver Clusters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Furini, L.; Constantino, C.; Sanchez-Cortes, S.; Otero, J.; López-Tocón, I. Adsorption of carbendazim pesticide on plasmonic nanoparticles studied by surface-enhanced Raman scattering. J. Colloid Interface Sci. 2016, 465, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tocón, I.L.; Otero, J.C.; Arenas, J.F.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Multicomponent Direct Detection of Polycyclic Aromatic Hydrocarbons by Surface-Enhanced Raman Spectroscopy Using Silver Nanoparticles Functionalized with the Viologen Host Lucigenin. Anal. Chem. 2011, 83, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Surface-enhanced Raman scattering (SERS)—A tool for single molecule detection in solution. In Single-Molecule Detection in Solution: Methods and Applications; Enderlein, J., Keller, R.A., Zander, C., Eds.; VCH-Wiley: Weinheim, Germany, 2001. [Google Scholar]
- Muehlethaler, C.; Leona, M.; Lombardi, J.R. Review of Surface Enhanced Raman Scattering Applications in Forensic Science. Anal. Chem. 2016, 88, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Morla-Folch, J.; Xie, H.-N.; Gisbert-Quilis, P.; Pedro, S.G.-D.; Pazos-Perez, N.; Alvarez-Puebla, R.A.; Guerrini, L. Ultrasensitive Direct Quantification of Nucleobase Modifications in DNA by Surface-Enhanced Raman Scattering: The Case of Cytosine. Angew. Chem. 2015, 127, 13854–13858. [Google Scholar] [CrossRef]
- Prado, E.; Colin, A.; Servant, L.; LeComte, S. SERS Spectra of Oligonucleotides as Fingerprints to Detect Label-Free RNA in Microfluidic Devices. J. Phys. Chem. C 2014, 118, 13965–13971. [Google Scholar] [CrossRef]
- Cailletaud, J.; De Bleye, C.; Dumont, E.; Sacré, P.-Y.; Netchacovitch, L.; Gut, Y.; Boiret, M.; Ginot, Y.-M.; Hubert, P.; Ziemons, E. Critical review of surface-enhanced Raman spectroscopy applications in the pharmaceutical field. J. Pharm. Biomed. Anal. 2018, 147, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, F.; Leona, M. Surface-enhanced Raman spectroscopy in art and archaeology. J. Raman Spectrosc. 2016, 47, 67–77. [Google Scholar] [CrossRef]
- Magdas, D.A.; Pinzaru, S.C.; Guyon, F.; Feher, I.; Cozar, B.I. Application of SERS technique in white wines discrimination. Food Control 2018, 92, 30–36. [Google Scholar] [CrossRef]
- Gukowsky, J.C.; Xie, T.; Gao, S.; Qu, Y.; He, L. Rapid identification of artificial and natural food colorants with surface enhanced Raman spectroscopy. Food Control 2018, 92, 267–275. [Google Scholar] [CrossRef]
- Creighton, J.A. The selection rules for surface-enhanced Raman spectroscopy. In Spectroscopy of Surfaces; Clark, R.J.H., Hester, R.E., Eds.; Wiley: Chichester, UK, 1988. [Google Scholar]
- Clark, R.J.H.; Dines, T.J. Resonance Raman Spectroscopy, and Its Application to Inorganic Chemistry. New Analytical Methods. Angew. Chem. Int. Ed. Engl. 1986, 25, 131–158. [Google Scholar] [CrossRef]
- Devine, T.M. Use of Surface-Enhanced Raman Spectroscopy in Studies of Electrode-Electrolyte Interfaces. In Electrochemical and Optical Techniques for the Study and Monitoring of Metallic Corrosion; Springer Science and Business Media LLC: Berlin, Germany, 1991; pp. 389–437. [Google Scholar]
- Shi, C.; Zhang, W.; Birke, R.L.; Lombardi, J.R. SERS investigation of the adsorption and electroreduction of 4-cyanopyridine on a silver electrode. J. Electroanal. Chem. 1997, 423, 67–81. [Google Scholar] [CrossRef]
- Allen, C.S.; Van Duyne, R.P. Orientational specificity of Raman scattering from molecules adsorbed on silver electrodes. Chem. Phys. Lett. 1979, 63, 455–459. [Google Scholar] [CrossRef]
- Furukawa, H.; Takahashi, M.; Ito, M. A surface-enhanced Raman study of the electrochemical reduction of 4-cyanopyridine. Chem. Phys. Lett. 1986, 132, 498–501. [Google Scholar] [CrossRef]
- Rubim, J.C. Surface-enhanced Raman scattering (SERS) on silver electrodes as a technical tool in the study of the electrochemical reduction of cyanopyridines and in quantitative analysis. J. Electroanal. Chem. Interfacial Electrochem. 1987, 220, 339–350. [Google Scholar] [CrossRef]
- Osaki, T.; Yoshikawa, T.; Satoh, Y.; Shimada, R. Adsorption of pyridine, ?-picoline and isonicotinonitrile on Ag colloidal particles studied by surface-enhanced Raman scattering spectroscopy. J. Raman Spectrosc. 2005, 36, 199–207. [Google Scholar] [CrossRef]
- Coyle, C.M.; Chumanov, G.; Jagodzinski, P.W. Surface-enhanced Raman spectra of the reduction product of 4-cyanopyridine on copper colloids. J. Raman Spectrosc. 1998, 29, 757–762. [Google Scholar] [CrossRef]
- Otto, A.; Pockrand, I.; Billmann, J.; Pettenkofer, C. Surface Enhanced Raman Scattering; Chang, R.K., Furtak, T.E., Eds.; Springer: Boston, MA, USA, 1982; pp. 147–172. [Google Scholar]
- Otto, A.; Billmann, J.; Eickmans, J.; Ertürk, U.; Pettenkofer, C. The “adatom model” of SERS (Surface Enhanced Raman Scattering): The present status. Surf. Sci. 1984, 138, 319–338. [Google Scholar] [CrossRef]
- Otto, A.J. The “chemical” (electronic) contribution to surface-enhanced Raman scattering. J Raman Spectrosc. 2005, 36, 497–509. [Google Scholar] [CrossRef]
- Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. Raman and Computational Study on the Adsorption of Xanthine on Silver Nanocolloids. ACS Omega 2018, 3, 13530–13537. [Google Scholar] [CrossRef]
- Wrzosek, B.; Cukras, J.; Dobrowolski, M.A.; Bukowska, J.J. Real Chemical States of the 3-Sulfur Derivative of 1,2,4-Triazole in Different Conditions: Complex Experimental and Theoretical Studies. Phys. Chem. C 2017, 121, 9282–9295. [Google Scholar] [CrossRef]
- Harroun, S.G.; Zhang, Y.; Chen, T.H.; Ku, C.R.; Chang, H.T. Biomarkers of cigarette smoking and DNA methylating agents: Raman, SERS and DFT study of 3-methyladenine and 7-methyladenine. Spectrochim.Acta A 2017, 176, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Miranda, M.; Muniz-Miranda, F.; Pedone, A. Raman and DFT study of methimazole chemisorbed on gold colloidal nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 5974–5980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shalalfeh, M.M.; Saleh, T.A.; Al-Saadi, A.A. Silver colloid and film substrates in surface-enhanced Raman scattering for 2-thiouracil detection. RSC Adv. 2016, 6, 75282–75292. [Google Scholar] [CrossRef]
- Owen, A.R.; Golden, J.W.; Price, A.S.; Henry, W.A.; Barker, W.R.; Perry, D.A.J. Surface-Enhanced Vibrational Spectroscopy and Density Functional Theory Study of Isoniazid Layers Adsorbed on Silver Nanostructures. Phys. Chem. C 2014, 118, 28959–28969. [Google Scholar] [CrossRef]
- Pagliai, M.; Caporali, S.; Muniz-Miranda, M.; Pratesi, G.; Schettino, V.J. SERS, XPS, and DFT Study of Adenine Adsorption on Silver and Gold Surfaces. Phys. Chem. Lett. 2012, 3, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, D.; Wang, M.; Dong, R.; Li, W. Cyanopyridine based bipolar host materials for phosphorescent light-emitting diodes with low efficiency roll-off: Importance of charge balance. Dye. Pigment. 2018, 159, 230–237. [Google Scholar] [CrossRef]
- Creighton, J.A.; Blatchford, C.G.; Albrecht, M.G. Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength. J. Chem. Soc., Faraday Trans. 2 1979, 75, 790. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Neto, N.; Sbrana, G. Surface enhanced Raman scattering of pyrazine adsorbed on silver colloidal particles. J. Mol. Struct. 1986, 143, 275–278. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Aranda, D.; Valdivia, S.; Soto, J.; López-Tocón, I.; Avila, F.J.; Otero, J.C. Theoretical Approaches for Modeling the Effect of the Electrode Potential in the SERS Vibrational Wavenumbers of Pyridine Adsorbed on a Charged Silver Surface. Front. Chem. 2019, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Centeno, S.P.; López-Tocón, I.; Roman-Perez, J.; Arenas, J.F.; Soto, J.; Otero, J.C. Franck−Condon Dominates the Surface-Enhanced Raman Scattering of 3-Methylpyridine: Propensity Rules of the Charge-Transfer Mechanism under Reduced Symmetry. J. Phys. Chem. C 2012, 116, 23639–23645. [Google Scholar] [CrossRef]
- Sardo, M.; Ruano, C.; Castro, J.L.; López-Tocón, I.; Soto, J.; Ribeiro-Claro, P.; Otero, J.C. Surface-enhanced Raman scattering of 5-fluorouracil adsorbed on silver nanostructures. Phys. Chem. Chem. Phys. 2009, 11, 7437. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Long, D.A. The Raman Effect: A Unified Treatment of the Theory of Raman Scattering by Molecules; John Wiley& Sons, Ltd.: West Sussex, UK, 2002. [Google Scholar]
- Arenas, J.F.; Castro, J.L.; Otero, J.C.; Marcos, J.I. Surface-enhanced Raman spectra of pyrazinecarboxamide and pyrazinecarbonitrile on silver sols. J. Raman Spectrosc. 1992, 23, 249–252. [Google Scholar] [CrossRef]
- Arenas, J.F.; Montañez, M.A.; Otero, J.C.; Marcos, J.I. Surface enhanced Raman spectra of 2-cyanopyridine and picolamide. J. Mol. Struct. 1993, 293, 341–344. [Google Scholar] [CrossRef]
- Otto, A.; Futamata, M. Electronic Mechanism of SERS. In Surface-Enhanced Raman Scattering, Topics in Applied Physics; Kneipp, K., Moskovits, M., Kneipp, H., Eds.; Springer: Berlin, Germany, 2006; pp. 147–182. [Google Scholar]
- Creighton, J.A. Spectroscopy of Surfaces; Clark, R.H.J., Hester, R.E., Eds.; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Green, J.; Harrison, D. Vibrational spectra of cyano-, formyl- and halogeno-pyridines. Spectrochim. Acta Part A Mol. Spectrosc. 1977, 33, 75–79. [Google Scholar] [CrossRef]
- Marquez, F.; Tocón, I.L.; Otero, J.; Marcos, J. A priori SQM vibrational spectrum of 2,2′-bipyridine. J. Mol. Struct. 1997, 410, 447–450. [Google Scholar] [CrossRef]
- Arenas, J.F.; López Tocón, I.; Otero, J.C.; Marcos, J.I. Vibrational spectra of methylpyridines. J. Mol. Struct. 1999, 476, 139–150. [Google Scholar] [CrossRef]
- Varsanyi, G. Vibrational Spectra of Benzene Derivatives; Academic Press: New York, NY, USA, 1969. [Google Scholar]
- Leopold, N.; Stefancu, A.; Herman, K.; Tódor, I.S.; Iancu, S.D.; Moisoiu, V.; Leopold, L.F. The role of adatoms in chloride-activated colloidal silver nanoparticles for surface-enhanced Raman scattering enhancement. Beilstein J. Nanotechnol. 2018, 9, 2236–2247. [Google Scholar] [CrossRef]
- Gellini, C.; Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. SERS active Ag–SiO2 nanoparticles obtained by laser ablation of silver in colloidal silica. Beilstein J. Nanotechnol. 2018, 9, 2396–2404. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Muniz-Miranda, M. SERS investigation on the adsorption and photoreaction of 4-nitroanisole in Ag hydrosols. J. Raman Spectrosc. 2013, 44, 1416–1421. [Google Scholar] [CrossRef]
- Arenas, J.F.; Tocón, I.L.; Otero, J.C.; Marcos, J.I. Charge Transfer Processes in Surface-Enhanced Raman Scattering. Franck−Condon Active Vibrations of Pyridine. J. Phys. Chem. 1996, 100, 9254–9261. [Google Scholar] [CrossRef]
- Roman-Perez, J.; Centeno, S.P.; López-Ramirez, M.R.; Arenas, J.F.; Soto, J.; López-Tocón, I.; Otero, J.C. On the dual character of charged metal-molecule hybrids and the opposite behavior of the forward and reverse CT processes. Phys. Chem. Chem. Phys. 2014, 16, 22958–22961. [Google Scholar] [CrossRef]
- Avila, F.; Ruano, C.; López-Tocón, I.; Arenas, J.F.; Soto, J.; Otero, J.C. How the electrode potential controls the selection rules of the charge transfer mechanism of SERS. Chem. Commun. 2011, 47, 4213. [Google Scholar] [CrossRef]
- Arenas, J.; Tocón, I.L.; Centeno, S.; Soto, J.; Otero, J.; Centeno, S. How a resonant charge transfer mechanism determines the relative intensities in the SERS spectra of 4-methylpyridine. Vib. Spectrosc. 2002, 29, 147–154. [Google Scholar] [CrossRef]
- Avila, F.; Fernandez, D.J.; Arenas, J.F.; Otero, J.C.; Soto, J. Modelling the effect of the electrode potential on the metal–adsorbate surface states: Relevant states in the charge transfer mechanism of SERS. Chem. Commun. 2011, 47, 4210. [Google Scholar] [CrossRef]
- Arenas, J.F.; Soto, J.; Pelaez, D.; Fernandez, D.J.; Otero, J.C. Understanding complex surface-enhanced Raman scattering, using quantum chemical calculations. Int. J. Quantum Chem. 2005, 104, 681–694. [Google Scholar] [CrossRef]
- Roman-Perez, J.; Ruano, C.; Centeno, S.P.; López-Tocón, I.; Arenas, J.F.; Soto, J.; Otero, J.C. Huge Energy Gain in Metal-to-Molecule Charge Transfer Processes: A Combined Effect of an Electrical Capacitive Enhancement in Nanometer-Size Hot Spots and the Electronic Structure of the Surface Complex. J. Phys. Chem. C 2014, 118, 2718–2725. [Google Scholar] [CrossRef]
- Aranda, D.; Valdivia, S.; Avila, F.J.; Soto, J.; Otero, J.C.; Tocón, I.L.; Ruiz, D.A.; Mantas, S.V. Charge transfer at the nanoscale and the role of the out-of-plane vibrations in the selection rules of surface-enhanced Raman scattering. Phys. Chem. Chem. Phys. 2018, 20, 29430–29439. [Google Scholar] [CrossRef]

| . | Experimental | Calculated B3LYP/LanL2DZ | ||||||
|---|---|---|---|---|---|---|---|---|
| Assignment | Solution | SERS 1 | SERS 2 | 4CNPy 3 | Ag2–N | Ag+–N | Ag2–CN | Ag+–CN |
| 2, ν(CH);A1 | 3084 | 4 | - | 3248 | 7 | 18 | 1 | 5 |
| ν(CN);A1 | 2250 | −2 | −2 | 2271 | 6 | 13 | 26 | 26 |
| 8a, νring;A1 | 1602 | 6 | 6 | 1623 | 15 | 28 | −3 | −20 |
| ν(CX), νring+ν(CN);A1 | 1198 | 4 | −2 | 1230 | 3 | 10 | 1 | −2 |
| 18a, δ(CH);A1 | 1072 | - | 0 | 1092 | −3 | −7 | 0 | −3 |
| 12;δring;A1 | 1004 | 6 | 8 | 987 | 23 | 40 | −1 | −4 |
| 1, νring+ν(CN);A1 | 784 | 6 | 6 | 774 | 12 | 23 | 4 | 8 |
| 6a, δring;A1 | 468 | - | 4 | 460 | 16 | 33 | 15 | 37 |
| 3, δ(CH);B2 | 1330 | 4 | 6 | 1369 | 1 | 8 | 1 | 1 |
| 6b, δring;B2 | 672 | 4 | 0 | 684 | −1 | −4 | 0 | −4 |
| δ(CCN);B2 | 566 | - | −4 | 563 | −1 | −3 | 12 | 20 |
| Experimental | Calculated B3LYP/LanL2DZ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Modes | ν (cm−1) | Sol. 1 | SERS 2 | SERS 3 | 4CNPy | Ag2-N | Ag+-N | Ag2-CN | Ag+-CN |
| ν(CN);A1 | 2250 | 1.37 | 0.70 | 0.57 | 2.66 | 2.33 | 11.54 | 11.30 | 5.73 |
| 8a, νring;A1 | 1602 | 0.51 | 1.22 | 0.63 | 0.77 | 1.70 | 2.29 | 1.70 | 1.18 |
| ν(CX);A1 | 1198 | 0.84 | 0.71 | 0.67 | 0.68 | 0.47 | 4.80 | 0.80 | 0.95 |
| 12, δring;A1 | 1004 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1, νring;A1 | 784 | 0.26 | 0.19 | 0.11 | 0.27 | 0.11 | 2.72 | 0.45 | 0.46 |
| 6a, δring;A1 | 468 | 0.18 | - | 0.43 | 0.31 | 0.36 | 0.18 | 1.70 | 0.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Tocón, I.; Valdivia, S.; Soto, J.; Otero, J.C.; Muniz-Miranda, F.; Menziani, M.C.; Muniz-Miranda, M. A DFT Approach to the Surface-Enhanced Raman Scattering of 4-Cyanopyridine Adsorbed on Silver Nanoparticles. Nanomaterials 2019, 9, 1211. https://doi.org/10.3390/nano9091211
López-Tocón I, Valdivia S, Soto J, Otero JC, Muniz-Miranda F, Menziani MC, Muniz-Miranda M. A DFT Approach to the Surface-Enhanced Raman Scattering of 4-Cyanopyridine Adsorbed on Silver Nanoparticles. Nanomaterials. 2019; 9(9):1211. https://doi.org/10.3390/nano9091211
Chicago/Turabian StyleLópez-Tocón, Isabel, Samuel Valdivia, Juan Soto, Juan Carlos Otero, Francesco Muniz-Miranda, Maria Cristina Menziani, and Maurizio Muniz-Miranda. 2019. "A DFT Approach to the Surface-Enhanced Raman Scattering of 4-Cyanopyridine Adsorbed on Silver Nanoparticles" Nanomaterials 9, no. 9: 1211. https://doi.org/10.3390/nano9091211