A Novel Route to Manufacture 2D Layer MoS2 and g-C3N4 by Atmospheric Plasma with Enhanced Visible-Light-Driven Photocatalysis
Abstract
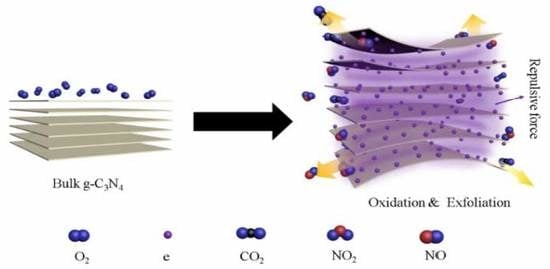
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterizations
2.3. Photocatalytic Activity
2.4. Photoelectrochemical Measurements
3. Results and Discussion
3.1. Sample Characterization
3.2. Morphology
3.3. Mechanism Analysis
3.4. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fu, J.W.; Yu, J.G.; Jiang, C.J.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 752–7535. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Wang, W.; Wang, F.; Di, L.B.; Yang, S.C.; Zhu, S.J.; Yao, Y.B.; Ma, C.H.; Dai, B.; Yu, F. Enhanced Photocatalytic Degradation of Organic Dyes via Defect-Rich TiO2 Prepared by Dielectric Barrier Discharge Plasma. Nanomaterials 2019, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Di, L.B.; Xu, Z.J.; Wang, K.; Zhang, X.L. A facile method for preparing Pt/TiO2 photocatalyst with enhanced activity using dielectric barrier discharge. Catal. Today 2013, 211, 109–113. [Google Scholar] [CrossRef]
- Haque, F.; Daeneke, T.; Kalantar-Zadeh, K.; Ou, J.Z. Two-Dimensional Transition Metal Oxide and Chalcogenide-Based Photocatalysts. Nano-Micro Lett. 2018, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.B.; Choudhary, N.; Han, G.H.; Park, J.H.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Tan, C.L.; Cao, X.H.; Wu, X.J.; He, Q.Y.; Yang, J.; Zhang, X.; Chen, J.Z.; Zhao, W.; Han, S.K.; Nam, G.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, H.; Peng, W. 2D Transition Metal Dichalcogenides and Graphene-Based Ternary Composites for Photocatalytic Hydrogen Evolution and Pollutants Degradation. Nanomaterials 2017, 7, 62. [Google Scholar] [CrossRef]
- Liu, P.B.; Zhang, Y.Q.; Yan, J.; Huang, Y.; Xia, L.; Guang, Z.X. Synthesis of lightweight N-doped graphene foams with open reticular structure for high-efficiency electromagnetic wave absorption. Chem. Eng. J. 2019, 368, 285–298. [Google Scholar] [CrossRef]
- Truong, L.; Jerng, S.; Roy, S.B.; Jeon, J.H.; Kim, K.; Akbar, K.; Yi, Y.; Chun, S. Chrysanthemum-Like CoP Nanostructures on Vertical Graphene Nanohills as Versatile Electrocatalysts for Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 4625–4630. [Google Scholar] [CrossRef]
- He, Q.G.; Liu, J.; Tian, Y.L.; Wu, Y.Y.; Magesa, F.; Deng, P.H.; Li, G.L. Facile Preparation of Cu2O Nanoparticles and Reduced Graphene Oxide Nanocomposite for Electrochemical Sensing of Rhodamine B. Nanomaterials 2019, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Nikokavoura, A.; Trapalis, C. Graphene and g-C3N4 based photocatalysts for NOx removal: A review. Appl. Surf. Sci. 2018, 430, 18–52. [Google Scholar] [CrossRef]
- Krasian, T.; Punyodom, W.; Worajittiphon, P. A hybrid of 2D materials (MoS2 and WS2) as an effective performance enhancer for poly (lactic acid) fibrous mats in oil adsorption and oil/water separation. Chem. Eng. J. 2019, 369, 563–575. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.J.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, Y.H.; Lee, S.Y.; Sohn, W.; Lee, J.E.; Kim, D.H.; Shim, Y.; Kwon, K.C.; Choi, K.S.; Yoo, H.J.; et al. Highly selective and sensitive chemoresistive humidity sensors based on rGO/MoS2 van der Waals composites. J. Mater. Chem. A 2018, 6, 5016–5024. [Google Scholar] [CrossRef]
- Xie, J.F.; Zhang, J.J.; Li, S.; Grote, F.B.; Zhang, X.D.; Zhang, H.; Wang, R.X.; Lei, Y.; Pan, B.C.; Xie, Y. Controllable Disorder Engineering in Oxygen-Incorporated MoS2 Ultrathin Nanosheets for Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.Y.; Liu, B.; Zou, X.L.; Cheng, H.M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.X.; Zhang, X.S.; Jang, B.W.L.; Liu, C.J. Catalyst Preparation with Plasmas: How Does It Work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
- Di, L.B.; Zhang, J.S.; Zhang, X.L. A review on the recent progress, challenges, and perspectives of atmospheric-pressure cold plasma for preparation of supported metal catalysts. Plasma Process. Polym. 2018, 15, 1700234. [Google Scholar] [CrossRef]
- Zhang, J.S.; Di, L.B.; Yu, F.; Duan, D.Z.; Zhang, X.L. Atmospheric-Pressure Cold Plasma Activating Au/P25 for CO Oxidation: Effect of Working Gas. Nanomaterials 2018, 8, 742. [Google Scholar] [CrossRef]
- Sun, Q.D.; Yu, B.; Liu, C.J. Characterization of ZnO Nanotube Fabricated by the Plasma Decomposition of Zn(OH)2 Via Dielectric Barrier Discharge. Plasma Chem. Plasma Process. 2012, 32, 201–209. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.K.; Sunkara, M.K.; Bogaerts, A. Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Y.Y.; Liu, D.D.; Zou, Y.Q.; Wang, S.Y. Water-Plasma-Enabled Exfoliation of Ultrathin Layered Double Hydroxide Nanosheets with Multivacancies for Water Oxidation. Adv. Mater. 2017, 29, 1701546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Yu, F.; Zhu, M.Y.; Ma, C.H.; Zhao, D.; Wang, C.; Zhou, A.M.; Dai, B.; Ji, J.Y.; Guo, X.H. N-Doping of plasma exfoliated graphene oxide via dielectric barrier discharge plasma treatment for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 2011–2017. [Google Scholar] [CrossRef]
- Peng, X.F.; Wang, Z.H.; Wang, Z.; Gong, J.B.; Hao, H.X. Electron reduction for the preparation of rGO with high electrochemical activity. Catal. Today 2019, 2, 1–6. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Z.; Marcus, K.; Li, Z.; Luo, B.; Zhou, L.; Wang, X.; Du, Y.; Yang, Y. MoS2/TiO2 heterostructures as nonmetal plasmonic photocatalysts for highly efficient hydrogen evolution. Energy Environ. Sci. 2018, 11, 106–114. [Google Scholar] [CrossRef]
- Li, Z.Z.; Meng, X.C.; Zhang, Z.S. Recent development on MoS2-based photocatalysis: A review. J. Photochem. Photobiol. C Photochem. Rev. 2018, 35, 39–55. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Yin, S.M.; Han, J.Y.; Zhou, T.H.; Xu, R. Recent progress in g-C3N4 based low cost photocatalytic system: Activity enhancement and emerging applications. Catal. Sci. Technol. 2015, 5, 5048–5061. [Google Scholar] [CrossRef]
- Wang, Y.G.; Li, Y.G.; Bai, X.; Cai, Q.; Liu, C.L.; Zuo, Y.H.; Kang, S.F.; Cui, L.F. Facile synthesis of Y-doped graphitic carbon nitride with enhanced photocatalytic performance. Catal. Commun. 2016, 84, 179–182. [Google Scholar] [CrossRef]
- Wang, G.Z.; Zhou, F.; Yuan, B.F.; Xiao, S.Y.; Kuang, A.L.; Zhong, M.M.; Dang, S.H.; Long, X.J.; Zhang, W.L. Strain-Tunable Visible-Light-Responsive Photocatalytic Properties of Two-Dimensional CdS/g-C3N4: A Hybrid Density Functional Study. Nanomaterials 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.F.; Zhang, H.; Li, S.; Wang, R.X.; Sun, X.; Zhou, M.; Zhou, J.F.; Lou, X.W.D.; Xie, Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Zhang, L.L.; Liu, G.; Cheng, H.M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated Graphitic Carbon Nitride Nanosheets as Efficient Catalysts for Hydrogen Evolution Under Visible Light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.T.; Li, M.M.J.; Ye, L.; Wise Man, S.; Liu, G.L.; Qu, J.; Nakagawa, K.; Tsang, S.C.E. The remarkable activity and stability of a dye-sensitized single molecular layer MoS2 ensemble for photocatalytic hydrogen production. Chem. Commun. 2015, 51, 13496–13499. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Islam, M.A.; Kim, J.H.; Ko, T.; Schropp, A.; Hurtado, L.; Weitzman, D.; Zhai, L.; Jung, Y. Two-dimensional transition metal dichalcogenide hybrid materials for energy applications. Nano Today 2018, 19, 16–40. [Google Scholar] [CrossRef]
- Lin, Q.Y.; Li, L.; Liang, S.J.; Liu, M.H.; Bi, J.H.; Wu, L. Efficient synthesis of monolayer carbon nitride 2D nanosheet with tunable concentration and enhanced visible-light photocatalytic activities. Appl. Catal. B Environ. 2015, 163, 135–142. [Google Scholar] [CrossRef]
- Zhang, J.S.; Chen, Y.; Wang, X.C. Two-dimensional covalent carbon nitride nanosheets: Synthesis, functionalization, and applications. Energy Environ. Sci. 2015, 8, 3092–3108. [Google Scholar] [CrossRef]
- Latorre Sánchez, M.; Esteve Adell, I.; Primo, A.; García, H. Innovative preparation of MoS2–graphene heterostructures based on alginate containing (NH4)2MoS4 and their photocatalytic activity for H2 generation. Carbon 2015, 81, 587–596. [Google Scholar] [CrossRef]
- Zheng, S.Z.; Zheng, L.J.; Zhu, Z.Y.; Chen, J.; Kang, J.L.; Huang, Z.L.; Yang, D.C. MoS2 Nanosheet Arrays Rooted on Hollow rGO Spheres as Bifunctional Hydrogen Evolution Catalyst and Supercapacitor Electrode. Nano-Micro Lett. 2018, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, G.; Jiang, C.; Bao, H.; Xu, Q. Synthesis of core-shell TiO2 @g-C3N4 hollow microspheres for efficient photocatalytic degradation of rhodamine B under visible light. Appl. Surf. Sci. 2018, 430, 263–272. [Google Scholar] [CrossRef]
- Li, F.T.; Liu, S.J.; Xue, Y.B.; Wang, X.J.; Hao, Y.J.; Zhao, J.; Liu, R.H.; Zhao, D.S. Structure Modification Function of g-C3N4 for Al2O3 in the In Situ Hydrothermal Process for Enhanced Photocatalytic Activity. Chem. A Eur. J. 2015, 21, 10149–10159. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.F.; Zhu, B.C.; Yu, J.G.; Cao, S.W.; Jaroniec, M. Ultra-thin nanosheet assemblies of graphitic carbon nitride for enhanced photocatalytic CO2 reduction. J. Mater. Chem. A 2017, 5, 3230–3238. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Su, C.S.; Ren, H.; Li, M.; Zhu, L.F.; Ge, S.; Wang, M.; Zhang, Z.L.; Li, L.; Cao, X.B. In-Situ Fabrication of g-C3N4/ZnO Nanocomposites for Photocatalytic Degradation of Methylene Blue: Synthesis Procedure Does Matter. Nanomaterials 2019, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, E.Z.; Ma, Y.N.; Hu, X.Y.; Wan, J.; Sun, L.; Fan, J. Synthesis of MoS2/g-C3N4 nanosheets as 2D heterojunction photocatalysts with enhanced visible light activity. Appl. Surf. Sci. 2016, 364, 694–702. [Google Scholar] [CrossRef]
- Zou, L.R.; Huang, G.F.; Li, D.F.; Liu, J.H.; Pan, A.L.; Huang, W.Q. A facile and rapid route for synthesis of g-C3N4 nanosheets with high adsorption capacity and photocatalytic activity. RSC Adv. 2016, 6, 86688–86694. [Google Scholar] [CrossRef]
- Cai, X.G.; He, J.Y.; Chen, L.; Chen, K.; Li, Y.L.; Zhang, K.S.; Jin, Z.; Liu, J.Y.; Wang, C.M.; Wang, X.G.; et al. A 2D-g-C3N4 nanosheet as an eco-friendly adsorbent for various environmental pollutants in water. Chemosphere 2017, 171, 192–201. [Google Scholar] [CrossRef]
- Ding, W.; Liu, S.Q.; He, Z. One-step synthesis of graphitic carbon nitride nanosheets for efficient catalysis of phenol removal under visible light. Chin. J. Catal. 2017, 38, 1711–1718. [Google Scholar] [CrossRef]
- Li, Y.F.; Fang, L.; Jin, R.X.; Yang, Y.; Fang, X.; Xing, Y.; Song, S.Y. Preparation and enhanced visible light photocatalytic activity of novel g-C3N4 nanosheets loaded with Ag2CO3 nanoparticles. Nanoscale 2015, 7, 758–764. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.W.; Shi, R.; Zhu, Y.F. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Miao, H.; Zhang, G.W.; Hu, X.Y.; Mu, J.L.; Han, T.X.; Fan, J.; Zhu, C.J.; Song, L.X.; Bai, J.T.; Hou, X. A novel strategy to prepare 2D g-C3N4 nanosheets and their photoelectrochemical properties. J. Alloys Compd. 2017, 690, 669–676. [Google Scholar] [CrossRef]
- Xiao, J.W.; Zhang, Y.; Chen, H.J.; Xu, N.S.; Deng, S.Z. Enhanced Performance of a Monolayer MoS2/WSe2 Heterojunction as a Photoelectrochemical Cathode. Nano-Micro Lett. 2018, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.S.; Zhai, C.Y.; Sun, M.J.; Hu, Y.F.; Yan, B.; Du, Y.K. Ultrathin graphitic C3N4 nanosheet as a promising visible-light-activated support for boosting photoelectrocatalytic methanol oxidation. Appl. Catal. B Environ. 2017, 203, 108–115. [Google Scholar] [CrossRef]
- Wang, M.; Shen, M.; Zhang, L.X.; Tian, J.J.; Jin, X.X.; Zhou, Y.J.; Shi, J.L. 2D-2D MnO2/g-C3N4 heterojunction photocatalyst: In-situ synthesis and enhanced CO2 reduction activity. Carbon 2017, 120, 23–31. [Google Scholar] [CrossRef]












© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Wang, Z.; Peng, X.; Wang, Z.; Zhou, L.; Yin, Q. A Novel Route to Manufacture 2D Layer MoS2 and g-C3N4 by Atmospheric Plasma with Enhanced Visible-Light-Driven Photocatalysis. Nanomaterials 2019, 9, 1139. https://doi.org/10.3390/nano9081139
Zhang B, Wang Z, Peng X, Wang Z, Zhou L, Yin Q. A Novel Route to Manufacture 2D Layer MoS2 and g-C3N4 by Atmospheric Plasma with Enhanced Visible-Light-Driven Photocatalysis. Nanomaterials. 2019; 9(8):1139. https://doi.org/10.3390/nano9081139
Chicago/Turabian StyleZhang, Bo, Zhenhai Wang, Xiangfeng Peng, Zhao Wang, Ling Zhou, and QiuXiang Yin. 2019. "A Novel Route to Manufacture 2D Layer MoS2 and g-C3N4 by Atmospheric Plasma with Enhanced Visible-Light-Driven Photocatalysis" Nanomaterials 9, no. 8: 1139. https://doi.org/10.3390/nano9081139
APA StyleZhang, B., Wang, Z., Peng, X., Wang, Z., Zhou, L., & Yin, Q. (2019). A Novel Route to Manufacture 2D Layer MoS2 and g-C3N4 by Atmospheric Plasma with Enhanced Visible-Light-Driven Photocatalysis. Nanomaterials, 9(8), 1139. https://doi.org/10.3390/nano9081139