Synthesis of Fe/Ni Bimetallic Nanoparticles and Application to the Catalytic Removal of Nitrates from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fe/Ni Nanoparticle Production
2.3. Nitrate Reduction Experiments
3. Results
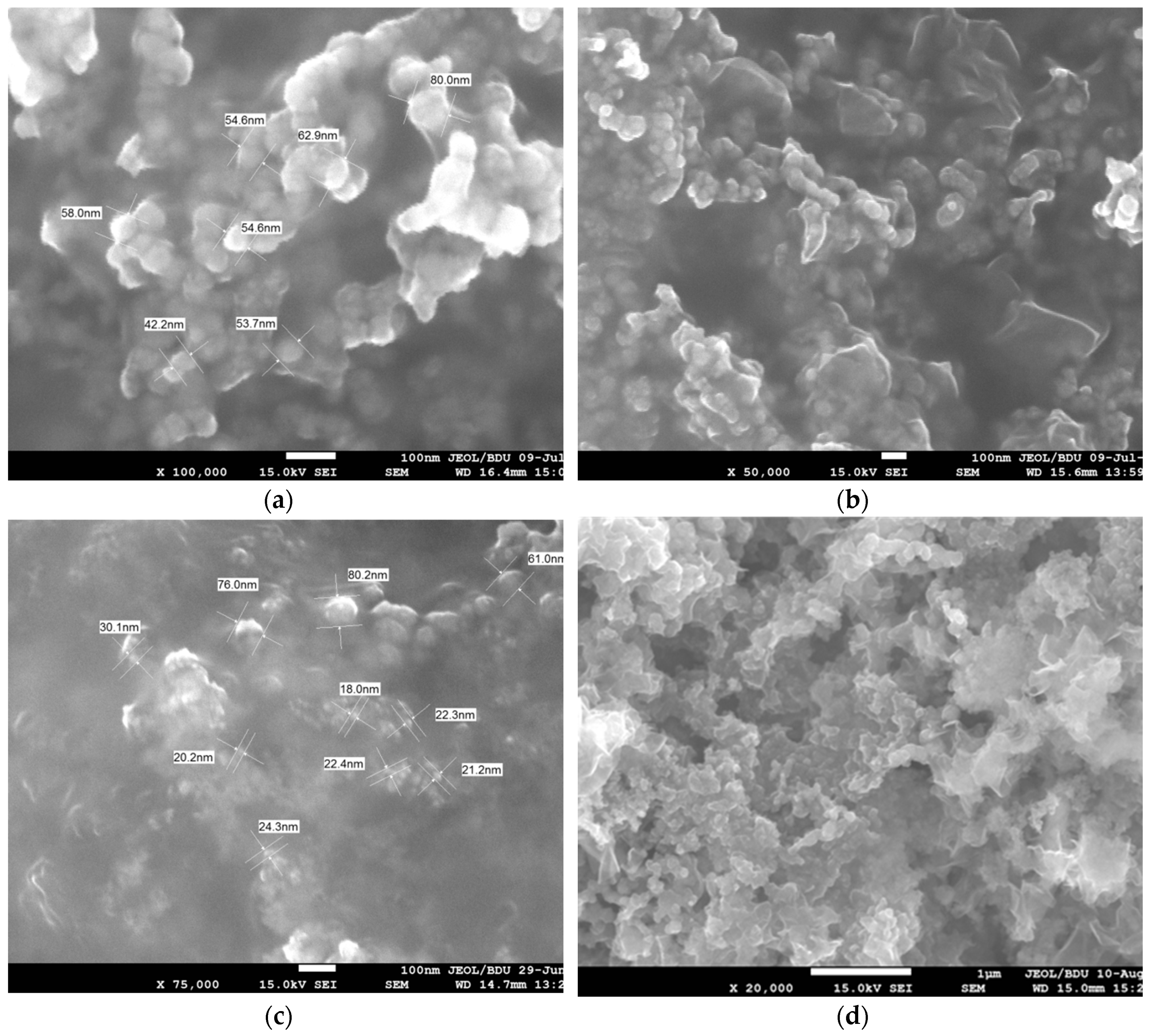
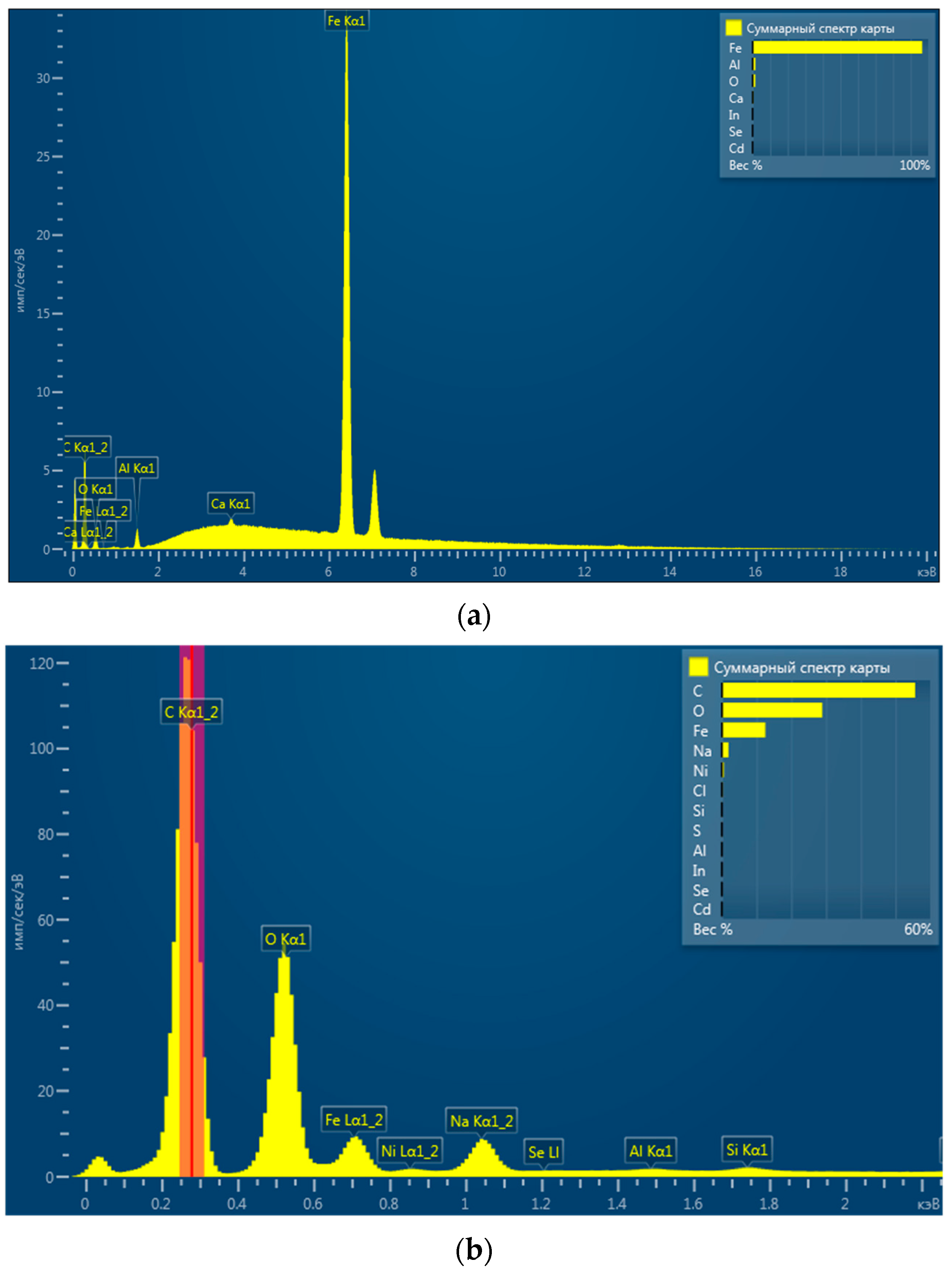
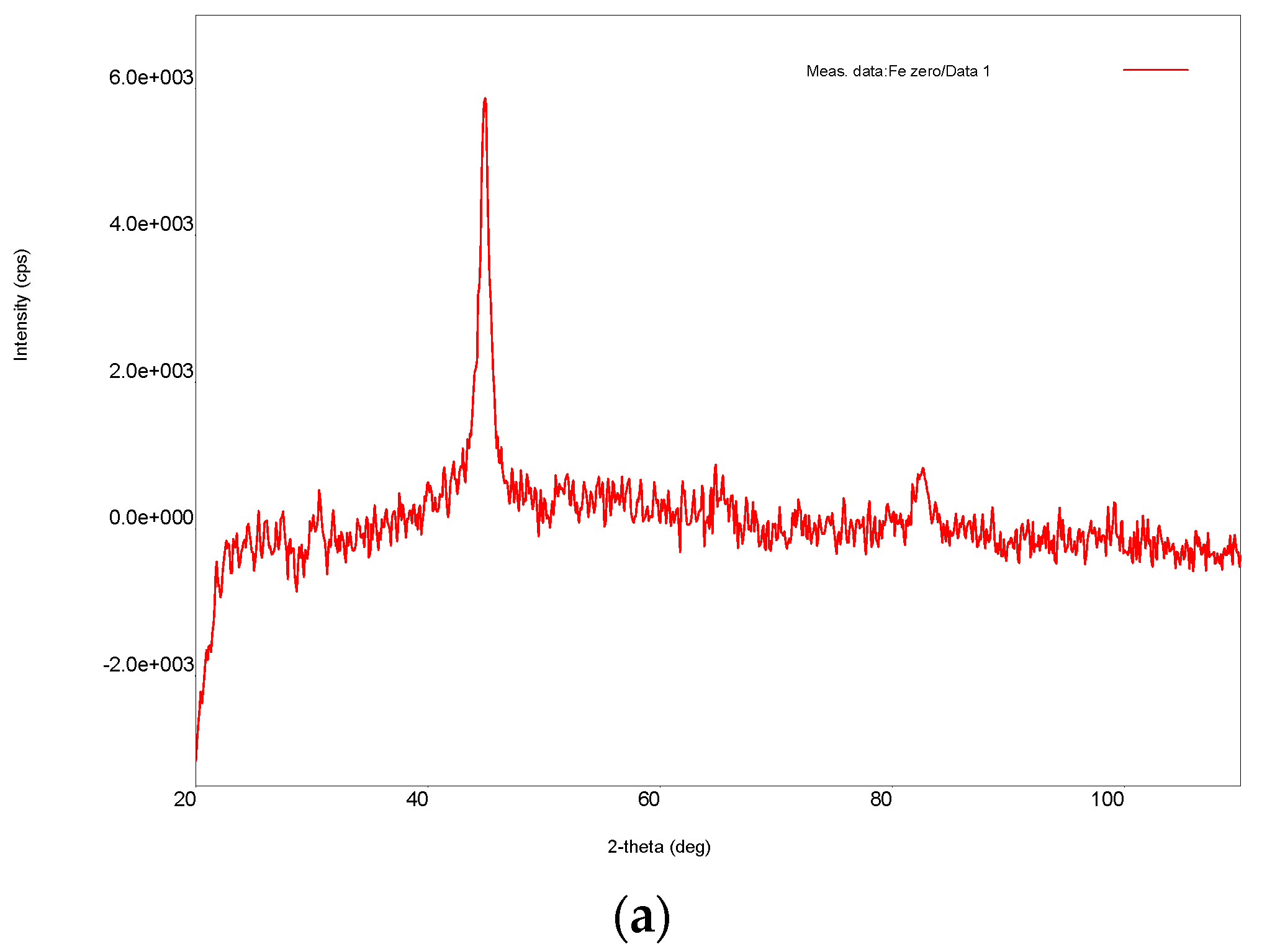
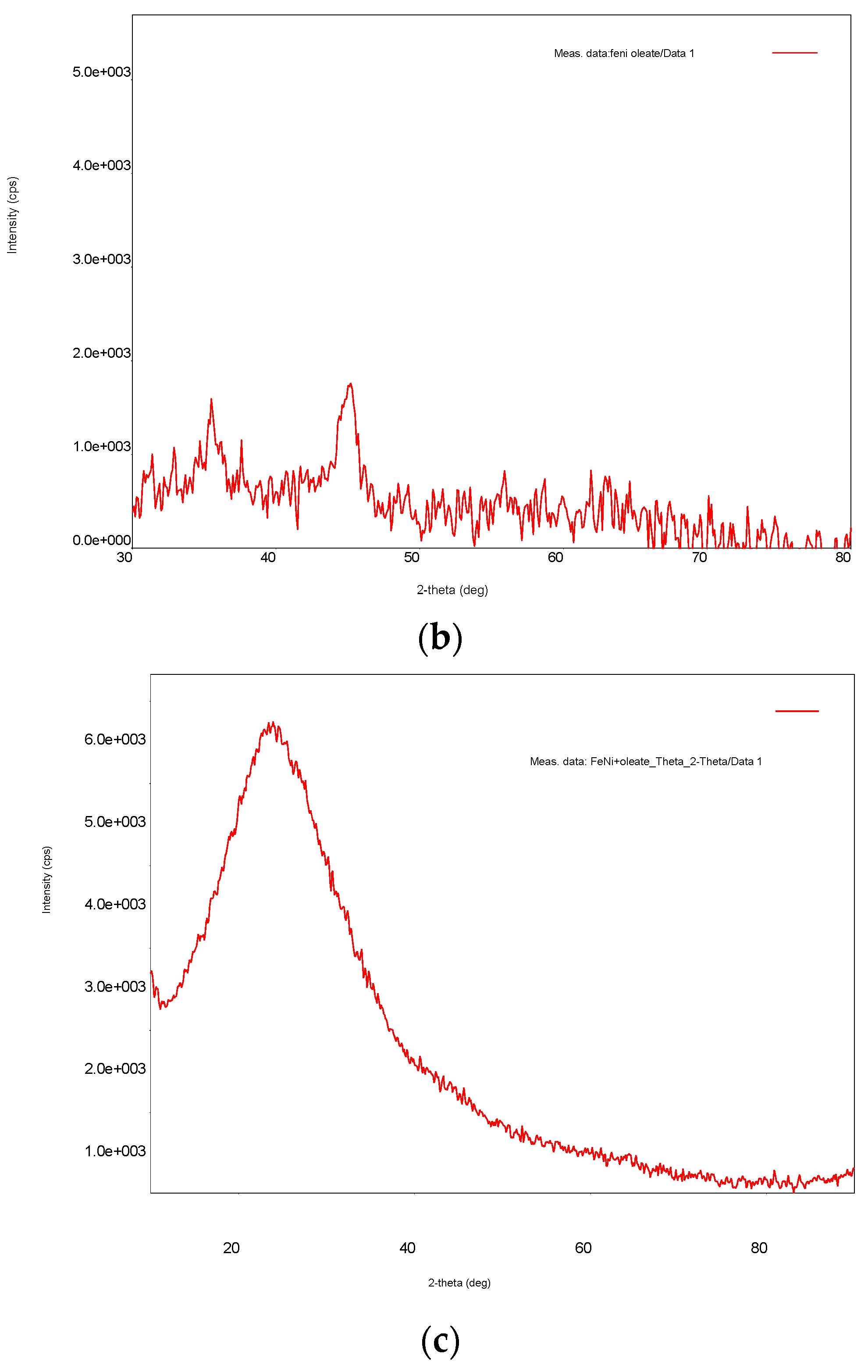
3.1. Ni/Fe Nanoparticle Characterization: SEM and XRD Analysis
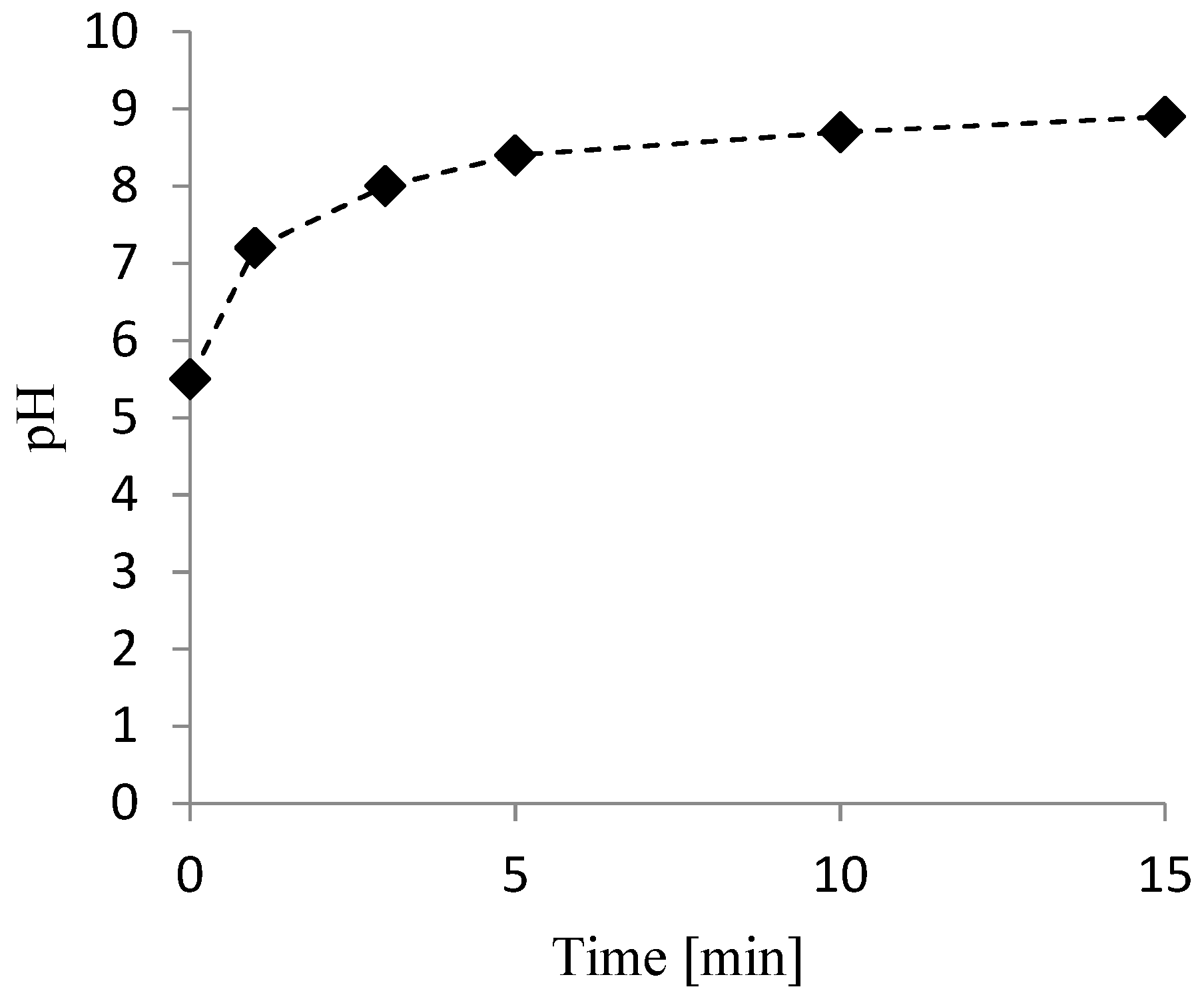
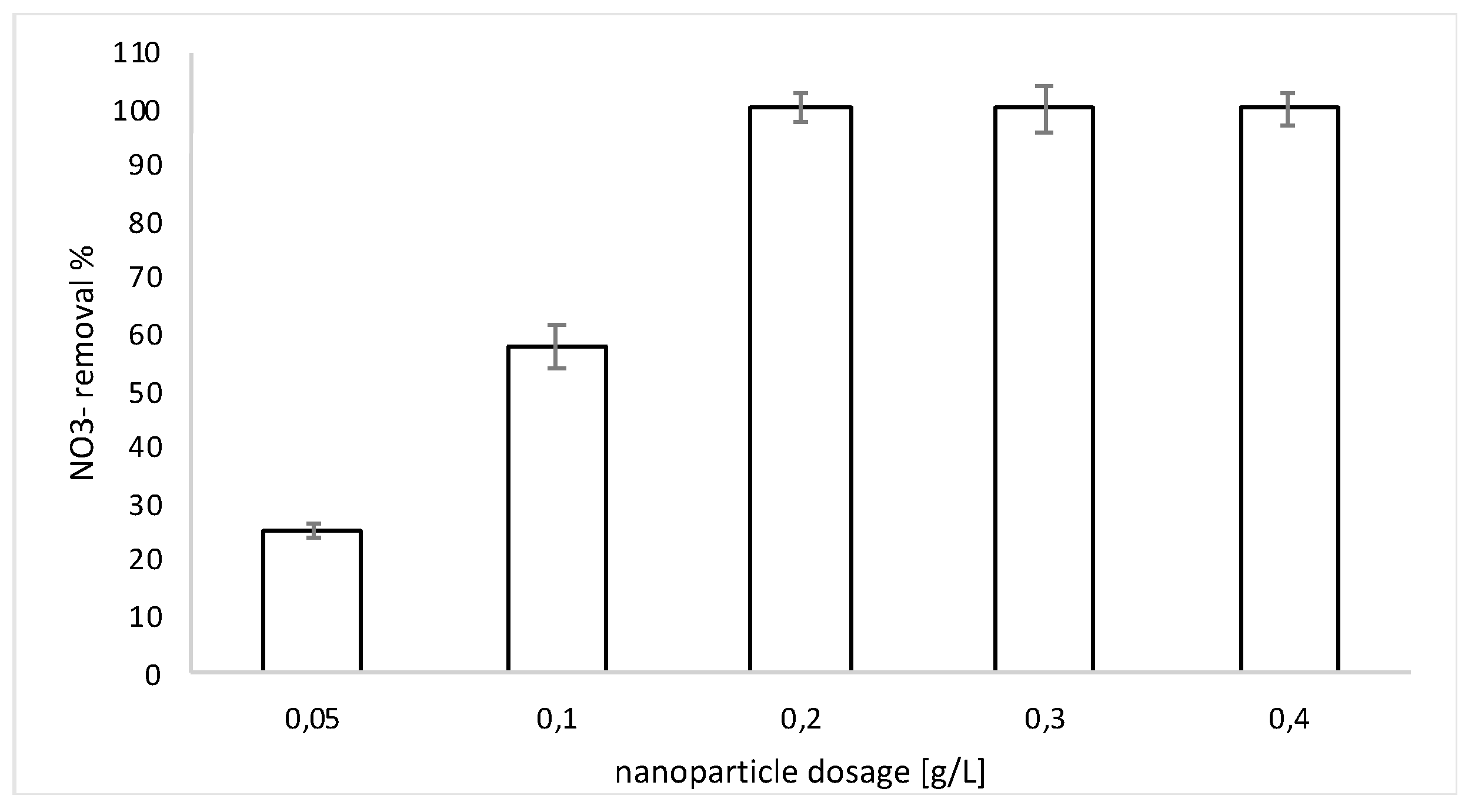
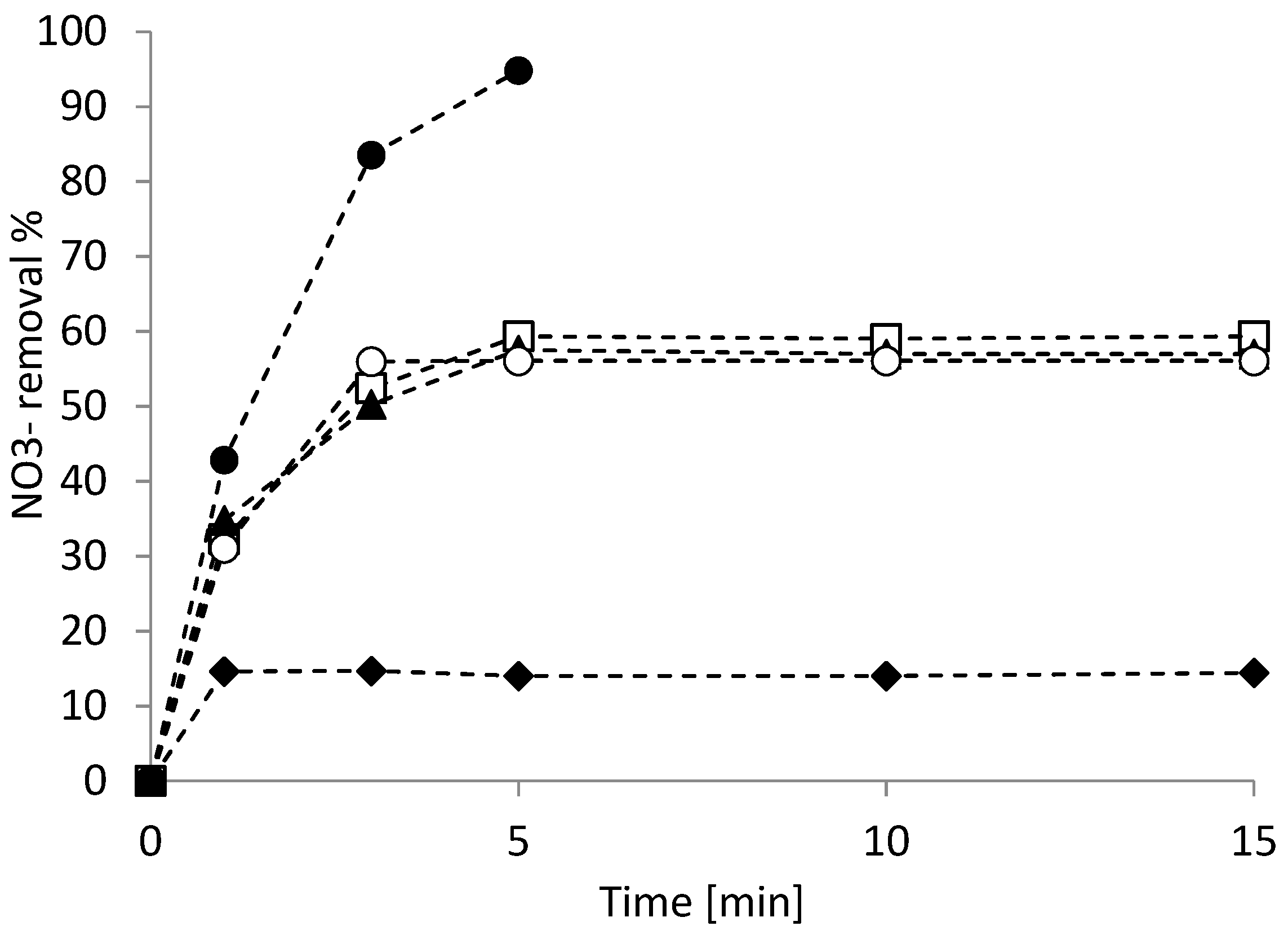
3.2. Nitrate Reduction Tests
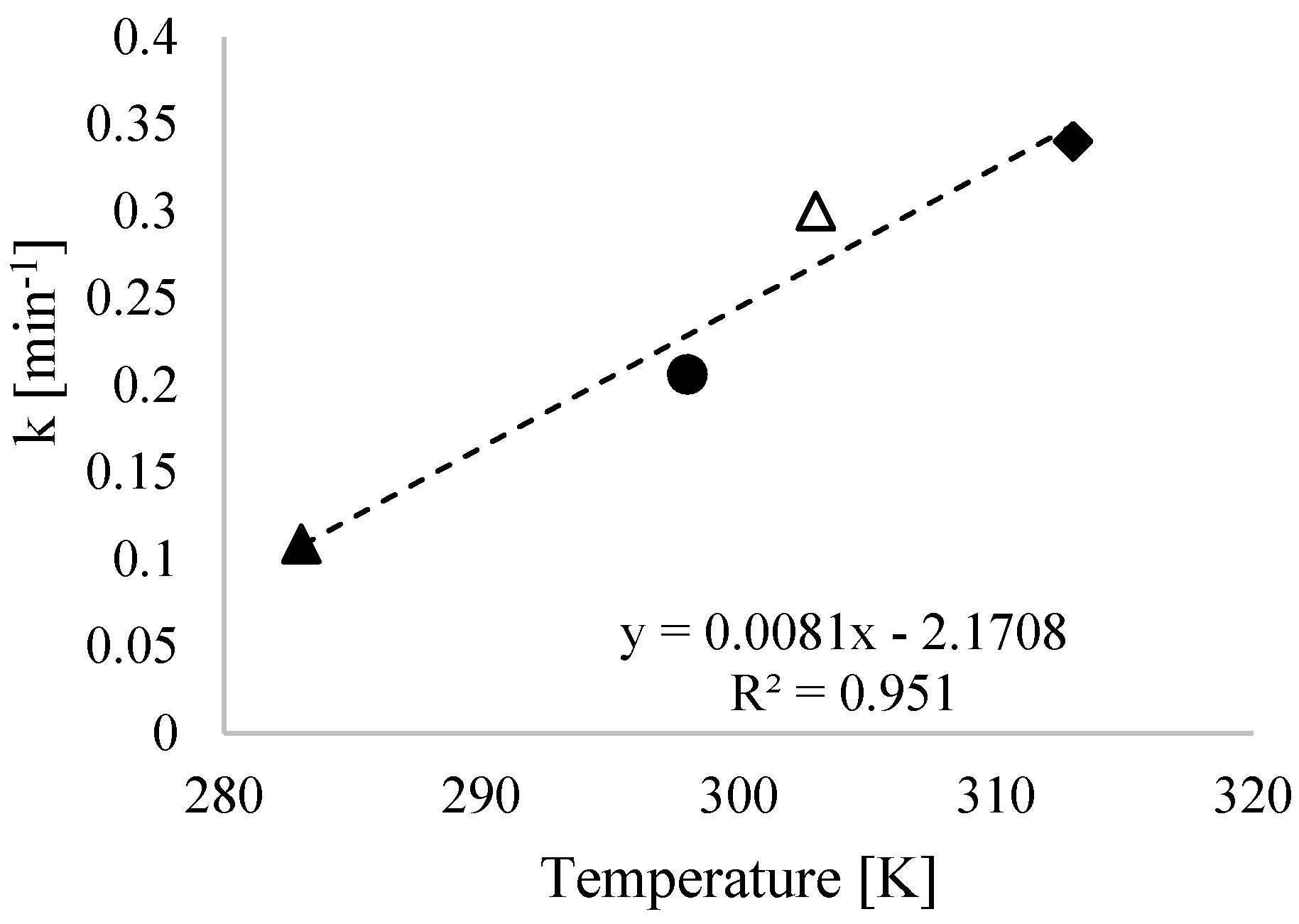
3.3. Kinetic Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhatnagar, A.; Sillanpää, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Liu, Y.; Majetich, S.A.; Tilton, R.D.; Sholl, D.S.; Lowry, G.V. TCE dechlorination rates, pathways, and efficiency of nano-scale iron particles with different properties. Environ. Sci. Technol. 2011, 39, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Prakasa Rao, E.V.S.; Puttanna, K. Nitrates, agriculture and environment. Curr. Sci. 2000, 79, 1163–1168. [Google Scholar]
- Majumdar, D.; Gupta, N. Nitrate pollution of groundwater and associated human health disorders. Indian J. Environ. Health 2000, 42, 28–39. [Google Scholar]
- Forde, B.G. Nitrate transporters in plants: Structure, function and regulation. Biochim. Biophys. Acta Biomembr. 2000, 1465, 219–235. [Google Scholar] [CrossRef]
- Kilfoy, B.A.; Ward, M.H.; Zheng, T.; Holford, T.R.; Boyle, P.; Zhao, P.; Dai, M.; Leaderer, B.; Zhang, Y. Risk of non-Hodgkin lymphoma and nitrate and nitrite from the diet in Connecticut women. Cancer Causes Control 2010, 21, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Fewtrell, L. Drinking-water nitrate, methemoglobinemia, and global burden of disease. Environ. Health Perspect. 2004, 112, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Sparis, D.; Mystrioti, C.; Xenidis, A.; Papassiopi, N. Reduction of nitrate by copper-coated ZVI nanoparticles. Desalin. Water Treat. 2013, 51, 2926–2933. [Google Scholar] [CrossRef]
- Schoeman, J.J.; Steyn, A. Nitrate removal with reverse osmosis in a rural area in South Africa. Desalination 2003, 155, 15–26. [Google Scholar] [CrossRef]
- Torabian, A.S.; Hassani, A.; Abedi, S.M. Comparing reverse osmosis and ion exchange methods of nitrate elimination. J. Environ. Sci. Technol. 2006, 8, 8–21. [Google Scholar]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 533–616. [Google Scholar] [PubMed]
- Kim, Y.H.; Hwang, E.D.; Shin, W.S.; Choi, J.H.; Ha, T.W.; Choi, S.J. Treatments of stainless steel wastewater containing a high concentration of nitrate using reverse osmosis and nanomembranes. Desalination 2007, 202, 286–292. [Google Scholar] [CrossRef]
- Choi, J.; Batchelor, B.; Won, C.; Chung, J. Nitrate reduction by green rusts modified with trace metals. Chemosphere 2012, 86, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Guo, P.; Yu, M.; Sun, Z.; Li, P.; Yang, T.; Liu, J.; Zhang, L. Chemical reduction of nitrate using nanoscale bimetallic iron/copper particles. Pol. J. Environ. Stud. 2018, 27, 2023–2028. [Google Scholar] [CrossRef]
- Kumar, M.; Chakraborty, S. Chemical denitrification of water by zero-valent magnesium powder. J. Hazard. Mater. 2006, 135, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.P. Chemical removal of nitrate from water. Nature 1991, 350, 223–225. [Google Scholar] [CrossRef]
- Sohn, K.; Kang, S.W.; Ahn, S.; Woo, M.; Yang, S.K. Fe (0) nanoparticles for nitrate reduction: Stability, reactivity, and transformation. Environ. Sci. Technol. 2006, 40, 5514–5519. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Hwang, Y.H.; Shin, H.S.; Ko, S.O. Kinetics of nitrate adsorption and reduction by nano-scale zero valent iron (NZVI): Effect of ionic strength and initial pH. KSCE J. Civil Eng. 2016, 20, 175–187. [Google Scholar] [CrossRef]
- Azzam, A.M.; El-Wakeel, S.T.; Mostafa, B.B.; El-Shahat, M. Removal of Pb, Cd, Cu and Ni from aqueous solution using nano scale zero valent iron particles. J. Environ. Chem. Eng. 2016, 4, 2196–2206. [Google Scholar] [CrossRef]
- Biterna, M.; Antonoglou, L.; Lazou, E.; Voutsa, D. Arsenite removal from waters by zero valent iron: Batch and column tests. Chemosphere 2010, 78, 7–12. [Google Scholar] [CrossRef]
- Biterna, M.; Arditsoglou, A.; Tsikouras, E.; Voutsa, D. Arsenate removal by zero valent iron: Batch and column tests. J. Hazard. Mater. 2007, 149, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Walker, H.; Zhu, Q. Reduction of nitrate by NaY zeolite supported Fe, Cu/Fe and Mn/Fe nanoparticles. J. Hazard. Mater. 2017, 324, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.; Eljamal, O.; Amen, T.W.; Sugihara, Y.; Matsunaga, N. Optimized nanoscale zero-valent iron supported on treated activated carbon for enhanced nitrate and phosphate removal from water. Chem. Eng. J. 2017, 309, 349–365. [Google Scholar] [CrossRef]
- Malakootian, M.; Yaghmaian, K.; Tahergorabi, M. The efficiency of nitrate removal in drinking water using iron nano-particle: Determination of optimum conditions. J. Yazd Univ. Med. Sci. 2011, 10, 35–44. [Google Scholar]
- Fenglian, F.; Dionysios, D.D.; Liu, L. The use of zero-valent iron for groundwater remediation and wastewater treatment. J. Hazard. Mater. 2017, 267, 194–205. [Google Scholar]
- Shanawar, H.; Sungjun, B.; Woojin, L.; Muhammad, T.A.; Abdulrahman, A.A. Catalytic nitrate removal in continuous bimetallic Cu−Pd/nanoscale zerovalent iron system. Ind. Eng. Chem. Res. 2015, 54, 6247–6257. [Google Scholar]
- Daud, M.; Khan, Z.; Ashgar, A.; Danish, M.I.; Qazi, I.A. Comparing and optimizing nitrate adsorption from aqueous solution using Fe/Pt bimetallic nanoparticles and anion exchange resins. J. Nanotechnol. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef]
- Shi, J.; Long, C.; Li, A. Selective reduction of nitrate into nitrogen using Fe-Pd bimetallic nanoparticle supported on chelating resin at near-neutral pH. Chem. Eng. J. 2016, 286, 408–415. [Google Scholar] [CrossRef]
- Huang, K.C.; Ehrman, S.H. Synthesis of iron nanoparticles via chemical reduction with palladium ion seeds. Langmuir 2007, 23, 1419–1426. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N. Preparation and characterization of zero valent iron nanoparticles. Dig. J. Nanomater. Biostruct. 2011, 6, 1771–1776. [Google Scholar]
- Kang, H.; Xiu, Z.; Chen, J.; Cao, W.; Guo, Y.; Li, T.; Jin, Z. Reduction of nitrate by bimetallic Fe/Ni nanoparticles. Environ. Technol. 2012, 33, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lin, K.; Fang, Z.; Wang, K. Enhanced nitrate removal by novel bimetallic Fe/Ni nanoparticles supported on biochar. J. Clean. Prod. 2017, 151, 21–33. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, Z.Y.; Cheng, B.; Zhu, Y.R.; Liu, H.J. The preparation, surface modification and characterization of metallic α-Fe nanoparticles. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 1999, 60, 223–226. [Google Scholar] [CrossRef]
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjipanayis, G.C. Chemistry of borohydride reduction of iron (II) and iron (III) ions in aqueous and nonaqueous media. Formation of nanoscale Fe, FeB, and Fe2B powders. Inorg. Chem. 1995, 34, 28–35. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Dong, Y.; Li, B.; Wang, L.; Chu, S.; Liu, J. Zeolite supported Fe/Ni bimetallic nanoparticles for simultaneous removal of nitrate and phosphate: Synergistic effect and mechanism. Chem. Eng. J. 2018, 347, 669–681. [Google Scholar] [CrossRef]
- Muradova, G.; Gadjieva, S.; Di Palma, L.; Vilardi, G. Nitrates removal by bimetallic nanoparticles in water. Chem. Eng. Trans. 2016, 47, 205–210. [Google Scholar]
- Cheng, I.F.; Muftikian, R.; Fernando, Q.; Korte, N. Reduction of nitrate to ammonia by zero-valent iron. Chemosphere 1997, 35, 2689–2695. [Google Scholar] [CrossRef]
- Ji, M.K.; Ahn, Y.T.; Ali Khan, M.; Abou-Shanab, R.A.I.; Cho, Y.; Choi, J.Y.; Je Kim, Y.; Song, H.; Jeon, B.H. Removal of nitrate and ammonium ions from livestock wastewater by hybrid systems composed of zero-valent iron and adsorbents. Environ. Technol. 2011, 32, 1851–1857. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, D.G.; Ahn, Y.T.; Moon, C.M.; Shin, H.S. Fate of nitrogen species in nitrate reduction by nanoscale zero valent iron and characterization of the reaction kinetics. Water Sci. Technol. 2010, 61, 705–712. [Google Scholar] [CrossRef]
- Ahn, S.C.; Oh, S.Y.; Cha, D.K. Enhanced reduction of nitrate by zero-valent iron at elevated temperatures. J. Hazard. Mater. 2008, 156, 17–22. [Google Scholar] [CrossRef] [PubMed]
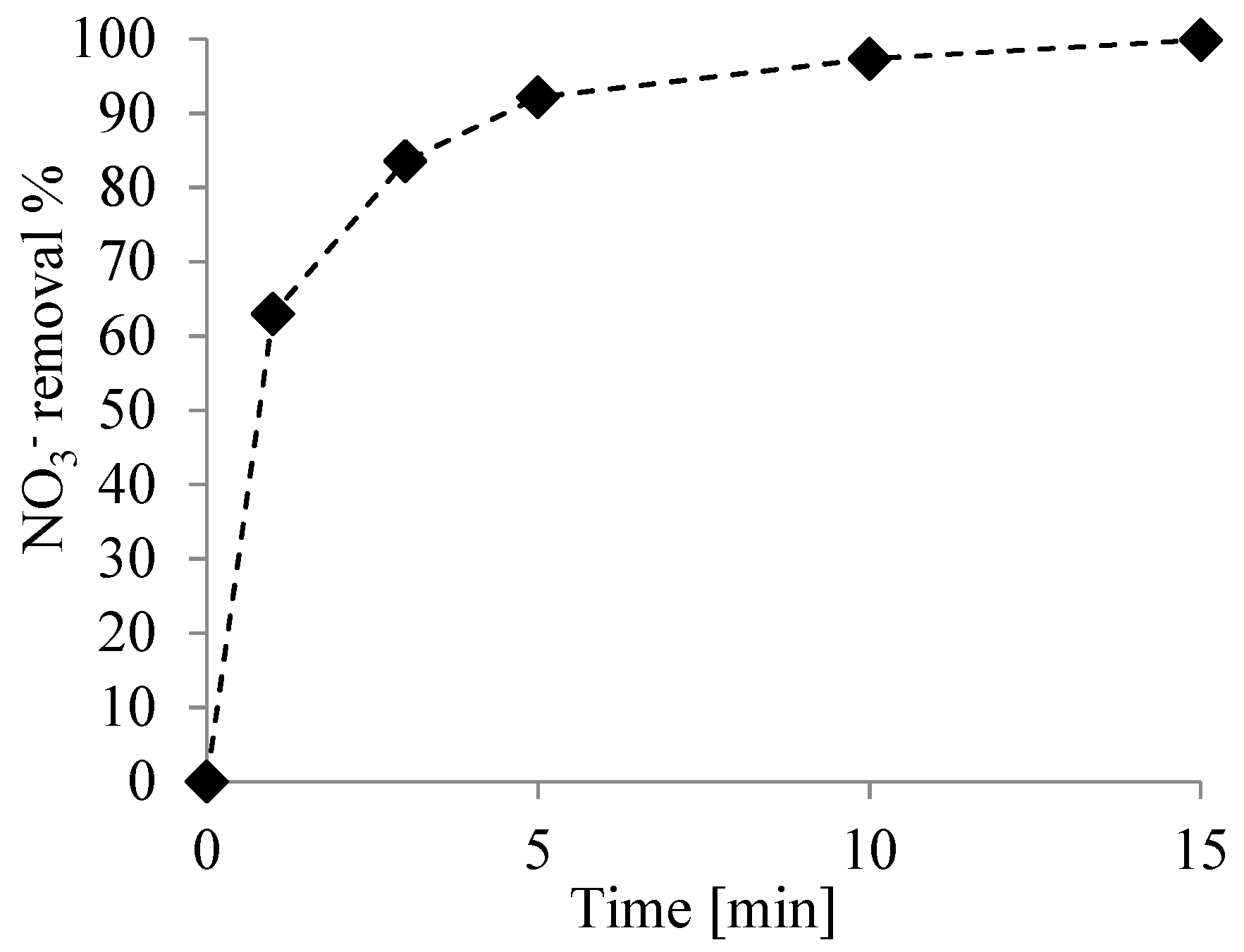

| Test Condition | ||||
|---|---|---|---|---|
| pH | T (K) | NO3− Removal (%) | TON | TOF (h−1) |
| unbuffered | 298 | 57 | 1.59 | 6.37 |
| 3 | 298 | 59.34 | 1.66 | 6.63 |
| buffered at 3 | 298 | 94.85 | 2.65 | 10.60 |
| 5 | 298 | 56.08 | 1.57 | 6.27 |
| 10 | 298 | 14.41 | 0.40 | 1.61 |
| unbuffered | 283 | 42.74 | 1.19 | 4.78 |
| unbuffered | 303 | 77.23 | 2.16 | 8.63 |
| unbuffered | 313 | 78.04 | 2.18 | 8.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valiyeva, G.G.; Bavasso, I.; Di Palma, L.; Hajiyeva, S.R.; Ramazanov, M.A.; Hajiyeva, F.V. Synthesis of Fe/Ni Bimetallic Nanoparticles and Application to the Catalytic Removal of Nitrates from Water. Nanomaterials 2019, 9, 1130. https://doi.org/10.3390/nano9081130
Valiyeva GG, Bavasso I, Di Palma L, Hajiyeva SR, Ramazanov MA, Hajiyeva FV. Synthesis of Fe/Ni Bimetallic Nanoparticles and Application to the Catalytic Removal of Nitrates from Water. Nanomaterials. 2019; 9(8):1130. https://doi.org/10.3390/nano9081130
Chicago/Turabian StyleValiyeva, Gunay G., Irene Bavasso, Luca Di Palma, Sevinj R. Hajiyeva, Mahammadali A. Ramazanov, and Flora V. Hajiyeva. 2019. "Synthesis of Fe/Ni Bimetallic Nanoparticles and Application to the Catalytic Removal of Nitrates from Water" Nanomaterials 9, no. 8: 1130. https://doi.org/10.3390/nano9081130





