Ferroelectric Polarization-Enhanced Photocatalysis in BaTiO3-TiO2 Core-Shell Heterostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterization
2.3. Photocatalytic Activity Measurements
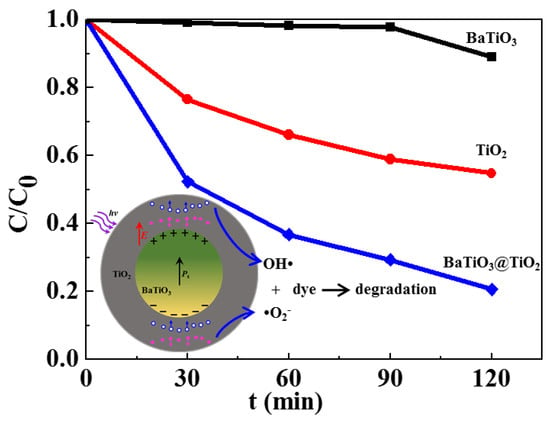
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Adsorption-Oriented Processes Using Conventional and Non-conventional Adsorbents for Wastewater Treatment; Springer: Berlin, Germany, 2018; pp. 23–71. [Google Scholar]
- Xie, Y.; Ren, L.; Zhu, X.; Xi, G.; Chen, S. Physical and Chemical Treatments for Removal of Perchlorate from Water—A Review. Process Saf. Environ. Prot. 2018, 116, 180–198. [Google Scholar] [CrossRef]
- Jin, W.; Tafen, D.N.; Lewis, J.P.; Hong, Z.; Manivannan, A.; Zhi, M.; Ming, L.; Wu, N. Origin of Photocatalytic Activity of Nitrogen-Doped TiO2 Nanobelts. J. Am. Chem. Soc. 2009, 131, 12290–12297. [Google Scholar]
- Huang, Z.A.; Sun, Q.; Lv, K.; Zhang, Z.; Li, M.; Li, B. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (001) vs (101) facets of TiO2. Appl. Catal. B Environ. 2015, 164, 420–427. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Hamzavi, R. Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst. J. Hazard. Mater. 2006, 133, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Guo, L. A novel method for the preparation of a highly stable and active CdS photocatalyst with a special surface nanostructure. J. Phys. Chem. B 2006, 110, 11139. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Araujo, C.M.; Luo, W.; Ahuja, R. Single-layer MoS2 as efficient photocatalyst. Catal. Sci. Technol. 2013, 3, 2214. [Google Scholar] [CrossRef]
- Lin, Y.C.; Liu, S.H.; Syu, H.R.; Ho, T.H. Synthesis, characterization and photocatalytic performance of self-assembled mesoporous TiO2 nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 95, 300–304. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, Y.; Ren, Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Albery, W.J. The Transport and Kinetics of Photogenerated Carriers in Colloidal Semiconductor Electrode Particles. J. Electrochem. Soc. 1984, 131, 315. [Google Scholar] [CrossRef]
- Carp, O. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Li, L.; Salvador, P.A.; Rohrer, G.S. Photocatalysts with internal electric fields. Nanoscale 2014, 6, 24–42. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Kurtz, S.K.; Jamieson, P.B. Atomic Displacement Relationship to Curie Temperature and Spontaneous Polarization in Displacive Ferroelectrics. Phys. Rev. 1968, 172, 551–553. [Google Scholar] [CrossRef]
- Huang, Z.; Xue, J.; Liu, S.; Yun, H.; Chu, J.; Zhang, D.H. Bound electrical charges in BaTiO 3 ferroelectric thin films: Evidence for spontaneous polarization. Phys. Rev. B 2006, 73. [Google Scholar] [CrossRef]
- Feng, K.; Liu, X.; Si, D.; Tang, X.; Xing, A.; Osada, M.; Xiao, P. Ferroelectric BaTiO 3 dipole induced charge transfer enhancement in dye-sensitized solar cells. J. Power Sources 2017, 350, 35–40. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Li, J.; Valanoor, N.; Tang, X.; Cao, G. Increase of power conversion efficiency in dye-sensitized solar cells through ferroelectric substrate induced charge transport enhancement. Sci. Rep. 2018, 8, 17389. [Google Scholar] [CrossRef]
- Wang, Z.; Song, J.; Gao, F.; Su, R.; Zhang, D.; Liu, Y.; Xu, C.; Lou, X.; Yang, Y. Developing a ferroelectric nanohybrid for enhanced photocatalysis. Chem. Commun. 2017, 53, 7596. [Google Scholar] [CrossRef]
- Li, H.; Sang, Y.; Chang, S.; Huang, X.; Zhang, Y.; Yang, R.; Jiang, H.; Liu, H.; Wang, Z.L. Enhanced ferroelectric-nanocrystal-based hybrid photocatalysis by ultrasonic-wave-generated piezophototronic effect. Nano Lett. 2015, 15, 2372–2379. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Y.; Li, C. Spatial distribution of active sites on a ferroelectric PbTiO3 photocatalyst for photocatalytic hydrogen production. Faraday Discuss. 2017, 198, 463–472. [Google Scholar] [CrossRef]
- Jing, L.; Qu, Y.; Wang, B.; Li, S.; Jiang, B.; Yang, L.; Fu, W.; Fu, H.; Sun, J. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Huang, X.; Wang, K.; Wang, Y.; Wang, B.; Zhang, L.; Gao, F.; Zhao, Y.; Feng, W.; Zhang, S.; Liu, P. Enhanced charge carrier separation to improve hydrogen production efficiency by ferroelectric spontaneous polarization electric field. Appl. Catal. B Environ. 2018, 227, 322–329. [Google Scholar] [CrossRef]
- Banizi, Z.T.; Seifi, M.; Askari, M.B.; Dehaghi, S.B.; Zadeh, M.H.R. Photoluminescence and photocatalytic studies of Cadmium sulfide/multiwall carbon nanotube (CdS/MWCNT) nanocomposites. Optik Int. J. Light Electron Opt. 2018, 158, 882–892. [Google Scholar] [CrossRef]
- Yu, J.G.; Yu, H.G.; Cheng, B.; Zhao, X.J.; Jimmy, C.Y.; HO, W.K. The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, L.; Li, J.; Lou, X.W. A universal cooperative assembly-directed method for coating of mesoporous TiO2 nanoshells with enhanced lithium storage properties. Sci. Adv. 2016, 2, e1501554. [Google Scholar] [CrossRef]
- Gruverman, A.; Alexe, M.; Meier, D. Piezoresponse force microscopy and nanoferroic phenomena. Nat. Commun. 2019, 10, 1661. [Google Scholar] [CrossRef]
- Husimi, K. Ultra-Low-Velocity Component of Spontaneous Polarization in BaTiO3 Single Crystal. J. Appl. Phys. 1958, 29, 1379–1380. [Google Scholar] [CrossRef]
- Polking, M.J.; Han, M.G.; Yourdkhani, A.; Petkov, V.; Kisielowski, C.F.; Volkov, V.V.; Zhu, Y.; Caruntu, G.; Alivisatos, A.P.; Ramesh, R. Ferroelectric order in individual nanometre-scale crystals. Nat. Mater. 2012, 11, 700–709. [Google Scholar] [CrossRef]
- Hafid, L.; Godefroy, G.; Idrissi, A.E.; Michel-Calendini, F. Absorption spectrum in the near U.V. and electronic structure of pure barium titanate. Solid State Commun. 1988, 66, 841–845. [Google Scholar] [CrossRef]
- Tang, H.; Lévy, F.; Berger, H.; Schmid, P.E. Urbach tail of anatase TiO2. Phys. Rev. B 1995, 52, 7771–7774. [Google Scholar] [CrossRef]
- Xia, Y.T.; Jia, Y.M.; Qian, W.Q.; Xu, X.L.; Wu, Z.; Han, Z.C.; Hong, Y.T.; You, H.L.; Ismail, M.; Bai, G.; et al. Pyroelectrically Induced Pyro-Electro-Chemical Catalytic Activity of BaTiO3 Nanofibers under Room-Temperature Cold–Hot Cycle Excitations. Metals 2017, 7, 122. [Google Scholar] [CrossRef]
- Shvydka, D.; Karpov, V.G. Nanodipole photovoltaics. Appl. Phys. Lett. 2008, 92, 053507. [Google Scholar] [CrossRef]
- Burbure, N.V.; Salvador, P.A.; Rohrer, G.S. Photochemical Reactivity of Titania Films on BaTiO3Substrates: Origin of Spatial Selectivity. Chem. Mater. 2010, 22, 5823–5830. [Google Scholar] [CrossRef]





| Catalysts | Pure BaTiO3 | Pure TiO2 | BaTiO3-TiO2 Core-Shell Heterostructures | ||
|---|---|---|---|---|---|
| 1:1 | 1.2:1 | 1.4:1 | |||
| Surface area (m2/g) | 25.66 | 70.79 | 36.81 | 35.77 | 34.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Lv, S.; Fan, B.; Xing, A.; Jia, B. Ferroelectric Polarization-Enhanced Photocatalysis in BaTiO3-TiO2 Core-Shell Heterostructures. Nanomaterials 2019, 9, 1116. https://doi.org/10.3390/nano9081116
Liu X, Lv S, Fan B, Xing A, Jia B. Ferroelectric Polarization-Enhanced Photocatalysis in BaTiO3-TiO2 Core-Shell Heterostructures. Nanomaterials. 2019; 9(8):1116. https://doi.org/10.3390/nano9081116
Chicago/Turabian StyleLiu, Xiaoyan, Siyi Lv, Baoyan Fan, An Xing, and Bi Jia. 2019. "Ferroelectric Polarization-Enhanced Photocatalysis in BaTiO3-TiO2 Core-Shell Heterostructures" Nanomaterials 9, no. 8: 1116. https://doi.org/10.3390/nano9081116
APA StyleLiu, X., Lv, S., Fan, B., Xing, A., & Jia, B. (2019). Ferroelectric Polarization-Enhanced Photocatalysis in BaTiO3-TiO2 Core-Shell Heterostructures. Nanomaterials, 9(8), 1116. https://doi.org/10.3390/nano9081116