Achieving Secondary Dispersion of Modified Nanoparticles by Hot-Stretching to Enhance Dielectric and Mechanical Properties of Polyarylene Ether Nitrile Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of PEN
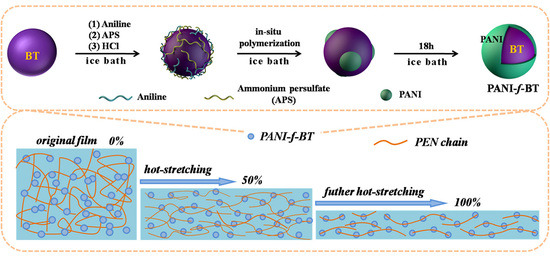
2.3. Preparation of PANI-f-BT Nanoparticles
2.4. Preparation of Nanocomposites
2.5. Preparation of Oriented Nanocomposites by Hot-Stretching
2.6. Characterization
3. Results and Discussion
3.1. Microstructure and Morphology of PANI-f-BT
3.2. Morphology of PEN/PANI-f-BT Composites
3.3. Thermal Properties and Crystallization of PEN/PANI-f-BT Composites
3.4. Mechanical Properties of PEN/PANI-f-BT Composites
3.5. Dielectric Properties of PEN/PANI-f-BT Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dang, Z.M.; Yuan, J.K.; Yao, S.H.; Liao, R.J. Flexible nanodielectric materials with high permittivity for power energy storage. Adv. Mater. 2013, 25, 6334–6365. [Google Scholar] [CrossRef]
- Chu, B.J.; Zhou, X.; Ren, K.L.; Neese, B.; Lin, M.R.; Wang, Q.; Bauer, F.; Zhang, Q.M. A dielectric polymer with high electric energy density and fast discharge speed. Science 2006, 313, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.N.; Wang, X.H.; O'Brien, S.; Lombardi, J.; Li, L.T. Flexible BaTiO3/PVDF gradated multilayer nanocomposite film with enhanced dielectric strength and high energy density. J. Mater. Chem. C 2015, 3, 9740–9747. [Google Scholar] [CrossRef]
- Kim, P.; Jones, S.C.; Hotchkiss, P.J.; Haddock, J.N.; Kippelen, B.; Marder, S.R.; Perry, J.W. Phosphonic acid-modified barium titanate polymer nanocomposites with high permittivity and dielectric strength. Adv Mater. 2007, 19, 1001–1005. [Google Scholar] [CrossRef]
- Maier, G. Low dielectric constant polymers for microelectronics. Prog. Polym. Sci. 2001, 26, 3–65. [Google Scholar] [CrossRef]
- Qi, L.; Lee, B.I.; Chen, S.; Samuels, W.D.; Exarhos, G.J. High-dielectric-constant silver-epoxy composites as embedded dielectrics. Adv. Mater. 2005, 17, 1777–1781. [Google Scholar] [CrossRef]
- Bi, K.; Bi, M.; Hao, Y.; Luo, W.; Cai, Z.; Wang, X.; Huang, Y. Ultrafine core-shell BaTiO3@SiO2 structures for nanocomposite capacitors with high energy density. Nano Energy 2018, 51, 513–523. [Google Scholar] [CrossRef]
- Maliakal, A.; Katz, H.; Cotts, P.M.; Subramoney, S.; Mirau, P. Inorganic oxide core, polymer shell nanocomposite as a high-k gate dielectric for flexible electronics applications. J. Am. Chem. Soc. 2005, 127, 14655–14662. [Google Scholar] [CrossRef]
- You, Y.; Zhan, C.H.; Tu, L.; Wang, Y.J.; Hu, W.B.; Wei, R.B.; Liu, X.B. Polyarylene ether nitrile-based high-k composites for dielectric applications. Int. J. Polym. Sci. 2018, 5161908. [Google Scholar] [CrossRef]
- Zotti, A.; Zuppolini, S.; Borriello, A.; Zarrelli, M. Thermal properties and fracture toughness of epoxy nanocomposites loaded with hyperbranched-polymers-based core/shell nanoparticles. Nanomaterials 2019, 9, 418. [Google Scholar] [CrossRef]
- Li, J.; Seok, S.I.; Chu, B.; Dogan, F.; Zhang, Q.; Wang, Q. Nanocomposites of ferroelectric polymers with TiO2 nanoparticles exhibiting significantly enhanced electrical energy density. Adv. Mater. 2009, 21, 217–221. [Google Scholar] [CrossRef]
- Wang, Y.J.; Tong, L.F.; You, Y.; Tu, L.; Zhou, M.R.; Liu, X.B. Polyethylenimine assisted bio-inspired surface functionalization of hexagonal boron nitride for enhancing the crystallization and the properties of poly(arylene ether nitrile). Nanomaterials 2019, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.B.; Wang, J.L.; Zhang, H.X.; Han, W.H.; Liu, X.B. Crosslinked polyarylene ether nitrile interpenetrating with zinc ion bridged graphene sheet and carbon nanotube network. Polymers 2017, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.D.; Liu, J.Y.; Cheng, Y.H.; Chen, S.Y.; Yang, M.M.; Huang, J.L.; Wang, H.K.; Wu, G.L.; Wu, H.J. Alignment of boron nitride nanofibers in epoxy composite films for thermal conductivity and dielectric breakdown strength improvement. Nanomaterials 2018, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Zhi, C.; Bando, Y.; Terao, T.; Tang, C.; Kuwahara, H.; Golberg, D. Boron nanotube–polymer composites: Towards thermoconductive, electrically insulating polymeric composites with boron nitride nanotubes as fillers. Adv. Funct. Mater. 2009, 19, 1857–1862. [Google Scholar] [CrossRef]
- Xu, M.Z.; Lei, Y.X.; Ren, D.X.; Chen, S.J.; Chen, L.; Liu, X.B. Synergistic effects of functional CNTs and h-BN on enhanced thermal conductivity of epoxy/cyanate matrix composites. Nanomaterials 2018, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Hu, C.; Zhao, H.; Haghi-Ashtiani, P.; He, D.; Yang, Y.; Yuan, J.; Bai, J. Core@double-shells nanowires strategy for simultaneously improving dielectric constants and suppressing losses of poly (vinylidene fluoride) nanocomposites. Carbon 2018, 132, 152–156. [Google Scholar] [CrossRef]
- Ma, J.; Azhar, U.; Zong, C.; Zhang, Y.; Xu, A.; Zhai, C.; Zhang, L.; Zhang, S. Core-shell structured PVDF@BT nanoparticles for dielectric materials: A novel composite to prove the dependence of dielectric properties on ferroelectric shell. Mater. Design 2019, 164, 107556. [Google Scholar] [CrossRef]
- Song, Y.; Shen, Y.; Liu, H.; Lin, Y.; Li, M.; Nan, C.W. Improving the dielectric constants and breakdown strength of polymer composites: Effects of the shape of the BaTiO3 nanoinclusions, surface modification and polymer matrix. J. Mater. Chem. 2012, 22, 16491–16498. [Google Scholar] [CrossRef]
- Niu, Y.; Bai, Y.; Yu, K.; Wang, Y.; Xiang, F.; Wang, H. Effect of the modifier structure on the performance of barium titanate/poly(vinylidene fluoride) nanocomposites for energy storage applications. ACS Appl. Mater. Interfaces 2015, 7, 24168–24176. [Google Scholar] [CrossRef]
- Wei, R.; Li, K.; Ma, J.; Zhang, H.; Liu, X. Improving dielectric properties of polyarylene ether nitrile with conducting polyaniline. J. Mater. Sci. Mater. Electron. 2016, 27, 9565–9571. [Google Scholar] [CrossRef]
- Yu, S.; Qin, F.; Wang, G. Improving the dielectric properties of poly (vinylidene fluoride) composites by using poly (vinyl pyrrolidone)-encapsulated polyaniline nanorods. J. Mater. Chem. C 2016, 4, 1504–1510. [Google Scholar] [CrossRef]
- Zhang, X.; He, Q.L.; Gu, H.B.; Wei, S.Y.; Guo, Z.H. Polyaniline stabilized barium titanate nanoparticles reinforced epoxy nanocomposites with high dielectric permittivity and reduced flammability. J. Mater. Chem. C 2013, 1, 2886–2899. [Google Scholar] [CrossRef]
- Li, Z.M.; Yang, M.B.; Lu, A.; Feng, J.M.; Huang, R. Tensile properties of poly(ethylene terephthalate) and polyethylene in-situ microfiber reinforced composite formed via slit die extrusion and hot-stretching. Mater. Lett. 2002, 56, 756–762. [Google Scholar] [CrossRef]
- You, Y.; Huang, X.; Pu, Z.; Jia, K.; Liu, X. Enhanced crystallinity, mechanical and dielectric properties of biphenyl polyarylene ether nitriles by unidirectional hot-stretching. J. Polym. Res. 2015, 22, 221. [Google Scholar] [CrossRef]
- Li, L.; Zhou, T.; Liu, J.Z.; Ran, Q.P.; Ye, G.D.; Zhang, J.H.; Liu, P.Q.; Zhang, A.; Yang, Z.Q.; Xu, D.G.; et al. Formation of a large-scale shish-kebab structure of polyoxymethylene in the melt spinning and the crystalline morphology evolution after hot stretching. Polym. Adv. Technol. 2015, 26, 77–84. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, C.Z.; Gong, J.H.; Yang, S.L.; Ma, J.H.; Xu, J. Lamellae break induced formation of shish-kebab during hot stretching of ultra-high molecular weight polyethylene precursor fibers investigated by in situ small angle X-ray scattering. Polymer 2014, 55, 4299–4306. [Google Scholar] [CrossRef]
- You, Y.; Du, X.; Mao, H.; Tang, X.; Wei, R.; Liu, X. Synergistic enhancement of mechanical, crystalline and dielectric properties of polyarylene ether nitrile-based nanocomposites by unidirectional hot stretching-quenching. Polym. Int. 2017, 66, 1151–1158. [Google Scholar] [CrossRef]
- Tian, C.; Du, Y.; Xu, P.; Qiang, R.; Wang, Y.; Ding, D.; Xue, J.; Ma, J.; Zhao, H.; Han, X. Constructing uniform core-shell PPy@PANI composites with tunable shell thickness toward enhancement in microwave absorption. ACS Appl. Mater. Interfaces 2015, 7, 20090–20099. [Google Scholar] [CrossRef]
- Tang, H.L.; Wang, P.; Zheng, P.L.; Liu, X.B. Core-shell structured BaTiO3@polymer hybrid nanofiller for poly(arylene ether nitrile) nanocomposites with enhanced dielectric properties and high thermal stability. Compos. Sci. Technol. 2016, 123, 134–142. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.; Kim, J.; Kim, Y.; Hui, K.N.; Lee, H. Selective substitution and tetragonality by co-doping of dysprosium and thulium on dielectric properties of barium titanate ceramics. Electron. Mater. Lett. 2011, 7, 155–159. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Gao, X.; Qi, S.; Ma, J.; Zhu, J. Synthesis of stabilized dispersion covalently-jointed SiO2@polyaniline with core-shell structure and anticorrosion performance of its hydrophobic coating for Mg-Li alloy. Appl. Surf. Sci. 2018, 462, 362–372. [Google Scholar] [CrossRef]
- You, Y.; Wang, Y.; Tu, L.; Tong, L.; Wei, R.; Liu, X. Interface modulation of core-shell structured BaTiO3@polyaniline for novel dielectric materials from its nanocomposite with polyarylene ether nitrile. Polymers 2018, 10, 1378. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, X.H.; Sun, C.K.; Li, L.T. Structure study of single crystal BaTiO3 nanotube arrays produced by the hydrothermal method. Nanotechnology 2009, 20, 055709. [Google Scholar] [CrossRef] [PubMed]
- Qaiser, A.A.; Hyland, M.M.; Patterson, D.A. Surface and charge transport characterization of polyaniline-cellulose acetate composite membranes. J. Phys. Chem. B 2011, 115, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Yang, R.; Xiong, Z.; Xiao, Q.; Li, K.; Liu, X. Enhanced dielectric properties of polyarylene ether nitriles filled with core-shell structured PbZrO3 around BaTiO3 nanoparticles. J. Electron. Mater. 2018, 47, 6177–6184. [Google Scholar] [CrossRef]
- You, Y.; Wei, R.; Yang, R.; Yang, W.; Hua, X.; Liu, X. Crystallization behaviors of polyarylene ether nitrile filled in multi-walled carbon nanotubes. RSC Adv. 2016, 6, 70877–70883. [Google Scholar] [CrossRef]
- Tu, L.; You, Y.; Tong, L.; Wang, Y.; Hu, W.; Wei, R.; Liu, X. Crystallinity of poly(arylene ether nitrile) copolymers containing hydroquinone and bisphenol A segments. J. Appl. Polym. Sci. 2018, 135, 46412. [Google Scholar] [CrossRef]
- Mollova, A.; Androsch, R.; Mileva, D.; Schick, C.; Benhamida, A. Effect of supercooling on crystallization of polyamide 11. Macromolecules 2013, 46, 828–835. [Google Scholar] [CrossRef]
- Mao, M.; Das, S.; Turner, S.R. Synthesis and characterization of poly(aryl ether sulfone) copolymers containing terphenyl groups in the backbone. Polymer 2007, 48, 6241–6245. [Google Scholar] [CrossRef]
- Wei, R.; Tu, L.; You, Y.; Zhan, C.; Wang, Y.; Liu, X. Fabrication of crosslinked single-component polyarylene ether nitrile composite with enhanced dielectric properties. Polymer 2019, 161, 162–169. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P. Core-shell structured high-k polymer nanocomposites for energy storage and dielectric applications. Adv. Mater. 2014, 27, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Elzatahry, A.; Aldhayan, D.; Zhao, D.Y. Core-shell structured titanium dioxide nanomaterials for solar energy utilization. Chem. Soc. Rev. 2018, 47, 8203–8237. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wei, R.; Tong, L.; Jia, K.; Liu, X.; Li, K. Crosslinked polyarylene ether nitrile film as flexible dielectric materials with ultrahigh thermal stability. Sci. Rep. 2016, 6, 36434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prateek; Thakur, V.K.; Gupta, R.K. Recent progress on ferroelectric polymer-based nanocomposites for high energy density capacitors: Synthesis, dielectric properties, and future aspects. Chem. Rev. 2016, 116, 4260–4317. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.F.; Thakur, V.K.; Tan, E.J.; Lee, P.S. Surface functionalization of BaTiO3 nanoparticles and improved electrical properties of BaTiO3/polyvinylidene fluoride composite. RSC Adv. 2011, 1, 576–578. [Google Scholar] [CrossRef]
- Ye, H.J.; Shao, W.Z.; Zhen, L. Tetradecylphosphonic acid modified BaTiO3 nanoparticles and its nanocomposite. Colloids Surf. A 2013, 427, 19–25. [Google Scholar] [CrossRef]
- Yu, K.; Niu, Y.; Bai, Y.; Zhou, Y.; Wang, H. Poly(vinylidene fluoride) polymer based nanocomposites with significantly reduced energy loss by filling with core-shell structured BaTiO3/SiO2 nanoparticles. Appl. Phys. Lett. 2013, 102, 102903. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, D.; Jiang, C.; Yuan, X.; Chen, C.; Zhou, K.C. Improved dielectric properties and energy storage density of poly(vinylidene fluoride-co-hexafluoropropylene) nanocomposite with hydantoin epoxy resin coated BaTiO3. ACS Appl. Mater. Interfaces 2015, 7, 8061–8069. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, C.; Fettkenhauer, C.; Glenneberg, J.; Münchgesang, W.; Pientschke, C.; Großmann, T.; Zenkner, M.; Wagner, G.; Leipner, H.S.; Buchsteiner, A.; et al. BaTiO3-P(VDF-HFP) nanocomposite dielectrics-influence of surface modification and dispersion additives. Mater. Sci. Eng. B 2013, 178, 881–888. [Google Scholar] [CrossRef]
- Xu, W.H.; Yang, G.; Jin, L.; Liu, J.; Zhang, Y.H.; Zhang, Z.C.; Jiang, Z.H. High-k polymer nanocomposites filled with hyperbranched phthalocyanine-coated BaTiO3 for high-temperature and elevated field applications. ACS Appl. Mater. Interfaces 2018, 10, 11233–11241. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Hou, X.X.; Zhang, L.Q.; Wu, S.Z. Enhancing crystallinity and orientation by hot-stretching to improve the mechanical properties of electrospun partially aligned polyacrylonitrile (PAN) nanocomposites. Materials 2011, 4, 621–632. [Google Scholar] [CrossRef] [PubMed]










| Samples | Tg (°C) | ΔHm (J/g) | Tensile Strength (MPa) | Tensile Modulus (GPa) |
|---|---|---|---|---|
| PEN/BT 0% 0% | 218.8 | - | 67.2 ± 3.9 | 1.93 ± 0.10 |
| PEN/BT 50% | 219.5 | 4.2 | 90.3 ± 5.7 | 2.25 ± 0.11 |
| PEN/BT 100% | 221.9 | 7.5 | 119.7 ± 6.3 | 2.75 ± 0.15 |
| PEN/PANI-f-BT-a 0% 0% | 218.5 | - | 78.5 ± 3.8 | 1.99 ± 0.09 |
| PEN/PANI-f-BT-a 50% | 219.4 | 5.9 | 108.6 ± 4.2 | 2.41 ± 0.13 |
| PEN/PANI-f-BT-a 100% | 221.7 | 8.4 | 141.2 ± 5.9 | 2.94 ± 0.12 |
| PEN/PANI-f-BT-b 0% 0% | 218.6 | - | 83.8 ± 3.6 | 2.11 ± 0.08 |
| PEN/PANI-f-BT-b 50% | 219.4 | 6.4 | 126.4 ± 4.6 | 2.77 ± 0.10 |
| PEN/PANI-f-BT-b 100% | 221.7 | 9.1 | 161.1 ± 5.3 | 3.37 ± 0.14 |
| PEN/PANI-f-BT-c 0% 0% | 218.1 | - | 79.6 ± 3.9 | 2.01 ± 0.10 |
| PEN/PANI-f-BT-c 50% 50% | 219.0 | 4.7 | 109.1 ± 5.1 | 2.57 ± 0.11 |
| PEN/PANI-f-BT-c 100% 100% | 221.4 | 7.6 | 144.3 ± 5.7 | 3.05 ± 0.13 |
| Samples | Content | Dielectric Constant (1 kHz, 25 °C) | Dielectric Loss (1 kHz, 25 °C) | Working Temperature (°C) | Ref. |
|---|---|---|---|---|---|
| PVDF/BT | 60 vol% | 95 | ~0.04 | <120 | [45] |
| PVDF/BT-PDOPA | 50 vol% | 56.8 | 0.04 | <120 | [46] |
| PVDF/BT-TDPA | 40 vol% | 48 | 0.03 | <120 | [47] |
| PVDF/BT-SiO2 | 10 vol% | 14.7 | 0.02 | <120 | [48] |
| hydantoin/BT-P(VDF-HFP) | 50 vol% | 48.9 | 0.06 | 120 | [49] |
| P(VDF-HFP)/BT-OPA | 30 vol% | ~15 | 0.08 | 120 | [50] |
| PES/BT-CuPc | 40 vol% | ~17 | ~0.12 | ~160 | [51] |
| PAEN/BT@CPAEN | 40 wt% | 13 | 0.023 | ~180 | [30] |
| PEN/PANI-f-BT | 40 wt% | 14 | 0.025 | ~200 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Y.; Tu, L.; Wang, Y.; Tong, L.; Wei, R.; Liu, X. Achieving Secondary Dispersion of Modified Nanoparticles by Hot-Stretching to Enhance Dielectric and Mechanical Properties of Polyarylene Ether Nitrile Composites. Nanomaterials 2019, 9, 1006. https://doi.org/10.3390/nano9071006
You Y, Tu L, Wang Y, Tong L, Wei R, Liu X. Achieving Secondary Dispersion of Modified Nanoparticles by Hot-Stretching to Enhance Dielectric and Mechanical Properties of Polyarylene Ether Nitrile Composites. Nanomaterials. 2019; 9(7):1006. https://doi.org/10.3390/nano9071006
Chicago/Turabian StyleYou, Yong, Ling Tu, Yajie Wang, Lifen Tong, Renbo Wei, and Xiaobo Liu. 2019. "Achieving Secondary Dispersion of Modified Nanoparticles by Hot-Stretching to Enhance Dielectric and Mechanical Properties of Polyarylene Ether Nitrile Composites" Nanomaterials 9, no. 7: 1006. https://doi.org/10.3390/nano9071006