Ethylenediamine-Catalyzed Preparation of Nitrogen-Doped Hierarchically Porous Carbon Aerogel under Hypersaline Condition for High-Performance Supercapacitors and Organic Solvent Absorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nitrogen-Doped Carbon Aerogels (N-CA)
2.3. Synthesis of Carbon Aerogel (CA)
2.4. Materials Characterization
2.5. Electrochemical Measurements
2.6. Absorption of Organic Solvents by the N-CA
3. Results and Discussion
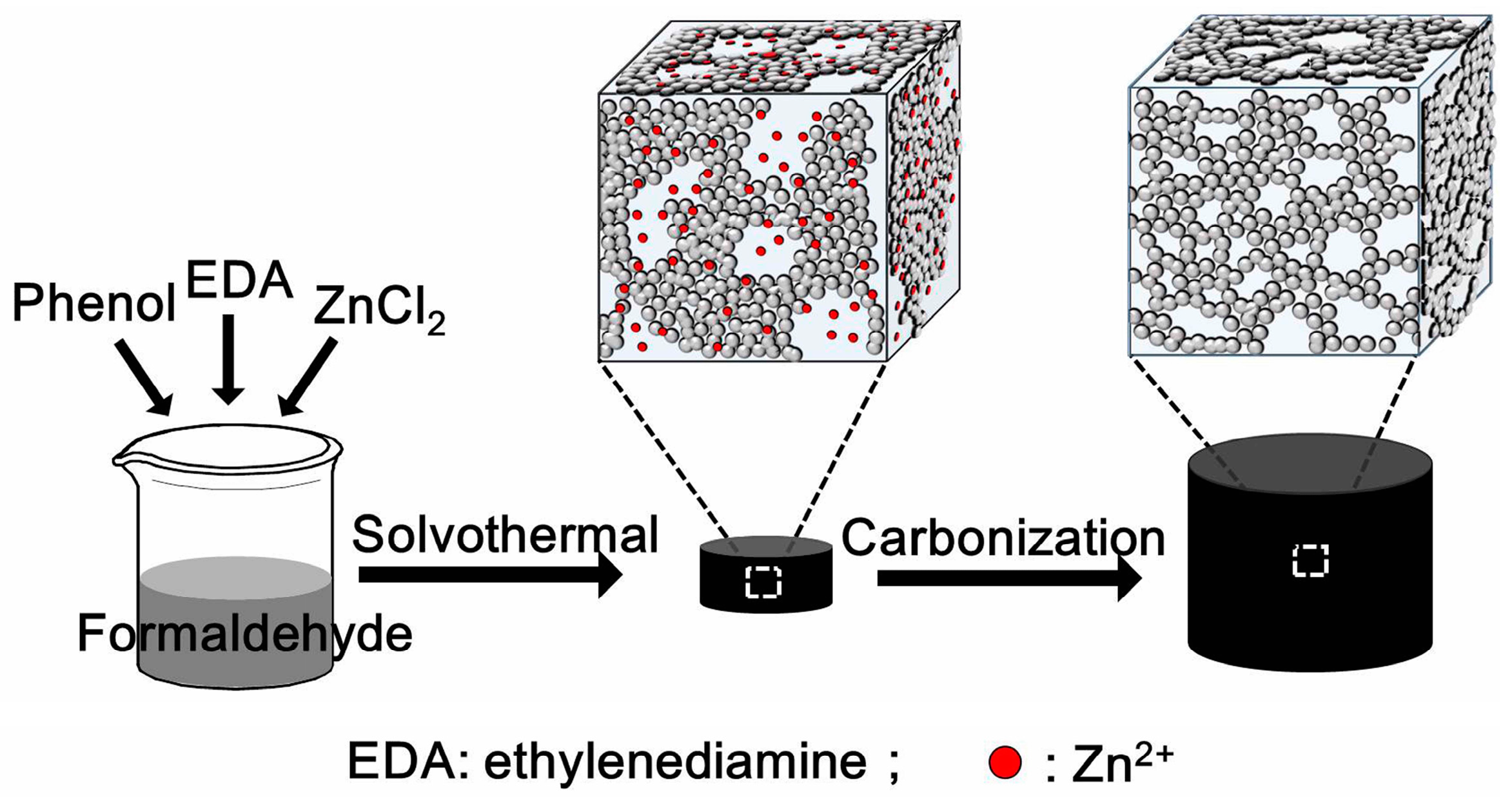
3.1. Fabrication of the N-CA
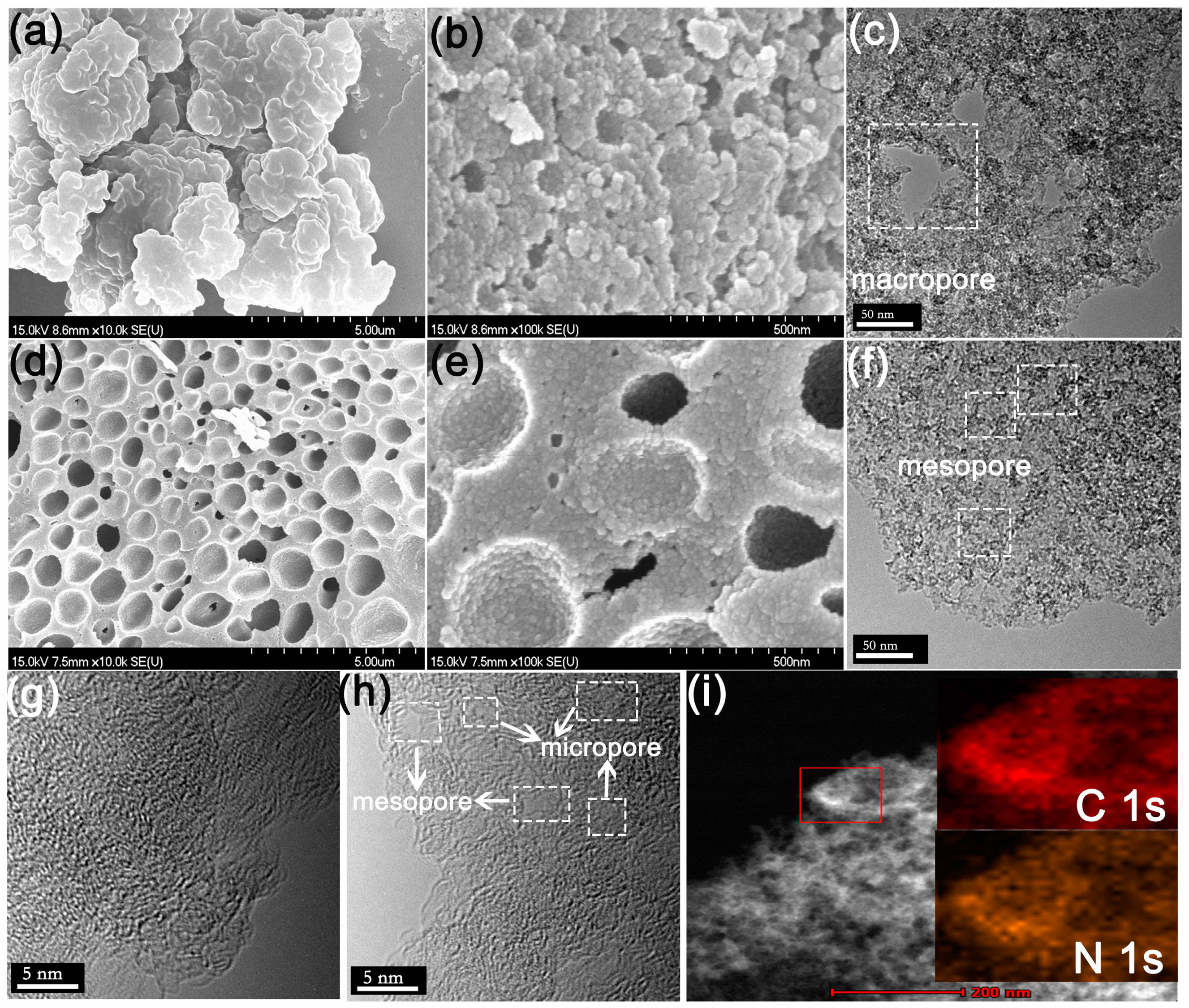
3.2. Morphology and Structure of the N-CA
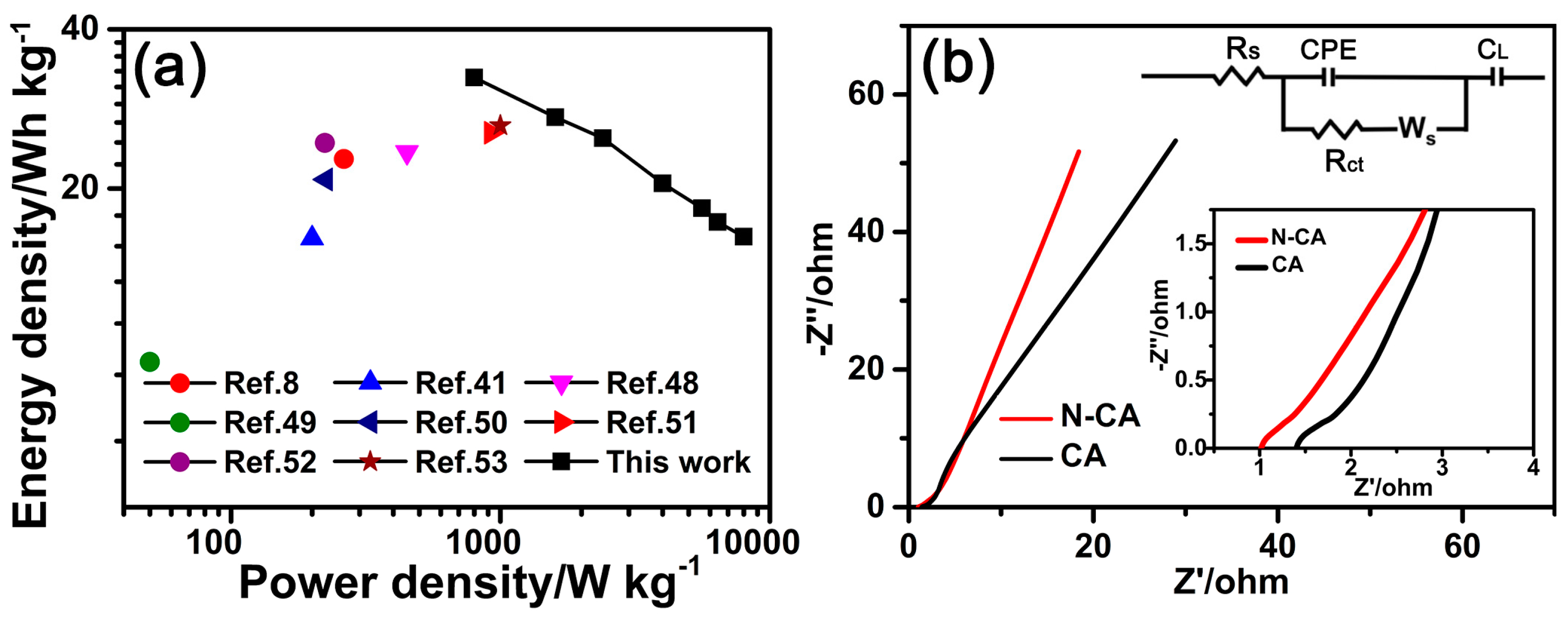
3.3. Electrochemical Performances of the N-CA
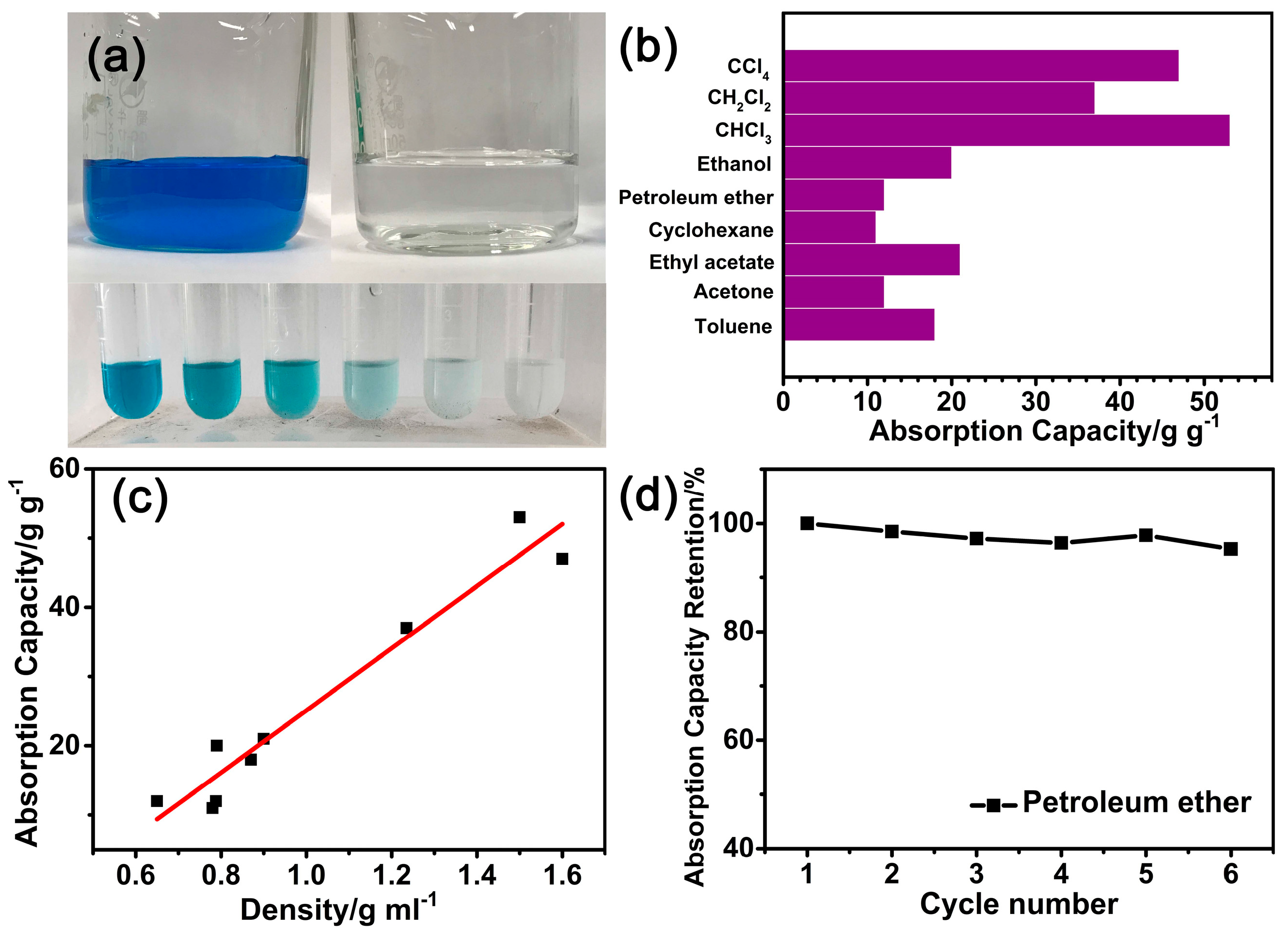
3.4. Absorption Capacity of the N-CA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Härmas, M.; Thomberg, T.; Kurig, H.; Romann, T.; Jänes, A.; Lust, E. Microporous-mesoporous carbons for energy storage synthesized by activation of carbonaceous material by zinc chloride, potassium hydroxide or mixture of them. J. Power Sources 2016, 326, 624–634. [Google Scholar] [CrossRef]
- Laheäär, A.; Peikolainen, A.-L.; Koel, M.; Jänes, A.; Lust, E. Comparison of carbon aerogel and carbide-derived carbon as electrode materials for non-aqueous supercapacitors with high performance. J. Solid State Electrochem. 2012, 16, 2717–2722. [Google Scholar] [CrossRef]
- Lei, W.; Liu, H.P.; Xiao, J.L.; Wang, Y.; Lin, L.X. Moss-derived mesoporous carbon as bi-functional electrode materials for lithium-sulfur batteries and supercapacitors. Nanomaterials 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.P.; Dou, Y.Q.; Zhao, D.Y.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sources 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Wang, H.Y.; Gong, Y.T.; Wang, Y. Cellulose-based hydrophobic carbon aerogels as versatile and superior adsorbents for sewage treatment. RSC Adv. 2014, 4, 45753–45759. [Google Scholar] [CrossRef]
- Wood, K.N.; O’Hayre, R.; Pylypenko, S. Recent progress on nitrogen/carbon structures designed for use in energy and sustainability applications. Energy Environ. Sci. 2014, 7, 1212–1249. [Google Scholar] [CrossRef]
- Wei, X.J.; Wan, S.G.; Gao, S.Y. Self-assembly-template engineering nitrogen-doped carbon aerogels for high-rate supercapacitors. Nano Energy 2016, 28, 206–215. [Google Scholar] [CrossRef]
- Zeng, F.Y.; Sui, Z.Y.; Liu, S.; Liang, H.P.; Zhan, H.H.; Han, B.H. Nitrogen-doped carbon aerogels with high surface area for supercapacitors and gas adsorption. Mater. Today Commun. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Zhao, L.; Baccile, N.; Gross, S.; Zhang, Y.J.; Wei, W.; Sun, Y.H.; Antonietti, M.; Titirici, M. Sustainable nitrogen-doped carbonaceous materials from biomass derivatives. Carbon 2010, 48, 3778–3787. [Google Scholar] [CrossRef]
- White, R.J.; Yoshizawa, N.; Antonietti, M.; Titirici, M.M. A sustainable synthesis of nitrogen-doped carbon aerogels. Green Chem. 2011, 13, 2428–2434. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Ma, L.B.; Lv, H.L.; Hu, Y.; Chen, T.; Chen, R.P.; Liang, J.; Wang, X.; Wang, Y.R.; Yan, C.Z.; et al. Pine needle-derived microporous nitrogen-doped carbon frameworks exhibit high performances in electrocatalytic hydrogen evolution reaction and supercapacitors. Nanoscale 2017, 9, 1237–1243. [Google Scholar] [CrossRef]
- Sethia, G.; Sayari, A. Comprehensive study of ultra-microporous nitrogen-doped activated carbon for CO2 capture. Carbon 2015, 93, 68–80. [Google Scholar] [CrossRef]
- Kitajima, M.; Sato, M.; Nishide, H. Preparation of flat porous carbon films from paper-thin wood shavings and control of their mechanical, electrical and magnetic properties. Carbon 2013, 61, 260–269. [Google Scholar] [CrossRef]
- Reuß, M.; Ratke, L. Subcritically dried RF-aerogels catalysed by hydrochloric acid. J. Sol-Gel Sci. Technol. 2008, 47, 74–80. [Google Scholar] [CrossRef]
- Quan, X.P.; Fu, Z.B.; Yuan, L.; Zhong, M.L.; Mi, R.; Yang, X.; Yi, Y.; Wang, C.Y. Capacitive deionization of NaCl solutions with ambient pressure dried carbon aerogel microsphere electrodes. RSC Adv. 2017, 7, 35875–35882. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Song, H.H.; Chen, X.H.; Du, X.; Zhong, L. Graphene oxide as an anti-shrinkage additive for resorcinol-formaldehyde composite aerogels. Phys. Chem. Chem. Phys. 2014, 16, 11603–11608. [Google Scholar] [CrossRef]
- Fechler, N.; Wohlgemuth, S.-A.; Philipp, J.; Antonietti, M. Salt and sugar: Direct synthesis of high surface area carbon materials at low temperatures via hydrothermal carbonization of glucose under hypersaline conditions. J. Mater. Chem. A 2013, 1, 9418–9421. [Google Scholar] [CrossRef]
- Yu, Z.L.; Li, G.C.; Fechler, N.; Yang, N.; Ma, Z.Y.; Wang, X.; Antonietti, M.; Yu, S.H. Polymerization under hypersaline conditions: A robust route to phenolic polymer-derived carbon aerogels. Angew. Chem. Int. Ed. 2016, 55, 14623–14627. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Xu, J.T.; Wang, M.; Zhu, L.; Dai, L.M.; Jaroniec, M. Nitrogen enriched porous carbon spheres: Attractive materials for supercapacitor electrodes and CO2 adsorption. Chem. Mater. 2014, 26, 2820–2828. [Google Scholar] [CrossRef]
- Hollink, E.; Simanek, E.E.; Bergbreiter, D.E. Strategies for protecting and manipulating triazine derivatives. Tetrahedron Lett. 2005, 46, 2005–2008. [Google Scholar] [CrossRef]
- Yue, Z.R.; Mangun, C.L.; Economy, J. Preparation of fibrous porous materials by chemical activation 1. ZnCl2 activation of polymer-coated fiber. Carbon 2002, 40, 1181–1191. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Li, P.G.; Gao, C. Strong, conductive, lightweight, neat graphene aerogel fibers with aligned pores. ACS Nano 2012, 6, 7103–7113. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Carbon aerogel from winter melon for highly efficient and recyclable oils and organic solvents absorption. ACS Sustain. Chem. Eng. 2014, 2, 1492–1497. [Google Scholar] [CrossRef]
- Mehare, R.S.; Ranganath, S.P.; Chaturvedi, V.; Badiger, M.V.; Shelke, M.V. In situ synthesis of nitrogen- and sulfur-enriched hierarchical porous carbon for high-performance supercapacitor. Energy Fuels 2018, 32, 908–915. [Google Scholar] [CrossRef]
- Fechler, N.; Fellinger, T.P.; Antonietti, M. One-pot synthesis of nitrogen-sulfur-co-doped carbons with tunable composition using a simple isothiocyanate ionic liquid. J. Mater. Chem. A 2013, 1, 14097–14102. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, H.W.; Xiao, Y.; Hu, H.; Cai, Y.J.; Liang, Y.R.; Sun, L.Y.; Liu, Y.L.; Zheng, M.T. Three-dimensional nitrogen-doped graphene as binder-free electrode materials for supercapacitors with high volumetric capacitance and the synergistic effect between nitrogen configuration and supercapacitive performance. Electrochim. Acta 2016, 218, 32–40. [Google Scholar] [CrossRef]
- Kim, T.; Kim, M.; Park, Y.; Kim, E.; Kim, J.; Ryu, W.; Jeong, H.; Kim, K. Cutting-processed single-wall carbon nanotubes with additional edge sites for supercapacitor electrodes. Nanomaterials 2018, 8, 464. [Google Scholar] [CrossRef]
- Chen, L.F.; Huang, Z.H.; Liang, H.W.; Yao, W.T.; Yu, Z.Y.; Yu, S.H. Flexible all-solid-state high-power supercapacitor fabricated with nitrogen-doped carbon nanofiber electrode material derived from bacterial cellulose. Energy Environ. Sci. 2013, 6, 3331–3338. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhao, J.Q.; He, X.; Li, Q.Y.; Ao, C.H.; Xia, T.; Zhang, W.; Lu, C.H.; Deng, Y.L. Mechanically robust and highly compressible electrochemical supercapacitors from nitrogen-doped carbon aerogels. Carbon 2018, 127, 236–244. [Google Scholar] [CrossRef]
- Chiu, P.W.; Duesberg, G.S.; Dettlaff-Weglikowska, U.; Roth, S. Interconnection of carbon nanotubes by chemical functionalization. Appl. Phys. Lett. 2002, 80, 3811–3813. [Google Scholar] [CrossRef]
- Miao, L.; Duan, H.; Liu, M.X.; Lu, W.J.; Zhu, D.Z.; Chen, T.; Li, L.C.; Gan, L.H. Poly(ionic liquid)-derived, N, S-codoped ultramicroporous carbon nanoparticles for supercapacitors. Chem. Eng. J. 2017, 317, 651–659. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Shanmugam, S. Nitrogen-doped porous multi-nano-channel nanocarbons for use in high-performance supercapacitor applications. ACS Sustain. Chem. Eng. 2016, 4, 2439–2448. [Google Scholar] [CrossRef]
- Ma, L.N.; Liu, R.; Niu, H.J.; Xing, L.X.; Liu, L.; Huang, Y.D. Flexible and freestanding supercapacitor electrodes based on nitrogen-doped carbon networks/graphene/bacterial cellulose with ultrahigh areal capacitance. ACS Appl. Mater. Interfaces 2016, 8, 33608–33618. [Google Scholar] [CrossRef]
- Liu, C.Z.; Chen, W.M.; Hong, S.; Pan, M.Z.; Jiang, M.; Wu, Q.L.; Mei, C.T. Fast microwave synthesis of hierarchical porous carbons from aste palm boosted by activated carbons for supercapacitors. Nanomaterials 2019, 9, 405. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, Y.C.; Yu, T.; Zhu, P.F.; Yan, X.Z.; Wang, Z.; Guan, S.Y. Boron and nitrogen co-doped porous carbon with a high concentration of boron and its superior capacitive behavior. Carbon 2017, 113, 266–273. [Google Scholar] [CrossRef]
- Hao, G.P.; Li, W.C.; Qian, D.; Wang, G.H.; Zhang, W.P.; Zhang, T.; Wang, A.Q.; Schuth, F.; Bongard, H.J.; Lu, A.H. Structurally designed synthesis of mechanically stable poly(benzoxazine-co-resol)-based porous carbon monoliths and their application as high-performance CO2 capture sorbents. J. Am. Chem. Soc. 2011, 133, 11378–11388. [Google Scholar] [CrossRef]
- Zhao, J.; Lai, H.W.; Lyu, Z.Y.; Jiang, Y.F.; Xie, K.; Wang, X.Z.; Wu, Q.; Yang, L.J.; Jin, Z.; Ma, Y.W.; et al. Hydrophilic hierarchical nitrogen-doped carbon nanocages for ultrahigh supercapacitive performance. Adv. Mater. 2015, 27, 3541–3545. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Szczurek, A.; Stein, N.; Parmentier, J.; Celzard, A. Hydrothermally treated aminated tannin as precursor of N-doped carbon gels for supercapacitors. Carbon 2015, 90, 63–74. [Google Scholar] [CrossRef]
- Chen, L.F.; Lu, Y.; Yu, L.; Lou, X.W. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors. Energy Environ. Sci. 2017, 10, 1777–1783. [Google Scholar] [CrossRef]
- Du, J.; Liu, L.; Hu, Z.P.; Yu, Y.F.; Zhang, Y.; Hou, S.L.; Chen, A.B. Raw-cotton-derived N-doped carbon fiber aerogel as an efficient electrode for electrochemical capacitors. ACS Sustain. Chem. Eng. 2018, 6, 4008–4015. [Google Scholar] [CrossRef]
- Choi, W.H.; Choi, M.J.; Bang, J.H. Nitrogen-doped carbon nanocoil array integrated on carbon nanofiber paper for supercapacitor electrodes. ACS Appl. Mater. Interfaces 2015, 7, 19370–19381. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, G.; Chen, L.; Dai, B.; Yu, F. Three-dimensional honeycomb-like porous carbon with both interconnected hierarchical porosity and nitrogen self-doping from cotton seed husk for supercapacitor electrode. Nanomaterials 2018, 8, 412. [Google Scholar] [CrossRef]
- Liu, M.Q.; Huo, S.L.; Xu, M.; Wu, L.L.; Liu, M.J.; Xue, Y.F.; Yan, Y.M. Structural engineering of N/S co-doped carbon material as high-performance electrode for supercapacitors. Electrochim. Acta 2018, 274, 389–399. [Google Scholar] [CrossRef]
- Meng, Q.S.; Qin, K.Q.; Ma, L.Y.; He, C.N.; Liu, E.Z.; He, F.; Shi, C.S.; Li, Q.Y.; Li, J.J.; Zhao, N.Q. N-doped porous carbon nanofibers/porous silver network hybrid for high-rate supercapacitor electrode. ACS Appl. Mater. Interfaces 2017, 9, 30832–30839. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.S.; Huang, Z.Y.; Zheng, L.; Han, B.; Gao, Q.; Zhou, C.G.; Wang, H.Q.; Wu, J.P. Facile and controllable synthesis of N/P co-doped graphene for high-performance supercapacitors. J. Power Sources 2017, 365, 380–388. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, H.; Li, L.; Yao, Y.; Qu, H.N.; Zhang, C.L.; Liu, S.H.; Zhou, Y.M. Double soft-template synthesis of nitrogen/sulfur-codoped hierarchically porous carbon materials derived from protic ionic liquid for supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 26088–26095. [Google Scholar] [CrossRef]
- Sun, G.L.; Ma, L.Y.; Ran, J.B.; Li, B.; Shen, X.Y.; Tong, H. Templated synthesis and activation of highly nitrogen-doped worm-like carbon composites based on melamine-urea-formaldehyde resins for high performance supercapacitors. Electrochim. Acta 2016, 194, 168–178. [Google Scholar] [CrossRef]
- Li, B.Q.; Cheng, Y.F.; Dong, L.P.; Wang, Y.M.; Chen, J.C.; Huang, C.F.; Wei, D.Q.; Feng, Y.J.; Jia, D.C.; Zhou, Y. Nitrogen doped and hierarchically porous carbons derived from chitosan hydrogel via rapid microwave carbonization for high-performance supercapacitors. Carbon 2017, 122, 592–603. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.F.; Sun, K.J.; Mu, J.J.; Lei, Z.Q. One-step preparation of ultrathin nitrogen-doped carbon nanosheets with ultrahigh pore volume for high-performance supercapacitors. J. Mater. Chem. A 2014, 2, 17297–17301. [Google Scholar] [CrossRef]
- Tang, C.G.; Liu, Y.J.; Yang, D.G.; Yang, M.; Li, H.M. Oxygen and nitrogen co-doped porous carbons with finely-layered schistose structure for high-rate-performance supercapacitors. Carbon 2017, 122, 538–546. [Google Scholar] [CrossRef]
- Mao, N.; Wang, H.L.; Sui, Y.; Cui, Y.P.; Pokrzywinski, J.; Shi, J.; Liu, W.; Chen, S.G.; Wang, X.; Mitlin, D. Extremely high-rate aqueous supercapacitor fabricated using doped carbon nanoflakes with large surface area and mesopores at near-commercial mass loading. Nano Res. 2017, 10, 1767–1783. [Google Scholar] [CrossRef]
- Xiang, X.X.; Liu, E.H.; Xie, H.; Tian, Y.Y.; Wu, Y.H.; Wu, Z.L.; Zhu, Y.H. Highly stable performance of supercapacitors using microporous carbon derive from phenol-melamine-formaldehyde resin. J. Solid State Electrochem. 2012, 16, 2661–2666. [Google Scholar] [CrossRef]
- Cheng, P.; Li, T.; Yu, H.; Zhi, L.; Liu, Z.H.; Lei, Z.B. Biomass-derived carbon fiber aerogel as a binder-free electrode for high-rate supercapacitors. J. Phys. Chem. C 2016, 120, 2079–2086. [Google Scholar] [CrossRef]
- Liu, R.L.; Wan, L.; Liu, S.Q.; Pan, L.X.; Wu, D.Q.; Zhao, D.Y. An interface-induced co-assembly approach towards ordered mesoporous carbon/graphene aerogel for high-performance supercapacitors. Adv. Funct. Mater. 2015, 25, 526–533. [Google Scholar] [CrossRef]
- Du, Y.X.; Liu, L.B.; Xiang, Y.; Zhang, Q. Enhanced electrochemical capacitance and oil-absorbability of N-doped graphene aerogel by using amino-functionalized silica as template and doping agent. J. Power Sources 2018, 379, 240–248. [Google Scholar] [CrossRef]



| Samples | SBET 1/m2·g−1 | VT 2/cm3·g−1 | Smicro 3/m2·g−1 | Vmicro 4/cm3·g−1 | SE 5/m2·g−1 | D 6/nm |
|---|---|---|---|---|---|---|
| CA | 1479.1 | 1.50 | 229.8 | 0.12 | 1049.92 | 5.0 |
| N-CA | 1201.1 | 0.75 | 624.6 | 0.35 | 576.5 | 3.7 |
| Items | Cg 1 | I 2 | Electrolyte | Ref. |
|---|---|---|---|---|
| N-enriched porous carbon spheres | 388 | 1 | 1M H2SO4 | [20] |
| N-doped carbon fiber aerogel | 365 | 0.5 | 6M KOH | [41] |
| N-doped carbon nanofiber paper | 209 | 1 | 1M KOH | [42] |
| B/N co-doped carbon | 304 | 0.1 | 1M H2SO4 | [36] |
| N-doped porous carbon | 238 | 0.5 | 6M KOH | [43] |
| N/S co-doped carbon | 322 | 1 | 6M KOH | [44] |
| N-doped porous carbon nanofibers | 245 | 1 | 6M KOH | [45] |
| N/P co-doped graphene | 219 | 0.25 | 6M KOH | [46] |
| N/S co-doped porous carbon | 302 | 1 | 6M KOH | [47] |
| N-doped carbon aerogel | 426 | 1 | 0.5M H2SO4 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Zhang, X.; Yang, J.; Zhou, J.; Tong, M.; Jin, Q.; Dai, F.; Li, G. Ethylenediamine-Catalyzed Preparation of Nitrogen-Doped Hierarchically Porous Carbon Aerogel under Hypersaline Condition for High-Performance Supercapacitors and Organic Solvent Absorbents. Nanomaterials 2019, 9, 771. https://doi.org/10.3390/nano9050771
Gao J, Zhang X, Yang J, Zhou J, Tong M, Jin Q, Dai F, Li G. Ethylenediamine-Catalyzed Preparation of Nitrogen-Doped Hierarchically Porous Carbon Aerogel under Hypersaline Condition for High-Performance Supercapacitors and Organic Solvent Absorbents. Nanomaterials. 2019; 9(5):771. https://doi.org/10.3390/nano9050771
Chicago/Turabian StyleGao, Jing, Xuan Zhang, Jiaxing Yang, Junxi Zhou, Mingxing Tong, Qiuyang Jin, Fangna Dai, and Guohua Li. 2019. "Ethylenediamine-Catalyzed Preparation of Nitrogen-Doped Hierarchically Porous Carbon Aerogel under Hypersaline Condition for High-Performance Supercapacitors and Organic Solvent Absorbents" Nanomaterials 9, no. 5: 771. https://doi.org/10.3390/nano9050771






