Plasmon-Enhanced Blue-Light Emission of Stable Perovskite Quantum Dot Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Perovskite Precursor Solutions
2.3. Synthesis of Ag NPs
2.4. Preparation of Ag NPs Electrospinning Solutions
2.5. Preparation of Polymer-Encapsulated Perovskite Electrospinning Membrane with and without Containing Plasmonic Nanoparticle Nanofibers
2.6. Characterizations
2.7. Theoretical Modeling
3. Results and Discussion
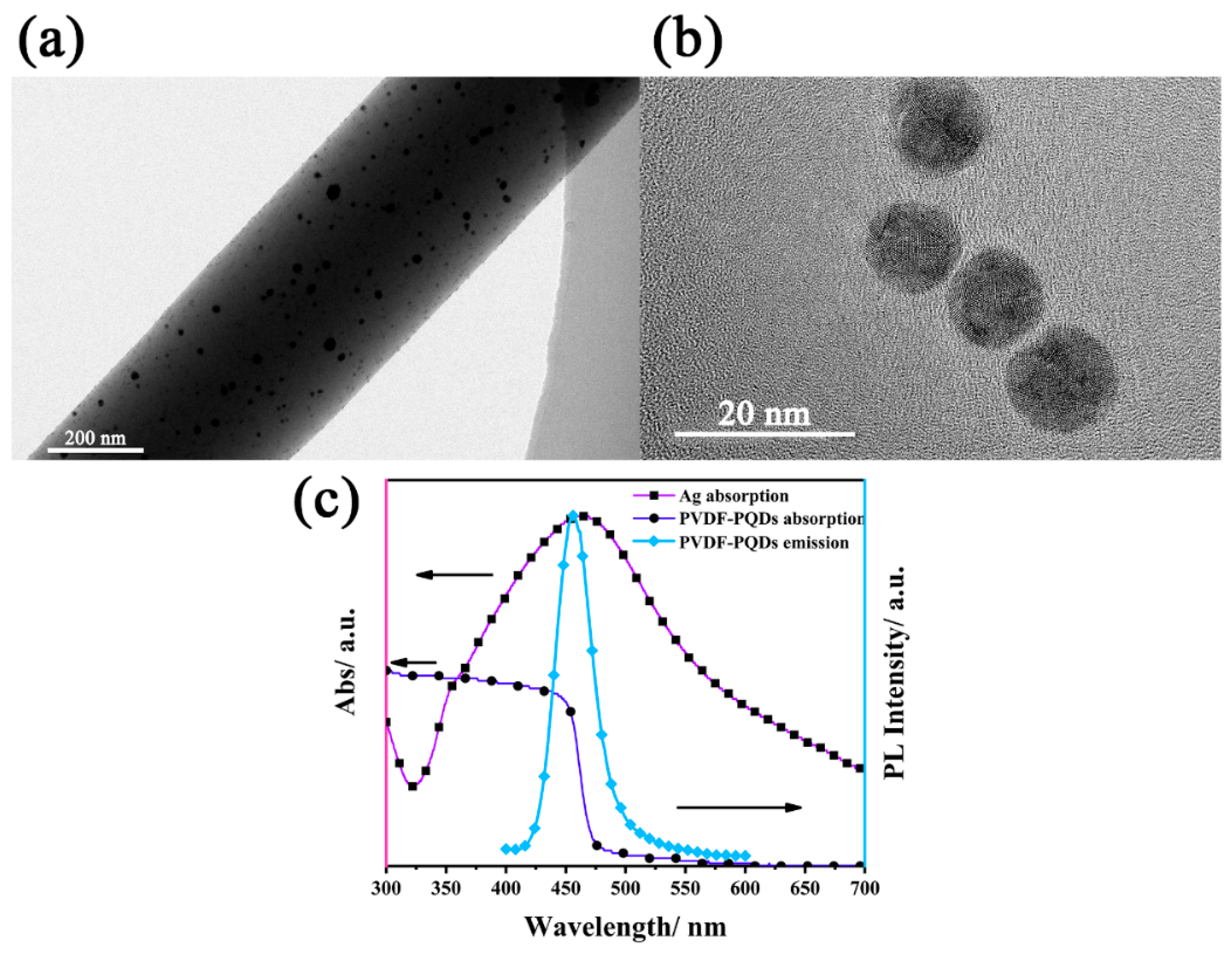
3.1. PVDF-Encapsulated MAPbBr3−xClx QDs
3.2. Effects of Halogens Composition in MAPbBr3−xClx QDs
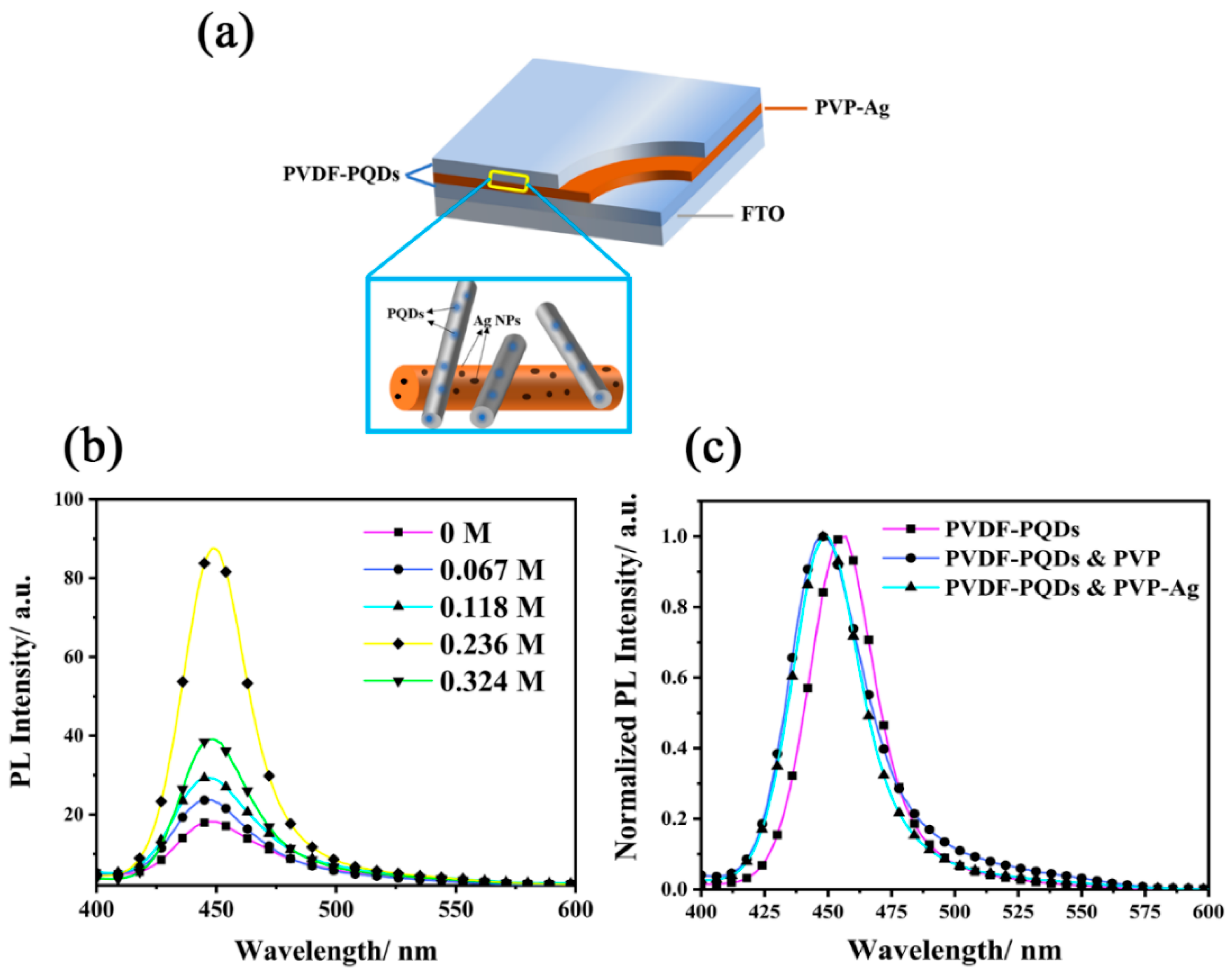
3.3. Plasmon-Enhanced Fluorescence
3.4. Theoretical Analysis of the Plasmon-Enhanced Fluorescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Y.; You, J.; Meng, L. Efficient and Stable Perovskite Solar Cells with All Solution Processed Metal Oxide Transporting Layers. U.S. Patent 15/553,483, 1 February 2018. [Google Scholar]
- Chang, S.; Bai, Z.; Zhong, H. In situ fabricated perovskite nanocrystals: A revolution in optical materials. Adv. Opt. Mater. 2018, 6, 1800380. [Google Scholar] [CrossRef]
- Imran, M.; Caligiuri, V.; Wang, M.; Goldoni, L.; Prato, M.; Krahne, R.; De, T.L.; Manna, L. Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead Based Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2018, 140, 2656–2664. [Google Scholar] [CrossRef]
- Brenner, T.M.; Egger, D.A.; Kronik, L.; Hodes, G.; Cahen, D. Hybrid organic—Inorganic perovskites: Low-cost semiconductors with intriguing charge-transport properties. Nat. Rev. Mater. 2016, 1, 15007. [Google Scholar] [CrossRef]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J. Highly Efficient Perovskite-Quantum-Dot Light-Emitting Diodes by Surface Engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef]
- Ling, Y.; Yuan, Z.; Tian, Y.; Wang, X.; Wang, J.C.; Xin, Y.; Hanson, K.; Ma, B.; Gao, H. Bright Light-Emitting Diodes Based on Organometal Halide Perovskite Nanoplatelets. Adv. Mater. 2016, 28, 305–311. [Google Scholar] [CrossRef]
- Wang, C.; Chesman, A.S.; Jasieniak, J.J. Stabilizing the cubic perovskite phase of CsPbI3 nanocrystals by using an alkyl phosphinic acid. Chem. Commun. 2017, 53, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Cheng, Z.; Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Wood, S.M.; Patel, J.B.; Nayak, P.K.; Huang, J.; Alexander-Webber, J.A.; Wenger, B.; Stranks, S.D.; Hörantner, M.T.; Wang, J.T. Structured Organic-Inorganic Perovskite toward a Distributed Feedback Laser. Adv. Mater. 2016, 28, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Liu, Y.; Li, J.; Liu, C.; Feng, R.; Jiang, F.; Li, Y.; Song, J.; Zeng, H.; Hong, M. Stabilizing Cesium Lead Halide Perovskite Lattice through Mn (II)-Substitution for Air-Stable Light-Emitting Diodes. J. Am. Chem. Soc. 2017, 139, 11443. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhao, F.; Liu, L.; Zhang, F.; Wu, X.G.; Shi, L.; Zou, B.; Pei, Q.; Zhong, H. Emulsion Synthesis of Size-Tunable CH3NH3PbBr3 Quantum Dots: An Alternative Route toward Efficient Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2015, 7, 28128. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yuan, Z.; Feng, G. Colloidal metal halide perovskite nanocrystals: Synthesis, characterization, and applications. J. Mater. Chem. C 2016, 4, 3898–3904. [Google Scholar] [CrossRef]
- Meng, Z.; Hua, Y.; Miaoqiang, L.; Qiong, W.; Jung-Ho, Y.; Lianzhou, W. Composition-dependent photoluminescence intensity and prolonged recombination lifetime of perovskite CH3NH3PbBr3−xClx films. Chem. Commun. 2014, 50, 11727–11730. [Google Scholar]
- Leng, M.; Yang, Y.; Chen, Z.; Gao, W.; Zhang, J.; Niu, G.; Li, D.; Song, H.; Zhang, J.; Jin, S. Surface passivation of bismuth-based perovskite variant quantum dots to achieve efficient blue emission. Nano Lett. 2018, 18, 6076–6083. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, J.; Chen, H.; Chen, J.; Zhu, R.; Ma, P.; Towers, A.; Lin, Y.; Gesquiere, A.J.; Wu, S.T. Ultrastable, Highly Luminescent Organic-Inorganic Perovskite-Polymer Composite Films. Adv. Mater. 2016, 28, 10710–10717. [Google Scholar] [CrossRef] [PubMed]
- Hengyue, L.I.; Gong, C.; Huang, K.; Yang, J. A Review on the Fabrication of Perovskite Solar Cells via Printing Techniques. Mater. Rev. 2018, 32, 1385–1400. [Google Scholar]
- Murphy, J.P.; Andriolo, J.M.; Sutton, N.J.; Brockway, M.C.; Skinner, J.L. Coaxial hybrid perovskite fibers: Synthesis and encapsulation in situ via electrospinning. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2017, 35, 06G402. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, Y. Electrospun Perovskite Nanofibers. Nanoscale Res. Lett. 2017, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.-M.; Tai, T.-Y.; Chen, Y.-Y.; Lin, P.-Y.; Fu, Y.-S. Fabrication of CH3NH3PbI3/PVP Composite Fibers via Electrospinning and Deposition. Materials 2015, 8, 5467–5478. [Google Scholar] [CrossRef]
- Tsai, P.C.; Chen, J.Y.; Ercan, E.; Chueh, C.C.; Tung, S.H.; Chen, W.C. Uniform Luminous Perovskite Nanofibers with Color-Tunability and Improved Stability Prepared by One-Step Core/Shell Electrospinning. Small 2018, 14, 1704379. [Google Scholar] [CrossRef]
- Lin, C.C.; Jiang, D.H.; Kuo, C.C.; Cho, C.J.; Tsai, Y.H.; Satoh, T.; Su, C. Water-Resistant Efficient Stretchable Perovskite-Embedded Fiber Membranes for Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 2210. [Google Scholar] [CrossRef]
- Aslan, K.; Wu, M.; Lakowicz, J.R.; Geddes, C.D. Fluorescent core-shell Ag@SiO2 nanocomposites for metal-enhanced fluorescence and single nanoparticle sensing platforms. J. Am. Chem. Soc. 2007, 129, 1524. [Google Scholar] [CrossRef] [PubMed]
- Dadı, S.; Altıntas, Y.; Beskazak, E.; Mutlugun, E. Plasmon Enhanced Emission of Perovskite Quantum Dot Films. MRS Adv. 2018, 3, 733–739. [Google Scholar] [CrossRef]
- Pompa, P.P.; Martiradonna, L.; Torre, A.D.; Sala, F.D.; Manna, L.; De Vittorio, M.; Calabi, F.; Cingolani, R.; Rinaldi, R. Metal-enhanced fluorescence of colloidal nanocrystals with nanoscale control. Nat. Nanotechnol. 2006, 1, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Tovmachenko, O.; Graf, C.; Heuvel, D.J.V.D.; Blaaderen, A.V.; Gerritsen, H.C. Fluorescence Enhancement by Metal-Core/Silica-Shell Nanoparticles. Adv. Mater. 2010, 18, 91–95. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Wu, L.; Fu, B.; Liu, G.; Zhang, D.; Zhao, J.; Chen, P.; Liu, L. Enhancing perovskite film fluorescence by simultaneous near- and far-field effects of gold nanoparticles. RSC Adv. 2017, 7, 35752–35756. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Bai, Z.; Lu, W.G.; Wang, Y.; Zou, B.; Zhong, H. In Situ Fabrication of Halide Perovskite Nanocrystal-Embedded Polymer Composite Films with Enhanced Photoluminescence for Display Backlights. Adv. Mater. 2016, 28, 9163–9168. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Pang, X.; He, Y.; Wang, Y.; Chen, G.; Wang, W.; Lin, Z. Precisely size-tunable magnetic/plasmonic core/shell nanoparticles with controlled optical properties. Angew. Chem. 2015, 127, 12259–12264. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Y.; Peng, H.S.; Chen, G.; Lin, Z. An integrated experimental and theoretical study on optical properties of uniform hairy noble metal nanoparticles. Nanoscale 2018, 10, 22750–22757. [Google Scholar] [CrossRef]
- Alias, A.; Kudin, T.; Zabidi, Z.; Harun, M.K.; Ali, A.M.M.; Yahya, M.F. In Refractive index dispersion and optical dielectric properties of poly(N-carbazole)/poly(vinylpyrrolidone) blends. Adv. Mater. Res. 2013, 652–654, 532–536. [Google Scholar] [CrossRef]
- Bai, M.; Sorokin, A.; Thompson, D.W.; Poulsen, M.; Ducharme, S.; Herzinger, C.; Palto, S.; Fridkin, V.; Yudin, S.; Savchenko, V. Determination of the optical dispersion in ferroelectric vinylidene fluoride (70%)/trifluoroethylene (30%) copolymer Langmuir–Blodgett films. J. Appl. Phys. 2004, 95, 3372–3377. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.B.; Christy, R.-W. Optical constants of the noble metals. Phys. Rev. B 1972, 6, 4370. [Google Scholar] [CrossRef]
- Baikie, T.; Barrow, N.S.; Fang, Y.; Keenan, P.J.; Slater, P.R.; Piltz, R.O.; Gutmann, M.; Mhaisalkar, S.G.; White, T.J. A combined single crystal neutron/X-ray diffraction and solid-state nuclear magnetic resonance study of the hybrid perovskites CH3NH3PbX3 (X = I, Br and Cl). J. Mater. Chem. A 2015, 3, 9298–9307. [Google Scholar] [CrossRef]
- Brennan, M.C.; Herr, J.E.; Nguyen-Beck, T.S.; Zinna, J.; Draguta, S.; Rouvimov, S.; Parkhill, J.; Kuno, M. Origin of the size-dependent stokes shift in CsPbBr3 perovskite nanocrystals. J. Am. Chem. Soc. 2017, 139, 12201–12208. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.J.; Lee, H.K.; Jeong, E.H.; Park, W.H.; Ji, H.Y. Preparation of Polymer Nanofibers Containing Silver Nanoparticles by Using Poly(N-vinylpyrrolidone). Macromol. Rapid Commun. 2010, 26, 1903–1907. [Google Scholar] [CrossRef]
- Guidelli, E.J.; Ramos, A.P.; Baffa, O. Enhancing and quenching luminescence with gold nanoparticle films: The influence of substrate on the luminescent properties. Nanotechnology 2015, 27, 015503. [Google Scholar] [CrossRef]
- Davletshin, Y.R.; Lombardi, A.; Cardinal, M.F.; Juvé, V.; Crut, A.; Maioli, P.; Liz-Marzan, L.M.; Vallée, F.; Fatti, N.D.; Kumaradas, J.C. A quantitative study of the environmental effects on the optical response of gold nanorods. ACS Nano 2012, 6, 8183–8193. [Google Scholar] [CrossRef]

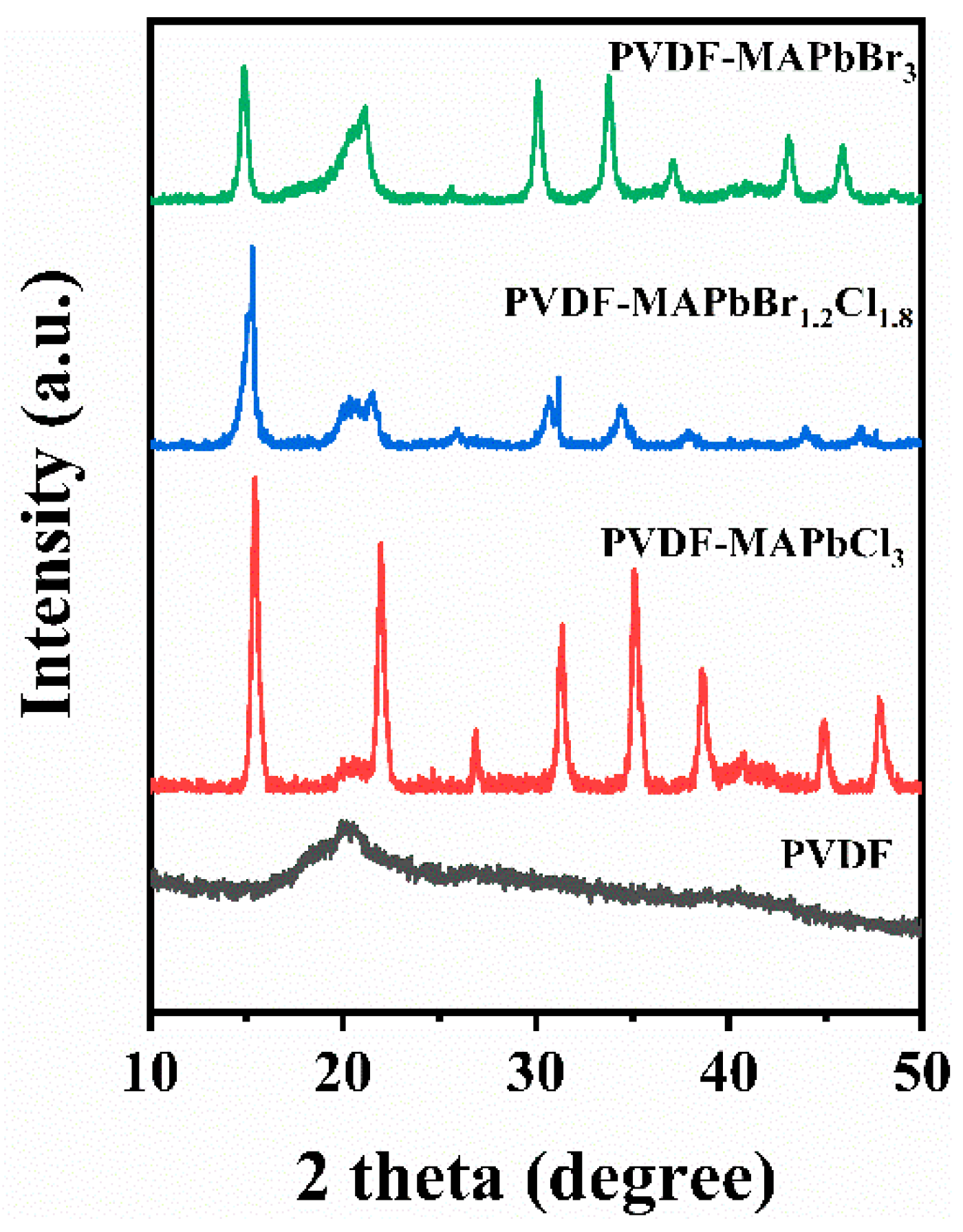


| Samples | Diffraction Peaks and Crystal Planes | |||||||
|---|---|---|---|---|---|---|---|---|
| MAPbBr3 | 14.82° | 21.04° | 25.98° | 30.11° | 33.72° | 37.09° | 43.09° | 45.91° |
| MAPbBr1.2Cl1.8 | 15.30° | 21.54° | 26.34° | 30.83° | 34.37° | 38.06° | 44.04° | 46.83° |
| MAPbCl3 | 15.42° | 21.97° | 26.89° | 31.30° | 35.11° | 38.60° | 44.93° | 47.85° |
| Crystal planes | (001) | (011) | (111) | (002) | (021) | (211) | (022) | (003) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, K.; Peng, H.; Hua, S.; Qu, Y.; Yang, D. Plasmon-Enhanced Blue-Light Emission of Stable Perovskite Quantum Dot Membranes. Nanomaterials 2019, 9, 770. https://doi.org/10.3390/nano9050770
Gu K, Peng H, Hua S, Qu Y, Yang D. Plasmon-Enhanced Blue-Light Emission of Stable Perovskite Quantum Dot Membranes. Nanomaterials. 2019; 9(5):770. https://doi.org/10.3390/nano9050770
Chicago/Turabian StyleGu, Kai, Hongshang Peng, Siwei Hua, Yusong Qu, and Di Yang. 2019. "Plasmon-Enhanced Blue-Light Emission of Stable Perovskite Quantum Dot Membranes" Nanomaterials 9, no. 5: 770. https://doi.org/10.3390/nano9050770





