Fabrication of Flexible, Lightweight, Magnetic Mushroom Gills and Coral-Like MXene–Carbon Nanotube Nanocomposites for EMI Shielding Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ti3AlC2
2.3. Preparation of Oxidized Carbon Nanotube (CNTO)
2.4. Preparation of Fe3O4 Decorated CNTO
2.5. Preparation of Nanoparticle Decorated CNTO
2.6. Preparation of Dispersed Solutions
2.7. Preparation of MXene and MXene Colloidal Solution
2.8. Synthesis of Carbon Fabric by Wet Laid Method (MC)
2.9. Fabrication of Fabric Composite
2.9.1. Preparation of Functionalized Carbon Fabric (FC)
2.9.2. Fabrication of MXCNTCx Composite by Spray Coating
2.9.3. Fabrication of MXene–CNTO Composite by Filtration
2.10. Characterization
3. Results and Discussion
3.1. Structural Analysis
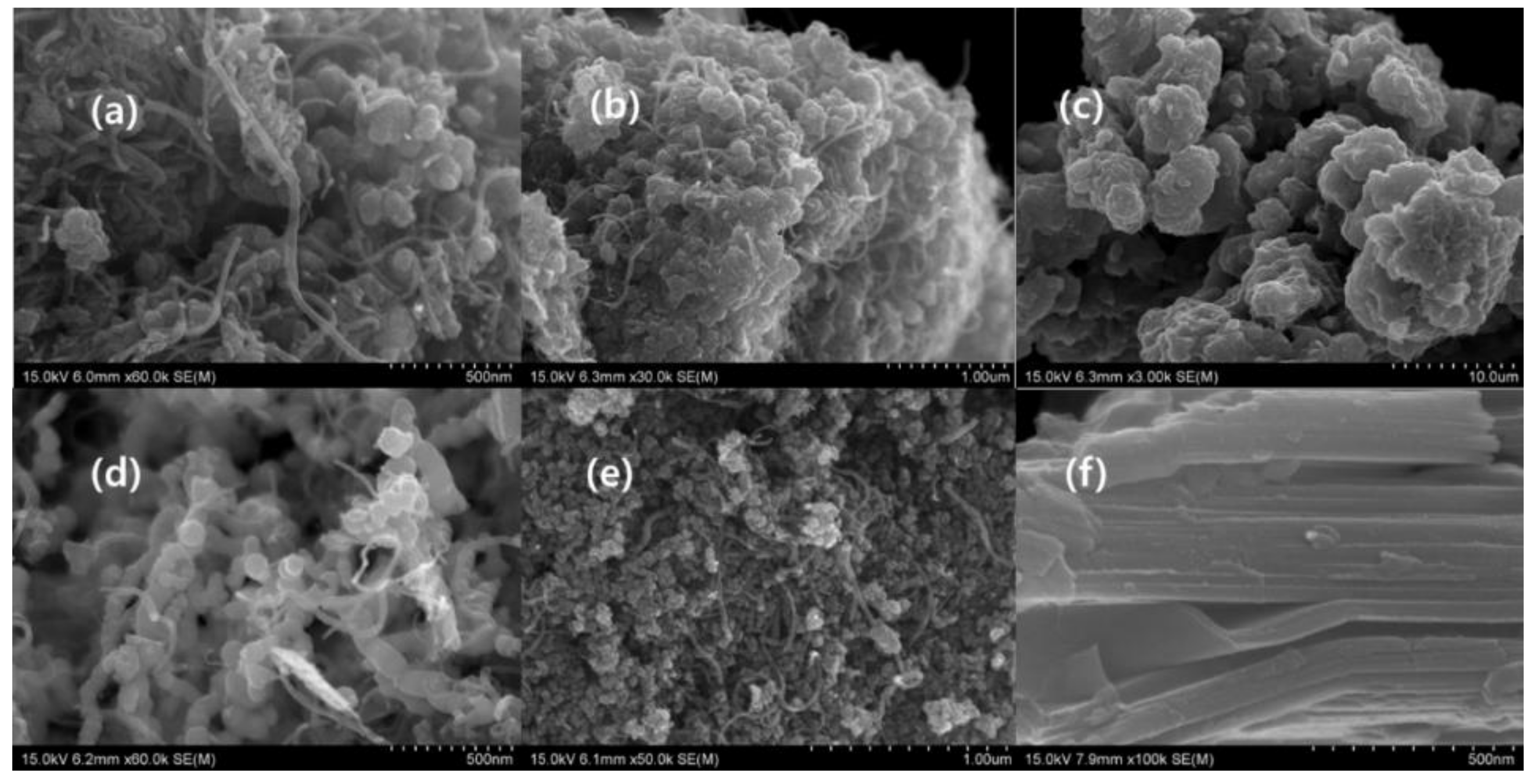
3.1.1. Scanning Electron Microscopic Analysis of Morphology
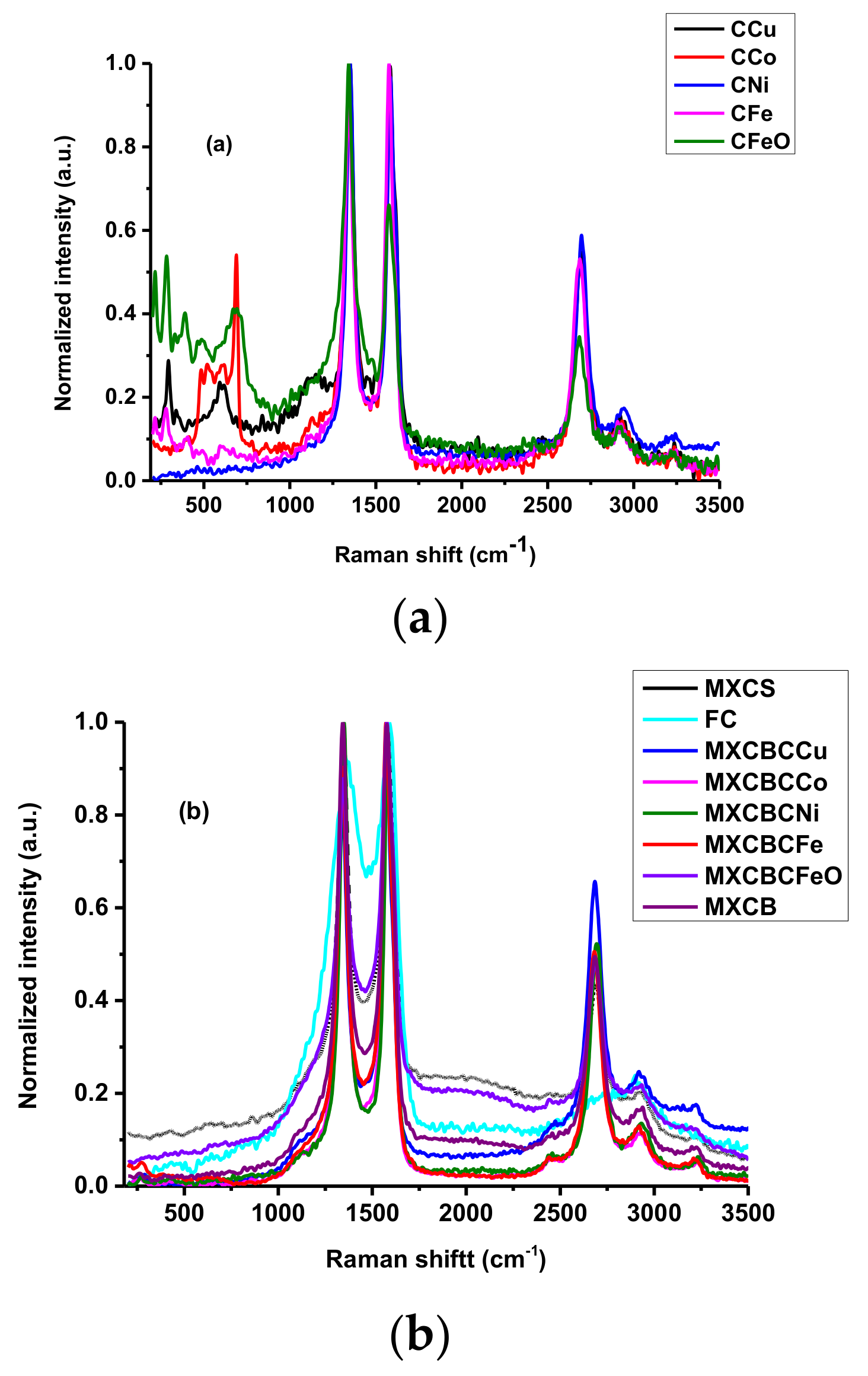
3.1.2. Raman Spectroscopic Analysis for Structure of Carbon-Based Material
3.1.3. X-ray Diffraction (XRD) Analysis
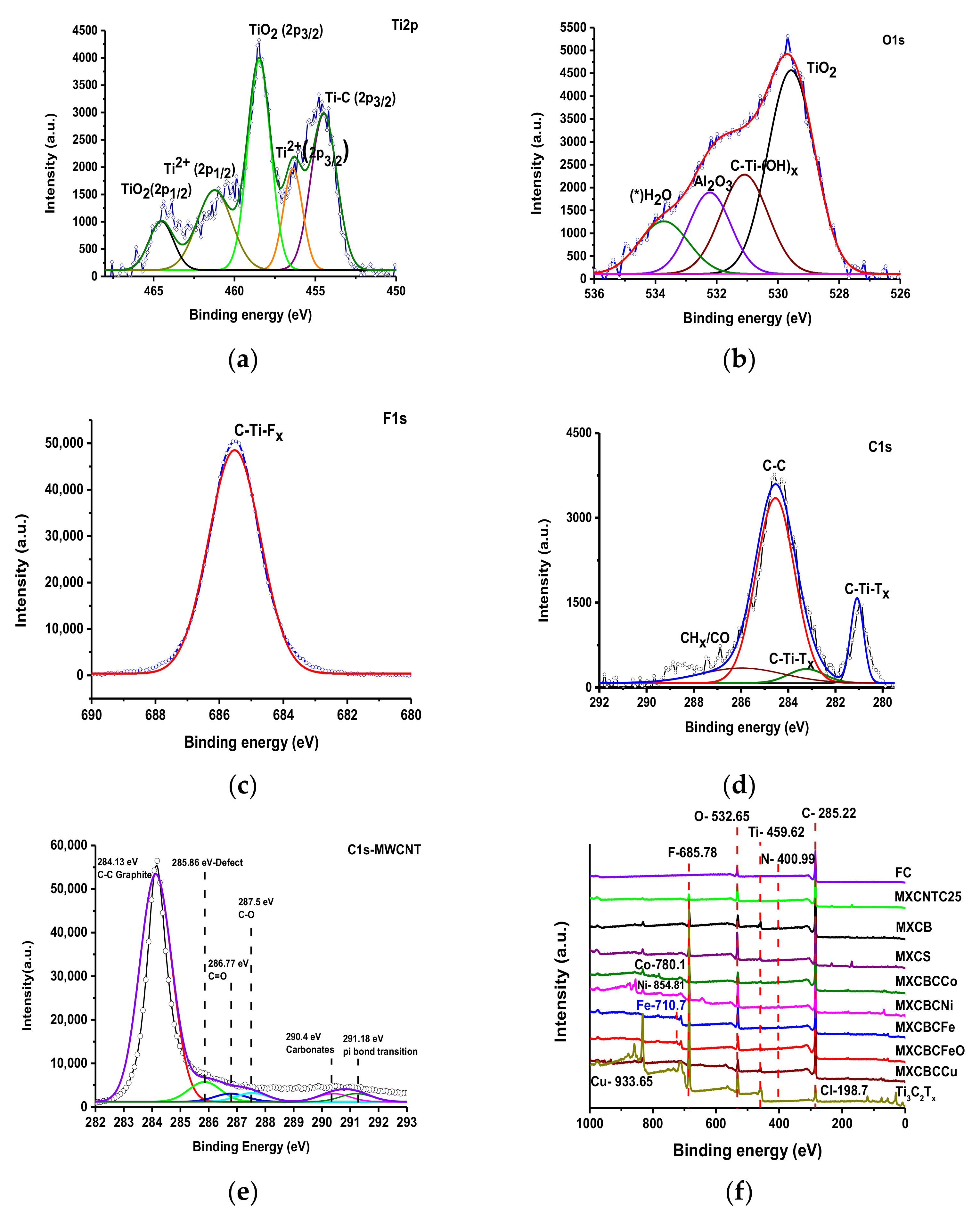
3.1.4. X-ray Photoelectron Spectroscopy Analysis
3.2. Electrical Conductivity (EC) and Surface Properties
3.3. Magnetic Properties of the Composites
3.4. Electromagnetic Shielding Effectiveness (EMI-SE) of Composites
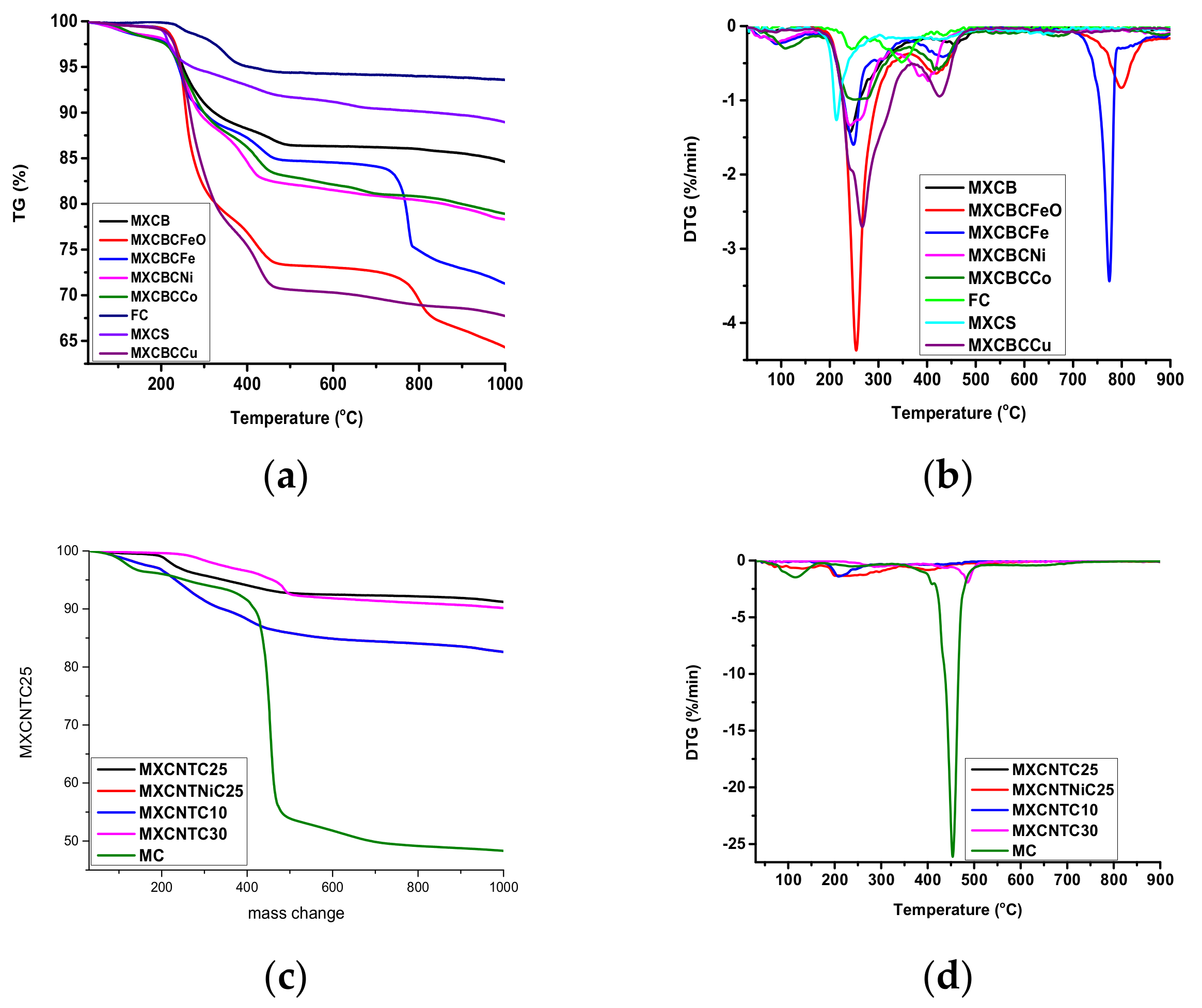
3.5. Thermal Stability and Thermo Gravimetric Analysis of Composites.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pawar, S.P.; Rzeczkowski, P.; Potschke, P.; Krause, B.; Bose, S. Does the Processing Method Resulting in Different States of an Interconnected Network of Multiwalled Carbon Nanotubes in Polymeric Blend Nanocomposites Affect EMI Shielding Properties? ACS Omega 2018, 3, 5771–5782. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Liu, G.; Li, J. Damage assessment of long-range rocket system by electromagnetic pulse weapon. AIP Conf. Proc. 2017, 1864, 020115. [Google Scholar]
- Choi, G.; Shahzad, F.; Bahk, Y.M.; Jhon, Y.M.; Park, H.; Alhabeb, M.; Anasori, B.; Kim, D.S.; Koo, C.M.; Gogotsi, Y.; et al. Enhanced Terahertz Shielding of MXenes with Nano-Metamaterials. Adv. Opt. Mater. 2018, 6, 1701076. [Google Scholar] [CrossRef]
- Pothupitiya Gamage, S.J.; Yang, K.; Braveenth, R.; Raagulan, K.; Kim, H.S.; Lee, Y.S.; Yang, C.M.; Moon, J.J.; Chai, K.Y. MWCNT coated free-standing carbon fiber fabric for enhanced performance in EMI shielding with a higher absolute EMI SE. Materials 2017, 10, 1350. [Google Scholar] [CrossRef]
- Raagulan, K.; Braveenth, R.; Jang, H.; Seon Lee, Y.; Yang, C.M.; Mi Kim, B.; Moon, J.; Chai, K. Electromagnetic Shielding by MXene-Graphene-PVDF Composite with Hydrophobic, Lightweight and Flexible Graphene Coated Fabric. Materials 2017, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xing, D.; Teh, K.S.; Liu, H.; Xie, Y.; Liu, X.; Tang, Y. Structural effects in a composite nonwoven fabric on EMI shielding. Mater. Des. 2018, 120, 354–362. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. Electromagnetic interference shielding mechanisms of CNT/polymer composites. Carbon 2009, 47, 1738–1746. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Skoda, D.; Urbánek, P.; Machovsky, M.; Masař, M.; Kalina, L.; Havlica, J. Lightweight NiFe2O4-Reduced Graphene Oxide-Elastomer Nanocomposite flexible sheet for electromagnetic interference shielding application. Compos. B Eng. 2019, 166, 95–111. [Google Scholar] [CrossRef]
- Ravindren, R.; Mondal, S.; Nath, K.; Das, N.C. Synergistic effect of double percolated co-supportive MWCNT-CB conductive network for high-performance EMI shielding application. Polym. Adv. Technol. 2019, 1–12. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, M.; He, X.; Long, Q.; Yu, J.; Xie, K.; Liao, L. The preparation of carbonized silk cocoon-Co-graphene composite and its enhanced electromagnetic interference shielding performance. Compos. Part A Appl. Sci. Manuf. 2019, 119, 111–118. [Google Scholar] [CrossRef]
- Wu, H.Y.; Jia, L.C.; Yan, D.X.; Gao, J.F.; Zhang, X.P.; Ren, P.G.; Li, Z.M. Simultaneously improved electromagnetic interference shielding and mechanical performance of segregated carbon nanotube/polypropylene composite via solid phase molding. Compos. Sci. Technol. 2018, 156, 87–94. [Google Scholar] [CrossRef]
- Ma, X.; Shen, B.; Zhang, L.; Liu, Y.; Zhai, W.; Zheng, W. Porous superhydrophobic polymer/carbon composites for lightweight and self-cleaning EMI shielding application. Compos. Sci. Technol. 2018, 158, 86–93. [Google Scholar] [CrossRef]
- Lu, S.; Shao, J.; Ma, K.; Chen, D.; Wang, X.; Zhang, L.; Meng, Q.; Ma, J. Flexible, mechanically resilient carbon nanotube composite films for high-efficiency electromagnetic interference shielding. Carbon 2018, 136, 387–394. [Google Scholar] [CrossRef]
- Mondal, S.; Das, P.; Ganguly, S.; Ravindren, R.; Remanan, S.; Bhawal, P.; Das, T.K.; Das, N.C. Thermal-air ageing treatment on mechanical, electrical, and electromagnetic interference shielding properties of lightweight carbon nanotube based polymer nanocomposites. Compos. Pt A. Appl. Sci. Manuf. 2018, 107, 447–460. [Google Scholar] [CrossRef]
- Lu, D.; Mo, Z.; Liang, B.; Yang, L.; He, Z.; Zhu, H.; Tang, Z.; Gui, X. Flexible, lightweight carbon nanotube sponges and composites for high-performance electromagnetic interference shielding. Carbon 2018, 133, 457–463. [Google Scholar] [CrossRef]
- Liu, Y.F.; Feng, L.M.; Chen, Y.F.; Shi, Y.D.; Chen, X.D.; Wang, M. Segregated polypropylene/cross-linked poly (ethylene-co-1-octene)/multi-walled carbon nanotube nanocomposites with low percolation threshold and dominated negative temperature coefficient effect: Towards electromagnetic interference shielding and thermistors. Compos. Sci. Technol. 2018, 159, 152–161. [Google Scholar]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- He, P.; Cao, M.; Shu, J.C.; Cai, Y.Z.; Wang, X.; Zhao, Q.; Yuan, J. Atomic Layer Tailoring Titanium Carbide MXene to Tune Transport and Polarization for Utilization of Electromagnetic Energy beyond Solar and Chemical Energy. ACS Appl. Mater. Interfaces 2019. [CrossRef]
- Xiang, C.; Guo, R.; Lin, S.; Jiang, S.; Lan, J.; Wang, C.; Cui, C.; Xiao, H.; Zhang, Y. Lightweight and ultrathin TiO2-Ti3C2TX/graphene film with electromagnetic interference shielding. Chem. Eng. J. 2019, 360, 1158–1166. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, J.; Zhang, X.; Tian, D.; Zhan, Z.; Lu, C. Ultrathin MXene/Calcium Alginate Aerogel Film for High-Performance Electromagnetic Interference Shielding. Adv. Mater. Interfaces 2019, 1802040–1802049. [Google Scholar] [CrossRef]
- Cui, C.; Xiang, C.; Geng, L.; Lai, X.; Guo, R.; Zhang, Y.; Xiao, H.; Lan, J.; Lin, S.; Jiang, S. Flexible and ultrathin electrospun regenerate cellulose nanofibers and d-Ti3C2Tx (MXene) composite film for electromagnetic interference shielding. J. Alloys Compd. 2019, 785, 1246–1255. [Google Scholar] [CrossRef]
- Li, X.; Yin, X.; Liang, S.; Li, M.; Cheng, L.; Zhang, L. 2D carbide MXene Ti2CTX as a novel high-performance electromagnetic interference shielding material. Carbon 2019, 146, 210–217. [Google Scholar] [CrossRef]
- Raagulan, K.; Braveenth, R.; Jang, H.J.; Lee, Y.S.; Yang, C.M.; Kim, B.M.; Moon, J.J.; Chai, K.Y. Fabrication of Nonwetting Flexible Free-Standing MXene-Carbon Fabric for Electromagnetic Shielding in S-Band Region. Bull. Korean Chem. Soc. 2018, 39, 1412–1419. [Google Scholar] [CrossRef]
- Georgakilas, V.; Gournis, D.; Tzitzios, V.; Pasquato, L.; Guldi, D.M.; Prato, M. Decorating carbon nanotubes with metal or semiconductor nanoparticles. J. Mater. Chem. 2017, 17, 2679–2694. [Google Scholar] [CrossRef]
- Megiel, E. Surface modification using TEMPO and its derivatives. Adv. Colloid Interface Sci. 2017, 250, 158–184. [Google Scholar] [CrossRef] [PubMed]
- Young, K.D. Bacterial morphology: Why have different shapes? Curr. Opin. Microbiol. 2017, 10, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.W.; Sihanonth, P.; Gartz, J.; Toro, G. An ethnopharmacological and ethnomycological update on the occurrence, use, cultivation, chemical analysis, and SEM photography of neurotropic fungi from Thailand, Cambodia and other regions of South and Southeast Asia, Indonesia and Bali. Ethnomycological Journals: Sacred Mushroom Studies, 2012, 9, 1–129. [Google Scholar]
- Clark, T.R.; Leonard, N.D.; Zhao, J.X.; Brodie, J.; McCook, L.J.; Wachenfeld, D.R.; Nguyen, A.D.; Markham, H.L.; Pandolfi, J.M. Historical photographs revisited: A case study for dating and characterizing recent loss of coral cover on the inshore Great Barrier Reef. Sci. Rep. 2016, 6, 19285. [Google Scholar] [CrossRef] [Green Version]
- Yan, P.; Zhang, R.; Jia, J.; Wu, C.; Zhou, A.; Xu, J.; Zhang, X. Enhanced supercapacitive performance of delaminated two-dimensional titanium carbide/carbon nanotube composites in alkaline electrolyte. J. Power Sources 2015, 284, 38–43. [Google Scholar] [CrossRef]
- Zhang, H.B.; Zhou, Y.C.; Bao, Y.W.; Li, M.S. Abnormal thermal shock behavior of Ti3SiC2 and Ti3AlC2. J. Mater. Res. 2006, 21, 2401–2407. [Google Scholar] [CrossRef]
- Ivasyshyn, A.; Ostash, O.; Prikhna, T.; Podhurska, V.; Basyuk, T. Oxidation Resistance of Materials Based on Ti3AlC2 Nanolaminate at 600 °C in Air. Nanoscale Res. Lett. 2016, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Wang, B.; Shen, C.; Zhang, C.; Hu, Q.; Zhou, A.; Liu, B. Synthesis and electrochemical performance of Ti3C2Tx with hydrothermal process. Electron. Mater. Lett. 2016, 12, 702–710. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Wu, H.; Hou, Z.; Song, C.; Li, X.; Zhang, L.; Cheng, L. Ti3C2 MXenes with modified surface for high-performance electromagnetic absorption and shielding in the X-band. ACS Appl. Mater. Interfaces 2016, 8, 21011–21019. [Google Scholar] [CrossRef]
- Qian, A.; Hyeon, S.E.; Seo, J.Y.; Chung, C.H. Capacitance changes associated with cation-transport in free-standing flexible Ti3C2Tx(TO,F,OH) MXene film electrodes. Electrochim. Acta 2018, 266, 86–93. [Google Scholar] [CrossRef]
- Saleh, T.A.; Agarwal, S.; Gupta, V.K. Synthesis of MWCNT/MnO2 and their application for simultaneous oxidation of arsenite and sorption of arsenate. Appl. Catal. B Environ. 2011, 106, 46–53. [Google Scholar] [CrossRef]
- Jia-Jia, C.; Xin, J.; Qiu-Jie, S.; Chong, W.; Qian, Z.; Ming-Sen, Z.; Quan-Feng, D. The preparation of nano-sulfur/MWCNTs and its electrochemical performance. Electrochim. Acta 2010, 55, 8062–8066. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J. Hazard. Mater. 2011, 185, 17–23. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Wang, N. Glycerol plasticized-starch/multiwall carbon nanotube composites for electroactive polymers. Compos. Sci. Technol. 2008, 68, 268–273. [Google Scholar] [CrossRef]
- Gupta, V.; Saleh, T.A. Syntheses of carbon nanotube-metal oxides composites; adsorption and photo-degradation. In Carbon Nanotubes-From Research to Applications; InTech: Shanghai, China, 2011. [Google Scholar]
- Łużny, W.; Bańka, E. Relations between the structure and electric conductivity of polyaniline protonated with camphorsulfonic acid. Macromolecules 2000, 33, 425–429. [Google Scholar] [CrossRef]
- Lorencová, L.; Bertok, T.; Dosekova, E.; Holazová, A.; Paprckova, D.; Vikartovská, A.; Sasinková, V.; Filip, J.; Kasák, P.; Jerigová, M.; et al. Electrochemical performance of Ti3C2Tx MXene in aqueous media: Towards ultrasensitive H2O2 sensing. Electrochim. Acta 2017, 235, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Garnero, C.; Rochard, G.; Magne, D.; Morisset, S.; Hurand, S.; Chartier, P.; Rousseau, J.; Cabioc’h, T.; Coutanceau, C.; et al. A new etching environment (FeF3/HCl) for the synthesis of two-dimensional titanium carbide MXenes: A route towards selective reactivity vs. water. J. Mater. Chem. A 2017, 5, 22012–22023. [Google Scholar] [CrossRef]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/graphene films for ultrafast supercapacitors with outstanding volumetric capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Sui, X.M.; Giordani, S.; Prato, M.; Wagner, H.D. Effect of carbon nanotube surface modification on dispersion and structural properties of electrospun fibers. Appl. Phys. Lett. 2009, 95, 233113. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Magnetite decorated multiwalled carbon nanotube based supercapacitor for arsenic removal and desalination of seawater. J. Phys. Chem. C 2017, 114, 2583–2590. [Google Scholar] [CrossRef]
- He, Y.; Huang, L.; Cai, J.S.; Zheng, X.M.; Sun, S.G. 2010. Structure and electrochemical performance of nanostructured Fe3O4/carbon nanotube composites as anodes for lithium ion batteries. Electrochimica Acta 2010, 55, 1140–1144. [Google Scholar] [CrossRef]
- Grassi, G.; Scala, A.; Piperno, A.; Iannazzo, D.; Lanza, M.; Milone, C.; Pistone, A.; Galvagno, S. A facile and ecofriendly functionalization of multiwalled carbon nanotubes by an old mesoionic compound. Chem. Comm. 2012, 48, 6836–6838. [Google Scholar] [CrossRef]
- Mali, S.S.; Betty, C.A.; Bhosale, P.N.; Patil, P.S. Synthesis, characterization of hydrothermally grown MWCNT–TiO2 photoelectrodes and their visible light absorption properties. ECS J. Solid State Sci. Technol. 2012, 1, M15–M23. [Google Scholar] [CrossRef]
- Dong, C.K.; Li, X.; Zhang, Y.; Qi, J.Y.; Yuan, Y.F. Fe3O4 nanoparticles decorated multi-walled carbon nanotubes and their sorption properties. Chem. Res. Chin. Univ. 2009, 25, 936–940. [Google Scholar]
- Jiao, Z.; Qiu, J. Microwave absorption performance of iron oxide/multiwalled carbon nanotubes nanohybrids prepared by electrostatic attraction. J. Mater. Sci. 2018, 53, 3640–3646. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. The Effect of Vacuum Annealing of Magnetite and Zero-Valent Iron Nanoparticles on the Removal of Aqueous Uranium. J. Nanotechnol. 2013, 2013, 11. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, J.; Jiang, D.; Wei, X.; Chen, M. Modifiers-assisted formation of nickel nanoparticles and their catalytic application to p-nitrophenol reduction. CrystEngComm 2013, 15, 560–569. [Google Scholar] [CrossRef]
- Balela, M.D.L.; Yagi, S.; Lockman, Z.; Aziz, A.; Amorsolo, A.V.; Matsubara, E. 2009. Electroless deposition of ferromagnetic cobalt nanoparticles in propylene glycol. J. Electrochem. Soc. 2009, 156, E139–E142. [Google Scholar] [CrossRef]
- Betancourt-Galindo, R.; Reyes-Rodriguez, P.Y.; Puente-Urbina, B.A.; Avila-Orta, C.A.; Rodríguez-Fernández, O.S.; Cadenas-Pliego, G.; Lira-Saldivar, R.H.; García-Cerda, L.A. Synthesis of copper nanoparticles by thermal decomposition and their antimicrobial properties. J. Nanomater. 2014, 2014, 10. [Google Scholar] [CrossRef]
- Graat, P.; Somers, M.A. Quantitative analysis of overlapping XPS peaks by spectrum reconstruction: Determination of the thickness and composition of thin iron oxide films. Surface and Interface Analysis: An International Journal devoted to the development and application of techniques for the analysis of surfaces. Interfaces Thin Films 1998, 26, 773–782. [Google Scholar]
- Lee, T.W.; Lee, S.E.; Jeong, Y.G. Carbon nanotube/cellulose papers with high performance in electric heating and electromagnetic interference shielding. Compos. Sci. Technol. 2016, 131, 77–87. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Halim, J. Synthesis and Characterization of 2D Nanocrystals and Thin Films of Transition Metal Carbides (MXenes). Doctoral Dissertation, Linköping University Electronic Press, Linköping, Sweden, 2014. [Google Scholar] [Green Version]
- Shen, C.; Wang, L.; Zhou, A.; Wang, B.; Wang, X.; Lian, W.; Hu, Q.; Qin, G.; Liu, X. Synthesis and Electrochemical Properties of Two-Dimensional RGO/Ti3C2Tx Nanocomposites. Nanomaterials 2018, 8, 80. [Google Scholar] [CrossRef]
- Shah, S.A.; Habib, T.; Gao, H.; Gao, P.; Sun, W.; Green, M.J.; Radovic, M. Template-free 3D titanium carbide (Ti3C2Tx) MXene particles crumpled by capillary forces. Chem. Commun. 2017, 53, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.B.; Sun, R.; Liu, Y.; Liu, Z.; Zhou, A.; Yu, Z.Z. Hydrophobic, Flexible, and Lightweight MXene Foams for High-Performance Electromagnetic-Interference Shielding. Adv. Mater. 2017, 29, 1702367. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.F.; El-Amoudy, E.S.; Shady, K.E. Thermal analysis and characterization of some cellulosic fabrics dyed by a new natural dye and mordanted with different mordants. Int. J. Chem. 2011, 3, 40. [Google Scholar] [CrossRef]
- Taira, M.; Araki, Y. DTG thermal analyses and viscosity measurements of three commercial agar impression materials. J. Oral Rehabil. 2002, 29, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Aslam, M. Roughness enhanced surface defects and photoconductivity of acid etched ZnO nanowires. In Proceedings of the 2012 International Conference on Emerging Electronics, Mumbai, India, 15–17 December 2012; pp. 1–4. [Google Scholar]
- Zhao, M.Q.; Ren, C.E.; Ling, Z.; Lukatskaya, M.R.; Zhang, C.; Van Aken, K.L.; Barsoum, M.W.; Gogotsi, Y. Flexible MXene/carbon nanotube composite paper with high volumetric capacitance. Adv. Mater. 2015, 27, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Innis, P.C.; Wallace, G.G.; Low, C.T.J.; Walsh, F.C.; Cho, W.J.; Yu, K.H. Electrically conductive coatings of nickel and polypyrrole/poly (2-methoxyaniline-5-sulfonic acid) on nylon Lycra® textiles. Prog. Org. Coat. 2013, 76, 1296–1301. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y. Fabrication and magnetic properties of Fe3O4 nanowire arrays in different diameters. Journal of Magnetism and Magnetic. Materials 2009, 321, L15–L20. [Google Scholar]
- Iida, H.; Takayanagi, K.; Nakanishi, T.; Osaka, T. Synthesis of Fe3O4 nanoparticles with various sizes and magnetic properties by controlled hydrolysis. J. Colloid Interface Sci. 2007, 314, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Su, H.T. Synthesis and magnetic properties of Ni nanoparticles. Rev. Adv. Mater. Sci. 2008, 18, 667–675. [Google Scholar]
- Alborzi, Z.; Hassanzadeh, A.; Golzan, M.M. Superparamagnetic behavior of the magnetic hysteresis loop in the Fe2O3@ Pt core-shell nanoparticles. Int. J. Nanosci. Nanotechnol. 2012, 8, 93–98. [Google Scholar]
- Hu, C.; Gao, Z.; Yang, X. Fabrication and magnetic properties of Fe3O4 octahedra. Chem. Phys. Lett. 2006, 429, 513–517. [Google Scholar] [CrossRef]
- Xing, D.; Lu, L.; Teh, K.S.; Wan, Z.; Xie, Y.; Tang, Y. Highly flexible and ultra-thin Ni-plated carbon-fabric/polycarbonate film for enhanced electromagnetic interference shielding. Carbon 2018, 132, 32–41. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Duan, H.; Gao, J.; Yan, D.X.; Zhao, G.; Liu, Y. Flexible and highly conductive sandwich nylon/nickel film for ultra-efficient electromagnetic interference shielding. Appl. Surf. Sci. 2018, 455, 856–863. [Google Scholar] [CrossRef]
- Bagotia, N.; Mohite, H.; Tanaliya, N.; Sharma, D.K. A comparative study of electrical, EMI shielding and thermal properties of graphene and multiwalled carbon nanotube filled polystyrene nanocomposites. Polym. Compos. 2018, 39, E1041–E1051. [Google Scholar] [CrossRef]
- Cao, W.T.; Chen, F.F.; Zhu, Y.J.; Zhang, Y.G.; Jiang, Y.Y.; Ma, M.G.; Chen, F. Binary Strengthening and Toughening of MXene/Cellulose Nanofiber Composite Paper with Nacre-Inspired Structure and Superior Electromagnetic Interference Shielding Properties. ACS Nano 2018, 12, 4583–4593. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, Y.J.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [PubMed]



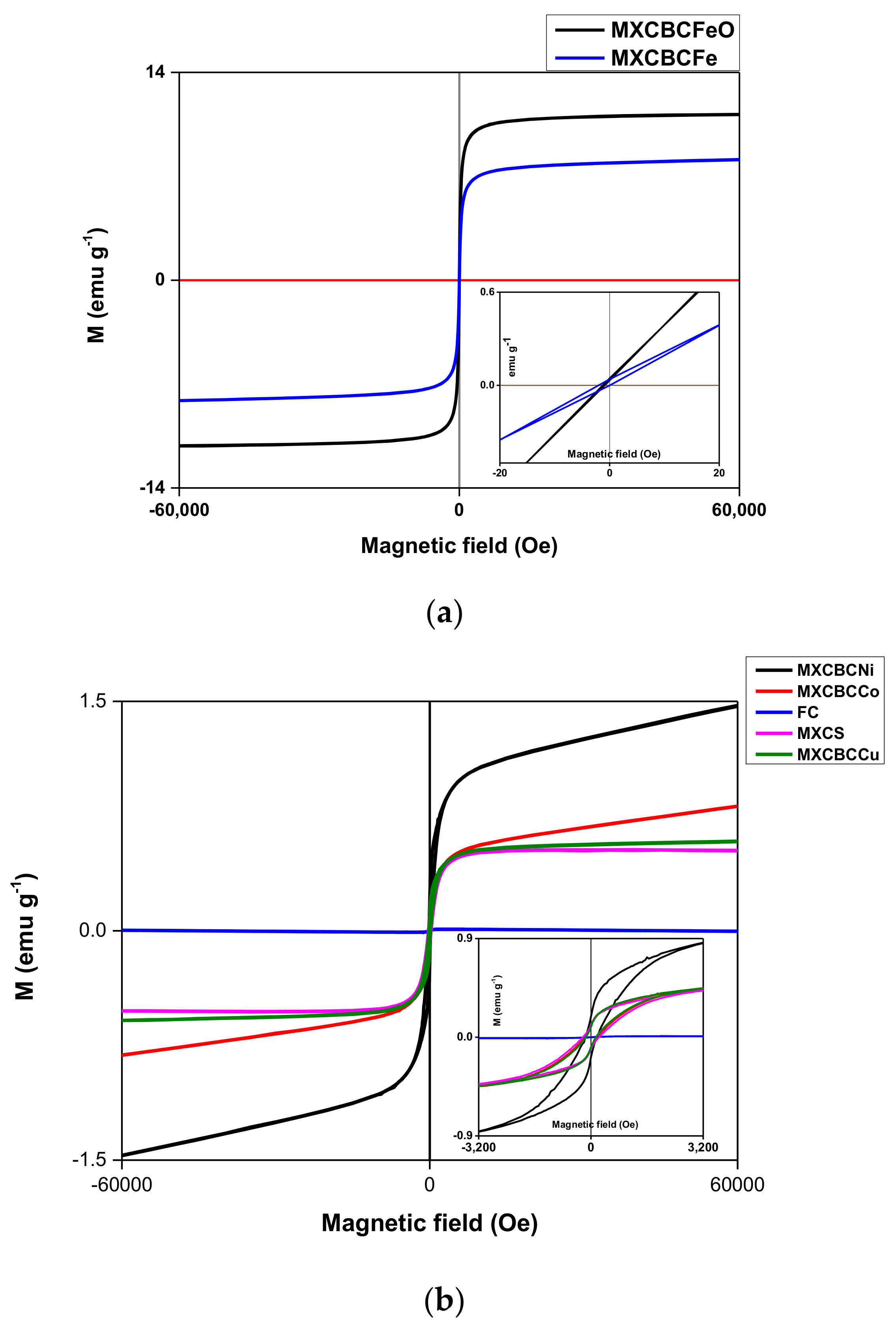

| Composites | Saturated Magnetic Strength (Oe) | Saturation Magnetization (Ms) (emu·g−1) | Remanence (Mr) (emu·g−1) | Coefficient of Squareness of Hysteresis Loops (Kp) = Mr/Ms (Dimensionless) | Coercivity (Hc) (Oe) |
|---|---|---|---|---|---|
| MXCBCCu | 3284.4 | 0.45 | 0.08 | 0.178 | 144.59 |
| MXCS | 3200 | 0.43 | 0.1 | 0.233 | 232.3 |
| FC | 2587.7 | 0.0099 | 2.2 × 10−4 | 0.022 | 22.34 |
| MXCBCCo | 3195.9 | 0.45 | 0.096 | 0.213 | 185.57 |
| MXCBCNi | 3184.9 | 0.86 | 0.187 | 0.217 | 152.58 |
| MXCBCFe | 21.4 | 0.41 | 0.039 | 0.095 | 2.02 |
| MXCBCFeO | 9.95 | 0.39 | 0.44 | 1.128 | 1.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raagulan, K.; Braveenth, R.; Lee, L.R.; Lee, J.; Kim, B.M.; Moon, J.J.; Lee, S.B.; Chai, K.Y. Fabrication of Flexible, Lightweight, Magnetic Mushroom Gills and Coral-Like MXene–Carbon Nanotube Nanocomposites for EMI Shielding Application. Nanomaterials 2019, 9, 519. https://doi.org/10.3390/nano9040519
Raagulan K, Braveenth R, Lee LR, Lee J, Kim BM, Moon JJ, Lee SB, Chai KY. Fabrication of Flexible, Lightweight, Magnetic Mushroom Gills and Coral-Like MXene–Carbon Nanotube Nanocomposites for EMI Shielding Application. Nanomaterials. 2019; 9(4):519. https://doi.org/10.3390/nano9040519
Chicago/Turabian StyleRaagulan, Kanthasamy, Ramanaskanda Braveenth, Lee Ro Lee, Joonsik Lee, Bo Mi Kim, Jai Jung Moon, Sang Bok Lee, and Kyu Yun Chai. 2019. "Fabrication of Flexible, Lightweight, Magnetic Mushroom Gills and Coral-Like MXene–Carbon Nanotube Nanocomposites for EMI Shielding Application" Nanomaterials 9, no. 4: 519. https://doi.org/10.3390/nano9040519





