Chiral Effect at Nano-Bio Interface: A Model of Chiral Gold Nanoparticle on Amylin Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. The Preparation of L(D)-NIBC-AuNPs
2.3. Sample Preparation of Amylin Solution
2.4. UV-Vis Spectra Measurement
2.5. Infrared (IR) Spectra Measurement
2.6. Dynamic Light Scattering (DLS) Measurement
2.7. Transmission Electron Microscopy (TEM) Measurements
2.8. Thioflavine-T (ThT) Binding Assay
2.9. Circular Dichroism (CD) Spectra Measurement
2.10. Atomic Force Microscopy (AFM) Characterization
2.11. Cell Experiment
3. Results and Discussion
3.1. The Characterization of L(D)-NIBC-AuNPs
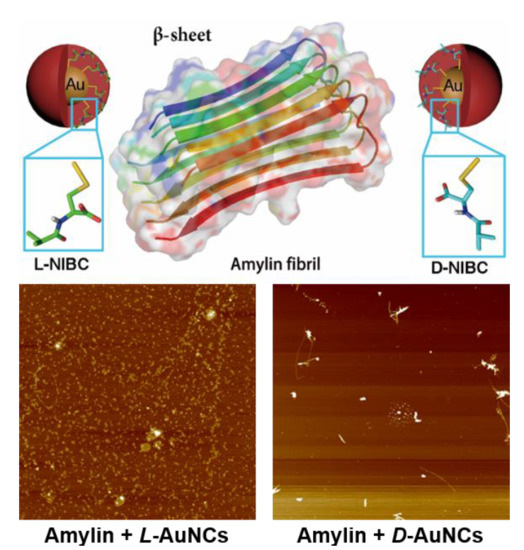
3.2. L(D)-NIBC-AuNPs on the Fibrillation of Amylin
3.3. The Similarities of L-and D-NIBC-AuNPs in Inhibition Mechanisms
3.4. The Differences of L-and D-NIBC-AuNPs in Inhibition Mechanisms
3.5. Cytotoxicity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Eisele, Y.S.; Monteiro, C.; Fearns, C.; Encalada, S.E.; Wiseman, R.L.; Powers, E.T.; Kelly, J.W. Targeting Protein Aggregation for the Treatment of Degenerative Diseases. Nat. Rev. Drug Discov. 2015, 14, 759–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maj, M.; Lomont, J.P.; Rich, K.L.; Alperstein, A.M.; Zanni, M.T. Site-specific detection of protein secondary structure using 2D IR dihedral indexing: A proposed assembly mechanism of oligomeric hIAPP. Chem. Sci. 2018, 9, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell. Biol. 2007, 9, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Misfolded, protease-resistant proteins in animal models and human neurodegenerative disease. J. Clin. Investig. 2002, 110, 1403–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhoades, E.; Gafni, A. Micelle formation by a fragment of human islet amyloid polypeptide. Biophys. J. 2003, 84, 3480–3487. [Google Scholar] [CrossRef]
- Bokvist, M.; Lindstrom, F.; Watts, A.; Grobner, G. Two types of Alzheimer’s beta-amyloid (1-40) peptide membrane interactions: Aggregation preventing transmembrane anchoring versus accelerated surface fibril formation. J. Mol. Biol. 2004, 335, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Skeby, K.K.; Schiøtt, B. Identification of key interactions in the initial self-assembly of amylin in a membrane environment. Biochemistry 2017, 56, 4884–4894. [Google Scholar] [CrossRef] [PubMed]
- Apostolidou, M.; Jayasinghe, S.A.; Langen, R. Structure of alpha-helical membrane-bound human islet amyloid polypeptide and its implications for membrane-mediated misfolding. J. Biol. Chem. 2008, 283, 17205–17210. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, M.F.M.; Tempra, C.; Scollo, F.; Milardi, D.; La Rosa, C. Amyloid growth and membrane damage: Current themes and emerging perspectives from theory and experiments on A beta and hIAPP. Bba-Biomembranes 2018, 1860, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Wilzius, J.J.; Sievers, S.A.; Sawaya, M.R.; Eisenberg, D. Atomic structures of IAPP (amylin) fusions suggest a mechanism for fibrillation and the role of insulin in the process. Protein Sci. 2009, 18, 1521–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaikaran, E.T.A.S.; Higham, C.E.; Serpell, L.C.; Zurdo, J.; Gross, M.; Clark, A.; Fraser, P.E. Identification of a novel human islet amyloid polypeptide beta-sheet domain and factors influencing fibrillogenesis. J. Mol. Biol. 2001, 308, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Gonzalez-Rubio, G.; Langer, J.; Tardajos, G.; Liz-Marzan, L.M.; Giraldo, R.; Guerrero-Martinez, A. Nucleation of amyloid oligomers by RepA-WH1-prionoid-functionalized gold nanorods. Angew. Chem. Int. Ed. 2016, 55, 11237–11241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Clair, J.R.S.; London, E.; Raleigh, D.P. Islet amyloid polypeptide membrance interaction: Effects of membrane composition. Biochemistry 2017, 56, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.A.; Lagen, R. Lipid membranes modulate the structure of islet amyloid polypeptide. Biochemistry 2005, 44, 12113–12119. [Google Scholar] [CrossRef]
- Profit, A.A.; Vedad, J.; Desamero, R.Z.B. Peptide conjugates of benzene carboxylic acids as agonists and antagonists of amylin aggregation. Bioconjug. Chem. 2017, 28, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Moores, B.; Drolle, E.; Attwood, S.J.; Simons, J.; Leonenko, Z. Effect of surfaces on amyloid fibril formation. PLoS ONE 2011, 6, e25954. [Google Scholar] [CrossRef] [PubMed]
- Österlund, N.; Kulkarni, Y.S.; Disiaszek, A.D.; Wallin, C.; Krüger, D.M.; Liao, Q.; Rad, F.M.; Jarvet, J.; Strodel, B.; Wärmländer, S.K.T.S.; et al. Amyloid-β peptide interactions with amphiphilic surfactants: Electrostatic and hydrophobic effects. ACS Chem. Neurosci. 2018, 9, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Chaudhury, S.; Pramanik, D.; DasGupta, S.; DasGupta, S. Hydrophobic tail length plays a pivotal role in amyloid beta (25–35) fibril–surfactant interactions. Proteins 2016, 84, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Qing, G.Y.; Zhao, S.L.; Xiong, Y.T.; Lv, Z.Y.; Jiang, F.L.; Liu, Y.; Chen, H.; Zhang, M.X.; Sun, T.L. Chiral effect at protein/graphene interface: A bioinspired perspective to understand amyloid formation. J. Am. Chem. Soc. 2014, 136, 10736–10742. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Guan, Y.J.; Ding, C.; Gao, N.; Ren, J.S.; Qu, X.G. Cross-fibrillation of insulin and amyloid beta on chiral surfaces: Chirality affects aggregation kinetics and cytotoxicity. Nano Res. 2018, 11, 4102–4110. [Google Scholar] [CrossRef]
- Sen, S.; Dasgupta, S.; DasGupta, S. Does surface chirality of gold nanoparticles affect fibrillation of HSA? J. Phys. Chem. C. 2017, 121, 18935–18946. [Google Scholar] [CrossRef]
- Kumar, J.; Erana, H.; Lopez-Martinez, E.; Claes, N.; Martin, V.F.; Solis, D.M.; Bals, S.; Cortajarena, A.L.; Castilla, J.; Liz-Marzan, L.M. Detection of amyloid fibrils in Parkinson’s disease using plasmonic chirality. Proc. Natl. Acad. Sci. USA 2018, 115, 3225–3230. [Google Scholar] [CrossRef] [PubMed]
- Christopher, P.S.; David, A.M.; Martin, V.; Raphaël, L. Amyloid-derived peptide forms self-assembled monolayers on gold nanoparticle with a curvature-dependent β-Sheet structure. ACS Nano 2012, 6, 1416–1426. [Google Scholar] [CrossRef]
- Gao, G.B.; Zhang, M.X.; Gong, D.J.; Chen, R.; Hu, X.J.; Sun, T.L. The size-effect of gold nanoparticles and nanoclusters in the inhibition of amyloid-β fibrillation. Nanoscale 2017, 9, 4107–4113. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Tang, Q.H.; Xiong, Y.T.; Qing, G.Y.; Sun, T.L. Protein/peptide aggregation and amyloidosis on biointerfaces. Materials 2016, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.B.; Zhang, M.X.; Lu, P.; Guo, G.L.; Wang, D.; Sun, T.L. Chirality assisted ring-like aggregation of Aβ(1-40) at liquid-solid interfaces: A stereoselective two-step assembly process. Angew. Chem. Int. Ed. 2015, 54, 2245–2250. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Ten Hove, J.B.; Schijven, L.M.I.; Wang, J.; Velders, A.H. Size-controlled and water-soluble gold nanoparticles using UV-induced ligand exchange and phase transfer. Chem. Commun. 2018, 54, 13355–13358. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Yang, Z.; Lee, K.H.; Chang, H.T. Synthesis of highly fluorescent gold nanoparticles for sensing Mercury(II). Angew. Chem. Int. Ed. 2007, 46, 6824–6828. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.B.; Tu, X.J.; Guo, X.Q. Fluorescent gold nanoparticles-based fluorescence sensor for Cu2+ ions. Chem. Commun. 2009, 13, 1736–1738. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Wang, S.H.; Sun, H.P.; Baker, J.R. Improved biocompatibility of surface functionalized dendrimer entrapped gold nanoparticles. Soft Matter. 2007, 3, 71–74. [Google Scholar] [CrossRef]
- Xing, Z.C.; Chang, Y.M.; Kang, I.K. Immobilization of biomolecules on the surface of inorganic nanoparticles for biomedical applications. Sci. Technol. Adv. Mat. 2010, 11, 014101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khemtémourian, L.; Casarramona, G.L.; Suylen, D.P.; Hackeng, T.M.; Meeldijk, J.D.; de Kruijff, B.; Killian, J.A. Impaired processing of human pro-islet amyloid polypeptide is not a causative factor for fibril formation or membrane damage in vitro. Biochemistry 2009, 48, 10918–10925. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Noe, Q.; Steve, B. Thioflavin T fluorescence to analyse amyloid formation kinetics: Measurement frequency as a factor explaining irreproducibility. Anal. Biochem. 2017, 532, 83–86. [Google Scholar] [CrossRef]
- Yang, J.; Lee, J.Y.; Too, H.P.; Chow, G.M.; Gan, L.M. Single stranded DNA stabilization and assembly of Au nanoparticles of different sizes. Chem. Phys. 2006, 323, 304–312. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Piella, J.; Bastus, N.G.; Puntes, V. Size-controlled synthesis of sub-10-nanometer citrate-stabilized gold nanoparticles and related optical properties. Chem. Mater. 2016, 28, 1066–1075. [Google Scholar] [CrossRef]
- Lopes, D.H.J.; Meister, A.; Gohlke, A.; Hauser, A.; Blume, A.; Winter, R. Mechanism of islet amyloid polypeptide fibrillation at lipid interfaces studied by infrared reflection absorption spectroscopy. Biophys. J. 2007, 93, 3132–3141. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Lee, C.C.; Mcrae, G.J.; Belfort, G. Osmolyte controlled fibrillation kinetics of insulin: New insight into fibrillation using the preferential exclusion principle. Biotechnol. Progr. 2009, 25, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Younan, N.D.; Viles, J.H. A Comparison of three fluorophores for the detection of amyloid fibers and prefibrillar oligomeric assemblies. ThT (Thioflavin T); ANS (1-Anilinonaphthalene-8-sulfonic Acid); and bisANS (4,4′-Dianilino-1,1′-binaphthyl-5,5′-disulfonic Acid). Biochemistry 2015, 54, 4297–4306. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chiu, C.C.; Reddy, A.S.; de Pablo, J.J. Alpha-helix to beta-hairpin transition of human amylin monomer. J. Chem. Phys. 2013, 138, 155101. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, R.; Zhang, S.; Ma, Z.; Luo, Z.; Gao, G. Chiral Effect at Nano-Bio Interface: A Model of Chiral Gold Nanoparticle on Amylin Fibrillation. Nanomaterials 2019, 9, 412. https://doi.org/10.3390/nano9030412
Li J, Chen R, Zhang S, Ma Z, Luo Z, Gao G. Chiral Effect at Nano-Bio Interface: A Model of Chiral Gold Nanoparticle on Amylin Fibrillation. Nanomaterials. 2019; 9(3):412. https://doi.org/10.3390/nano9030412
Chicago/Turabian StyleLi, Jing, Rui Chen, Shasha Zhang, Zhongjie Ma, Zhuoying Luo, and Guanbin Gao. 2019. "Chiral Effect at Nano-Bio Interface: A Model of Chiral Gold Nanoparticle on Amylin Fibrillation" Nanomaterials 9, no. 3: 412. https://doi.org/10.3390/nano9030412