Single-Crystalline Metal Oxide Nanostructures Synthesized by Plasma-Enhanced Thermal Oxidation
Abstract
:1. Introduction
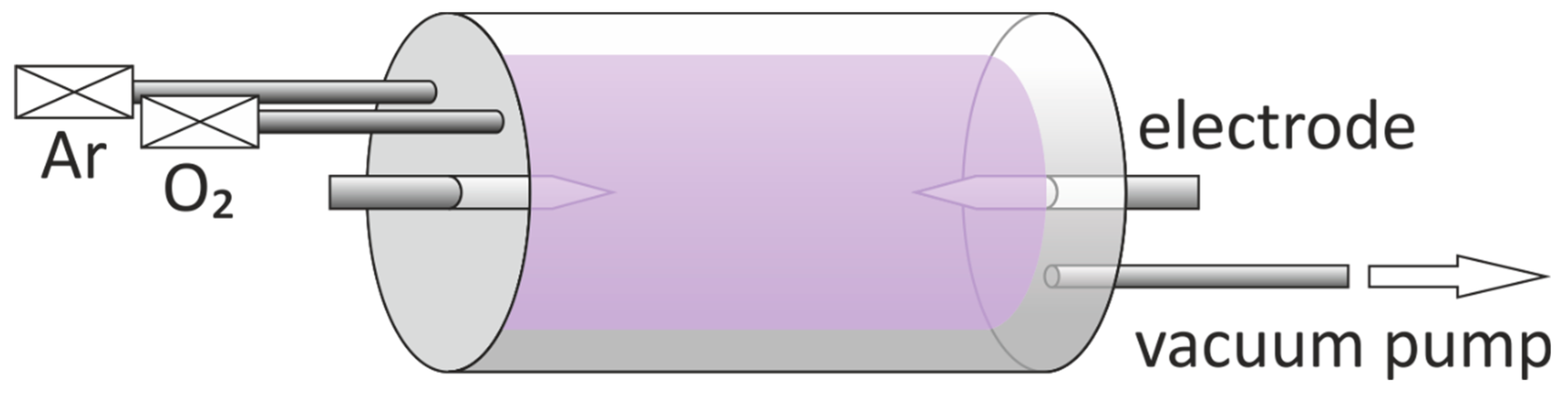
2. Materials and Methods
3. Results and Discussion
3.1. XRD Analysis
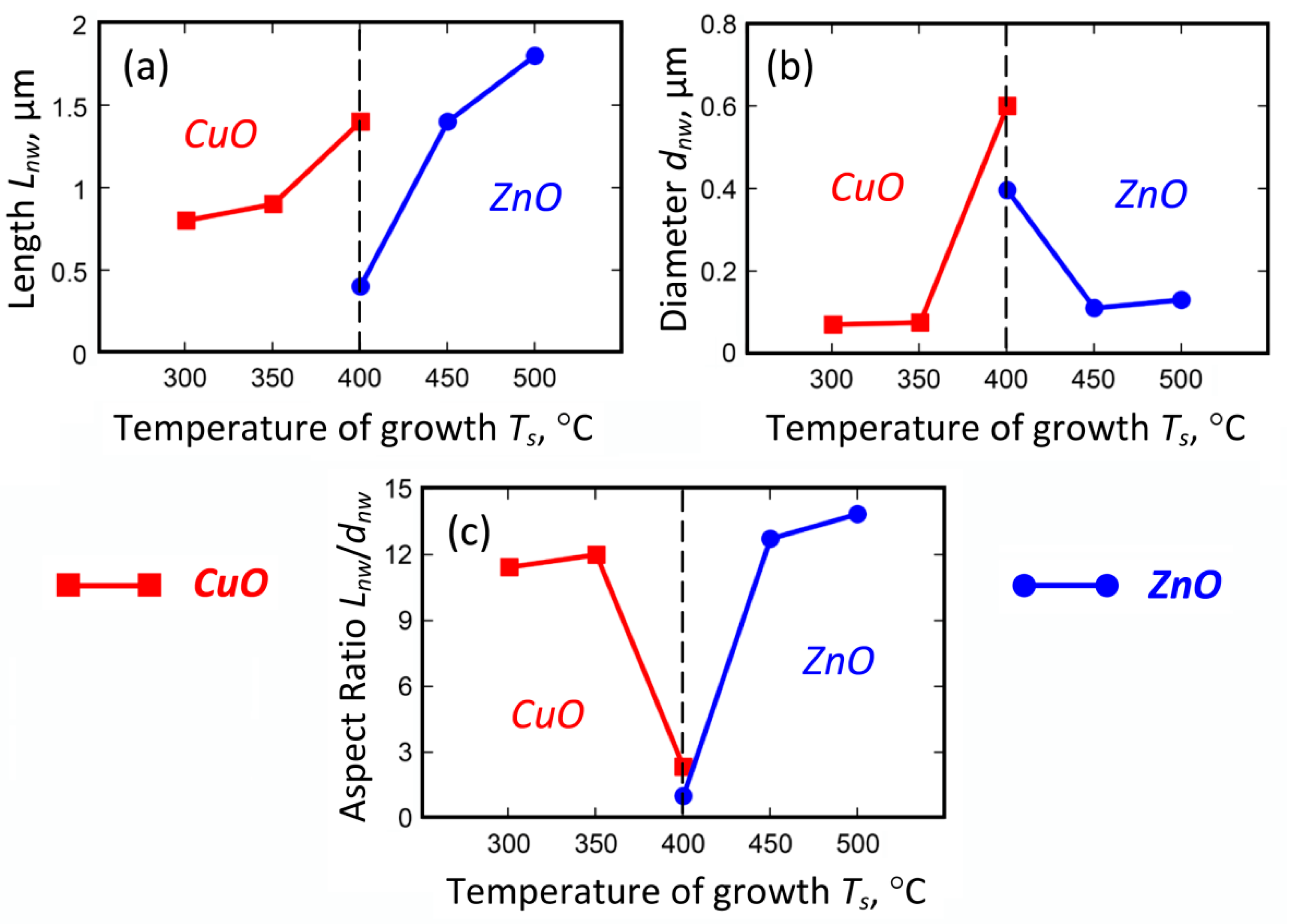
3.2. Electron Microscopy Characterization
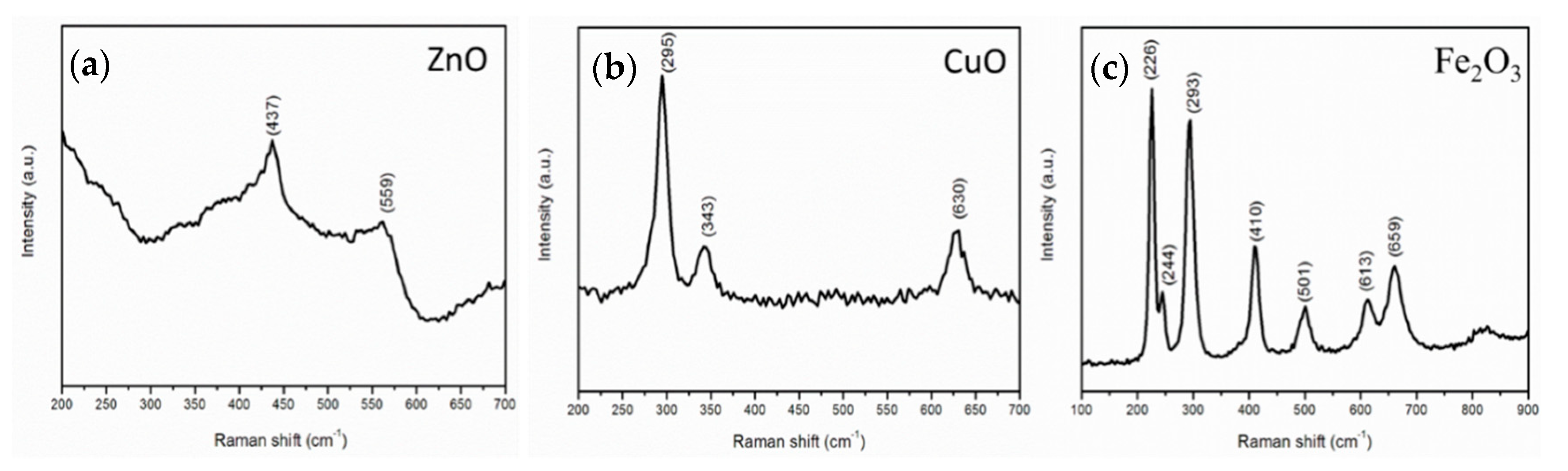
3.3. Raman Analysis
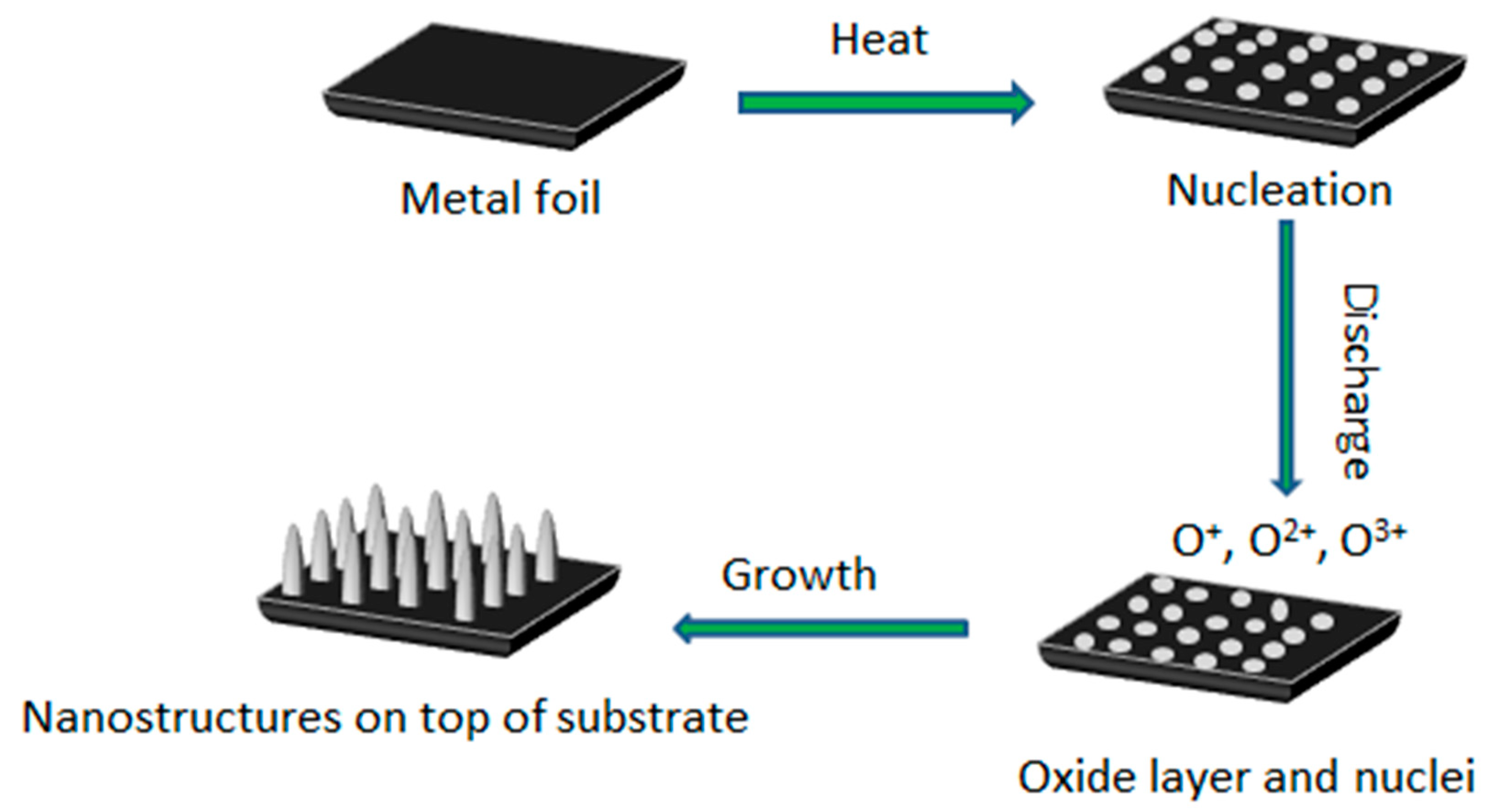
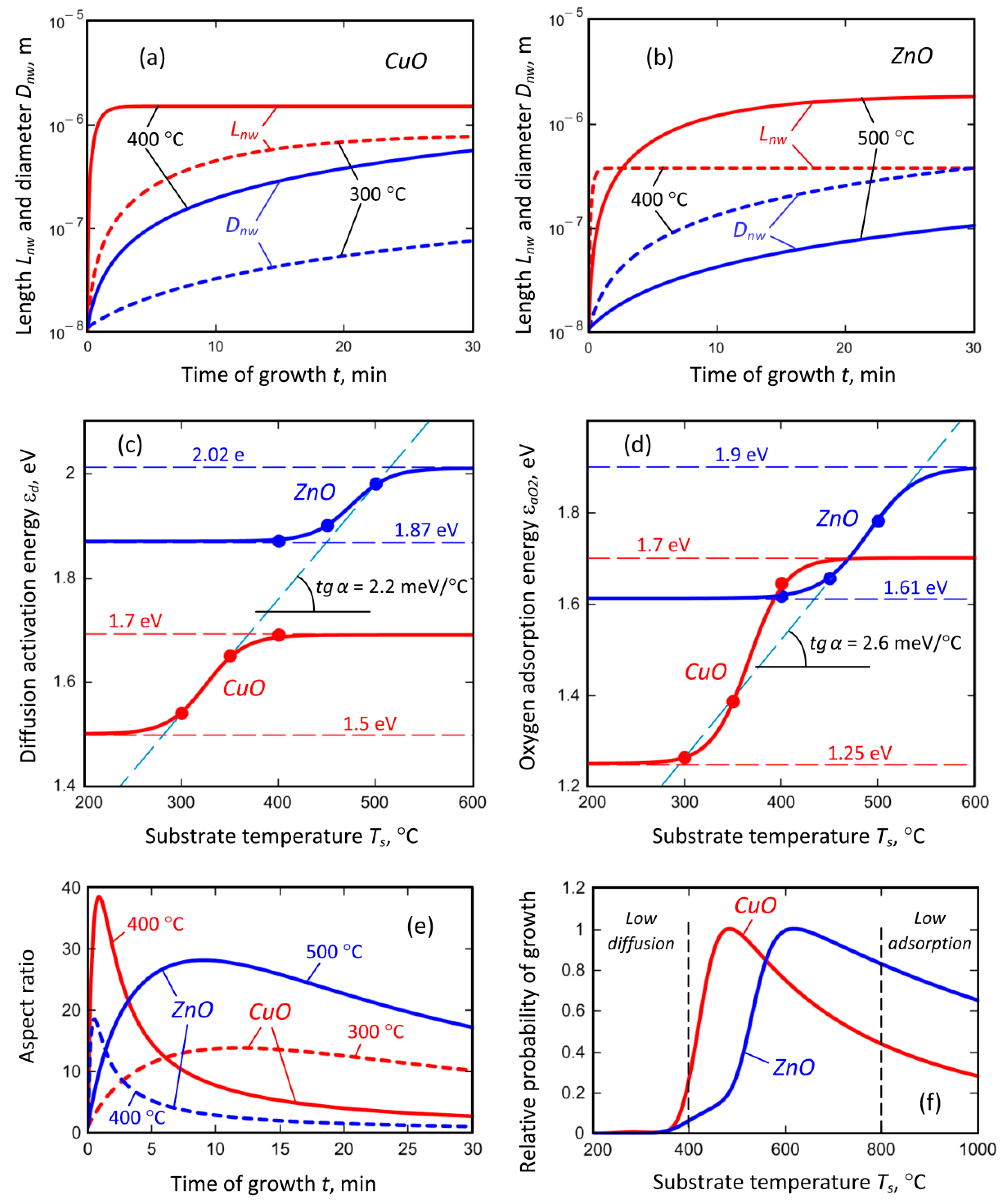
3.4. Growth Mechanism
3.5. Advantages of Plasma-Based Process and Outlook of This Work
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Su, B.; Wu, Y.; Jiang, L. The art of aligning One-Dimensional (1D) nanostructures. Chem. Soc. Rev. 2012, 41, 7832–7856. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.J.; Ostrikov, K. Temperature-Dependent growth mechanisms of Low-Dimensional ZnO nanostructures. Crystengcomm 2011, 13, 3455–3461. [Google Scholar] [CrossRef]
- Garino, N.; Limongi, T.; Dumontel, B.; Canta, M.; Racca, L.; Laurenti, M.; Castellino, M.; Casu, A.; Falqui, A.; Cauda, V.A. Microwave-Assisted synthesis of zinc oxide nanocrystals finely tuned forbiological applications. Nanomaterials 2019, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Q.; Zhang, L.; Sui, L.; Qian, D.J.; Chen, M. Morphology-Controllable synthesis of ZnO Nano-Microstructures by a solvothermal process in ethanol solution. Cryst. Res. Technol. 2013, 48, 947–955. [Google Scholar] [CrossRef]
- Choopun, S.; Tubtimtae, A.; Santhaveesuk, T.; Nilphai, S.; Wongrat, E.; Hongsith, N. Zinc oxide nanostructures for applications as ethanol sensors and Dye-Sensitized solar cells. Appl. Surf. Sci. 2009, 256, 998–1002. [Google Scholar] [CrossRef]
- Guo, K.W. Property of zinc oxide (ZnO) nanostructures potential for biomedical system and its common growth mechanism. J. Appl. Biotechnol. Bioeng. 2017, 2, 197–202. [Google Scholar] [CrossRef]
- Cha, S.N.; Song, B.G.; Jang, J.E.; Jung, J.E.; Han, I.T.; Ha, J.H.; Hong, J.P.; Kang, D.J.; Kim, J.M. Controlled growth of vertically aligned ZnO nanowires with different crystal orientation of the ZnO seed layer. Nanotechnology 2008, 19, 235601. [Google Scholar] [CrossRef]
- Cheng, Q.J.; Ostrikov, K. Property-Performance control of multidimensional, hierarchical, Single-Crystalline ZnO nanoarchitectures. Chemphyschem 2012, 13, 1535–1541. [Google Scholar] [CrossRef]
- Yu, L.B.; Li, Z. Synthesis of ZnxCd1−xSe@ZnO hollow spheres indifferent sizes for quantum dots sensitized solarcells application. Nanomaterials 2019, 9, 132. [Google Scholar] [CrossRef]
- Elhag, S.; Ibupoto, Z.H.; Khranovskyy, V.; Willander, M.; Nur, O. Habit-Modifying additives and their morphological consequences on photoluminescence and glucose sensing properties of ZnO nanostructures, grown via aqueous chemical synthesis. Vacuum 2015, 116, 21–26. [Google Scholar] [CrossRef]
- Cao, M.; Li, Z.; Wang, J.; Ge, W.P.; Yue, T.L.; Li, R.H.; Colvin, V.L. Food related applications of magnetic iron oxide nanoparticles: Enzyme immobilization, protein purification, and food analysis. Trends Food Sci. Tech. 2012, 27, 47–56. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Hosseinkhani, H.; Hosseinkhani, M.; Boutry, B.; Simchi, A.; Journeay, W.S.; Subramani, K.; Laurent, S. Magnetic resonance imaging tracking of stem cells in vivo using iron oxide nanoparticles as a tool for the advancement of clinical regenerative medicine. Chem. Rev. 2011, 111, 253–280. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, A.; Brintakis, K.; Fragogeorgi, E.; Anthousi, A.; Manna, L.; Colin, S.B.; Billotey, C.; Ranella, A.; Loudos, G.; Athanassakis, I.; et al. Iron oxide colloidal nanoclusters as theranosticvehicles and their Interactions at the cellular level. Nanomaterials 2018, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Li, Y.H.; Ma, R.Z.; Roe, M.J.; McCartney, D.G.; Zhu, Y.Q. Two-Dimensional tungsten oxide nanowire networks. Appl. Phys. Lett. 2006, 89, 133116. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Wang, R.M.; Xu, J.; Chen, J.; Yan, Y.; Narlikar, A.; Zhang, H. Synthesis of large arrays of aligned alpha-Fe2O3 nanowires. Chem. Phys. Lett. 2003, 379, 373–379. [Google Scholar] [CrossRef]
- Fortunato, E.; Figueiredo, V.; Barquinha, P.; Elamurugu, E.; Barros, R.; Goncalves, G.; Park, S.H.K.; Hwang, C.S.; Martins, R. Thin-Film transistors based on P-Type Cu2O thin films produced at room temperature. Appl. Phys. Lett. 2010, 96, 191101. [Google Scholar] [CrossRef]
- Jiang, X.C.; Herricks, T.; Xia, Y.N. CuO nanowires can be synthesized by heating copper substrates in air. Nano Lett. 2002, 2, 1333–1338. [Google Scholar] [CrossRef]
- Tan, J.; Dun, M.; Li, L.; Zhao, J.; Li, X.; Hu, Y.; Huang, G.; Tan, W.; Huang, X. Self-template derived CuO nanowires assembled microspheres and its gas sensing properties. Sens. Actuators B: Chem. 2017, 252, 1–8. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, J.; Zhang, T.; Wang, Y.; Liu, D.; Chen, H.; Ji, L.; Liu, C.; Ahmad, W.; Chen, Z.D.; et al. Perovskite solar cells with ZnO Electron-Transporting materials. Adv. Mater. 2018, 30, 1703737. [Google Scholar] [CrossRef]
- Sun, X.M.; Liu, J.F.; Li, Y.D. Use of carbonaceous polysaccharide microspheres astemplates for fabricating metal oxide hollow spheres. Chem.-Eur. J. 2006, 12, 2039–2047. [Google Scholar] [CrossRef]
- Wu, W.P.; Wang, Y.Y.; Wu, Y.P.; Liu, J.Q.; Zeng, X.R.; Shi, Q.Z.; Peng, S.M. Hydro(solvo) thermal synthesis, structures, luminescence of 2-D zinc(II) and 1-D copper(II) complexes constructed from pyridine-2,6-Dicarboxylic acid N-Oxide and decarboxylation of the ligand. Crystengcomm 2007, 9, 753–757. [Google Scholar] [CrossRef]
- Zhang, D.H.; Li, C.; Han, S.; Liu, X.L.; Tang, T.; Jin, W.; Zhou, C.W. Electronic transport studies of Single-Crystalline In2O3 nanowires. Appl. Phys. Lett. 2003, 82, 112–114. [Google Scholar] [CrossRef]
- Shen, G.; Chen, P.C.; Ryu, K.; Zhou, C. Devices and chemical sensing applications of metal oxide nanowires. J. Mater. Chem. 2009, 19, 828–839. [Google Scholar] [CrossRef]
- Sun, Z.; Liao, T.; Kou, L. Strategies for designing metal oxide nanostructures. Sci. China Mater. 2017, 60, 1–24. [Google Scholar] [CrossRef]
- Devan, R.S.; Patil, R.A.; Lin, J.H.; Ma, Y.R. One-Dimensional Metal-Oxide nanostructures: Recentdevelopments in synthesis, characterization, andapplications. Adv. Funct. Mater. 2012, 22, 3326–3370. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.W.; Oh, T.; Petrosko, S.H.; Mirkin, C.A.; Jang, J.-W. Direct observation of Plasmon-Induced interfacial charge separation in metal/semiconductor hybrid nanostructures by measuring surface potentials. Nano Lett. 2018, 18, 109–116. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, C.; Ma, J.Z. A Two-Step hydrothermal route for synthesis hollow Urchin-Like ZnO microspheres. Ceram. Int. 2016, 42, 10289–10296. [Google Scholar] [CrossRef]
- Zhong, M.; Guo, W.M.; Li, C.L.; Chai, L. Morphology-Controllable growth of vertical ZnO nanorod arrays by a polymer soft template method: Growth mechanism and optical properties. J. Alloy. Compd. 2017, 725, 1018–1026. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-Inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@AgCl/ZnO nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef]
- Kumar, V.; Kim, J.H.; Pendyala, C.; Chernomordik, B.; Sunkara, M.K. Gas-Phase, bulk production of metal oxide nanowires and nanoparticles using a microwave plasma jet reactor. Phys. Chem. C 2008, 112, 17750–17754. [Google Scholar] [CrossRef]
- Ostrikov, K.; Levchenko, I.; Cvelbar, U.; Sunkara, M.; Mozetic, M. From nucleation to nanowires: A Single-Step process in reactive plasmas. Nanoscale 2010, 2, 2012–2027. [Google Scholar] [CrossRef] [PubMed]
- Morrish, R.; Silverstein, R.; Wolden, C.A. Synthesis of stoichiometric FeS2 through Plasma-Assisted sulfurization of Fe2O3 nanorods. J. Am. Chem. Soc. 2012, 134, 17854–17857. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhou, G. The growth of One-Dimensional oxide nanostructures by thermal oxidation of metals. Int. J. Nano Sci. Nao Eng. Nanotechnol. 2012, 4, 1–22. [Google Scholar]
- Cheng, Q.J.; Yan, W.; Randeniya, L.; Zhang, F.; Ostrikov, K. Plasma-Produced Phase-Pure cuprous oxide nanowires for methane gas sensing. J. Appl. Phys. 2014, 115, 124310. [Google Scholar] [CrossRef]
- Altaweel, A.; Imam, A.; Ghanbaja, J.; Mangin, D.; Miska, P.; Gries, T.; Belmonte, T. Fast synthesis of ultrathin ZnO nanowires by oxidation of Cu/Zn stacks in low-pressure afterglow. Nanotechnology 2017, 28, 085602. [Google Scholar] [CrossRef]
- Altaweel, A.; Filipic, G.; Gries, T.; Belmonte, T. Controlled growth of copper oxide nanostructures by atmospheric pressure micro-afterglow. J. Cryst. Growth 2014, 407, 17–24. [Google Scholar] [CrossRef]
- Filipic, G.; Cvelbar, U. Copper oxide nanowires: A review of growth. Nanotechnology 2012, 23, 194001. [Google Scholar] [CrossRef]
- Filipic, G.; Baranov, O.; Mozetic, M.; Cvelbar, U. Growth dynamics of copper oxide nanowires in plasma at low pressures. J. Appl. Phys. 2015, 117, 043304. [Google Scholar] [CrossRef]
- Baranov, O.; Filipič, G.; Cvelbar, U. Towards a Highly-Controllable synthesis of copper oxide nanowires in Radio-Frequency reactive plasma: Fast saturation at the targeted size. Plasma Sources Sci. Technol. 2019, 28, 084002. [Google Scholar] [CrossRef]
- Filipic, G.; Baranov, O.; Mozetic, M.; Ostrikov, K.; Cvelbar, U. Uniform surface growth of copper oxide nanowires in radiofrequency plasma discharge and limiting factors. Phys. Plasmas 2014, 21, 113506. [Google Scholar] [CrossRef]
- Cvelbar, U.; Chen, Z.; Sunkara, M.K.; Mozetic, M. Spontaneous growth of superstructure alpha-Fe2O3 nanowire and nanobelt arrays in reactive oxygen plasma. Small 2008, 4, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Liu, S.C. Catalyst-Free growth and characterization of ZnO nanorods. J. Phys. Chem. B 2002, 106, 9546–9551. [Google Scholar] [CrossRef]
- Choopun, S.; Hongsith, N.; Wongrat, E.; Kamwanna, T.; Singkarat, S.; Mangkorntong, P.; Mangkorntong, N.; Chairuangsri, T. Growth kinetic and characterization of RF-Sputtered ZnO: Al nanostructures. J. Am. Ceram. Soc. 2008, 91, 174–177. [Google Scholar] [CrossRef]
- Chen, W.Z.; Wang, B.B.; Qu, Y.Z.; Huang, X.; Ostrikov, K.; Levchenko, I.; Xu, S.; Cheng, Q.J. Catalyst-Free growth and tailoring morphology of zinc oxide nanostructures by Plasma-Enhanced deposition at low temperature. J. Nanopart. Res. 2017, 19, 95. [Google Scholar] [CrossRef]
- Mozetic, M.; Cvelbar, U.; Sunkara, M.K.; Vaddiraju, S. A method for the rapid synthesis of large quantities of metal oxide nanowires at low temperatures. Adv. Mater. 2005, 17, 2138–2142. [Google Scholar] [CrossRef]
- Kittel, C.; Kroemer, H. Thermal Physics; W. H. Freeman and Co.: New York, NY, USA, 1980. [Google Scholar]
- Baranov, O.; Levchenko, I.; Xu, S.; Lim, J.W.M.; Cvelbar, U.; Bazaka, K. Formation of vertically oriented graphenes: What are the key drivers of growth? 2D Mater. 2018, 5, 044002. [Google Scholar] [CrossRef]
- Baranov, O.; Fang, J.; Ostrikov, K.; Cvelbar, U. TiN deposition and morphology control by scalable Plasma-Assisted surface treatments. Mater. Chem. Phys. 2017, 188, 143–153. [Google Scholar] [CrossRef]

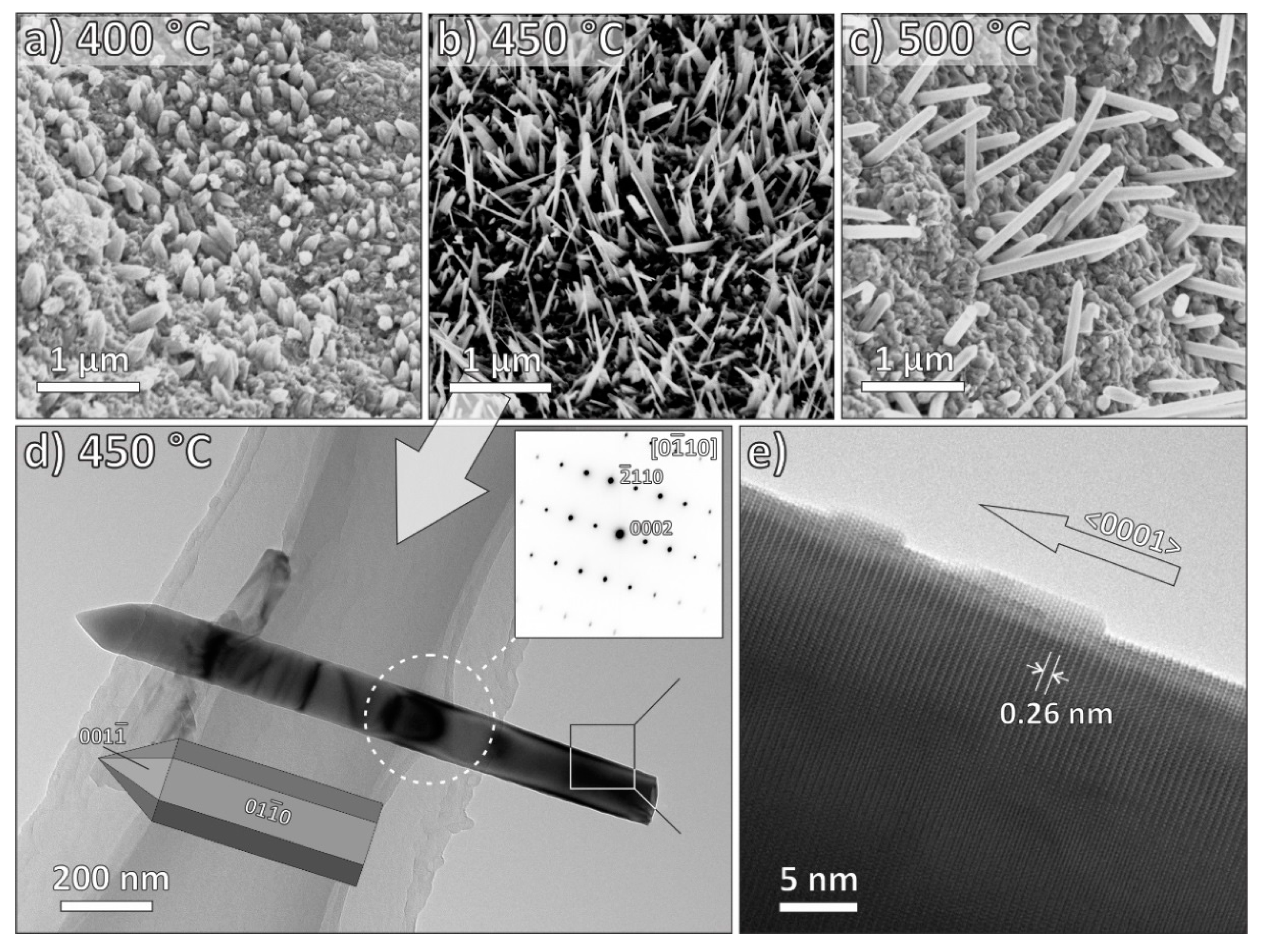
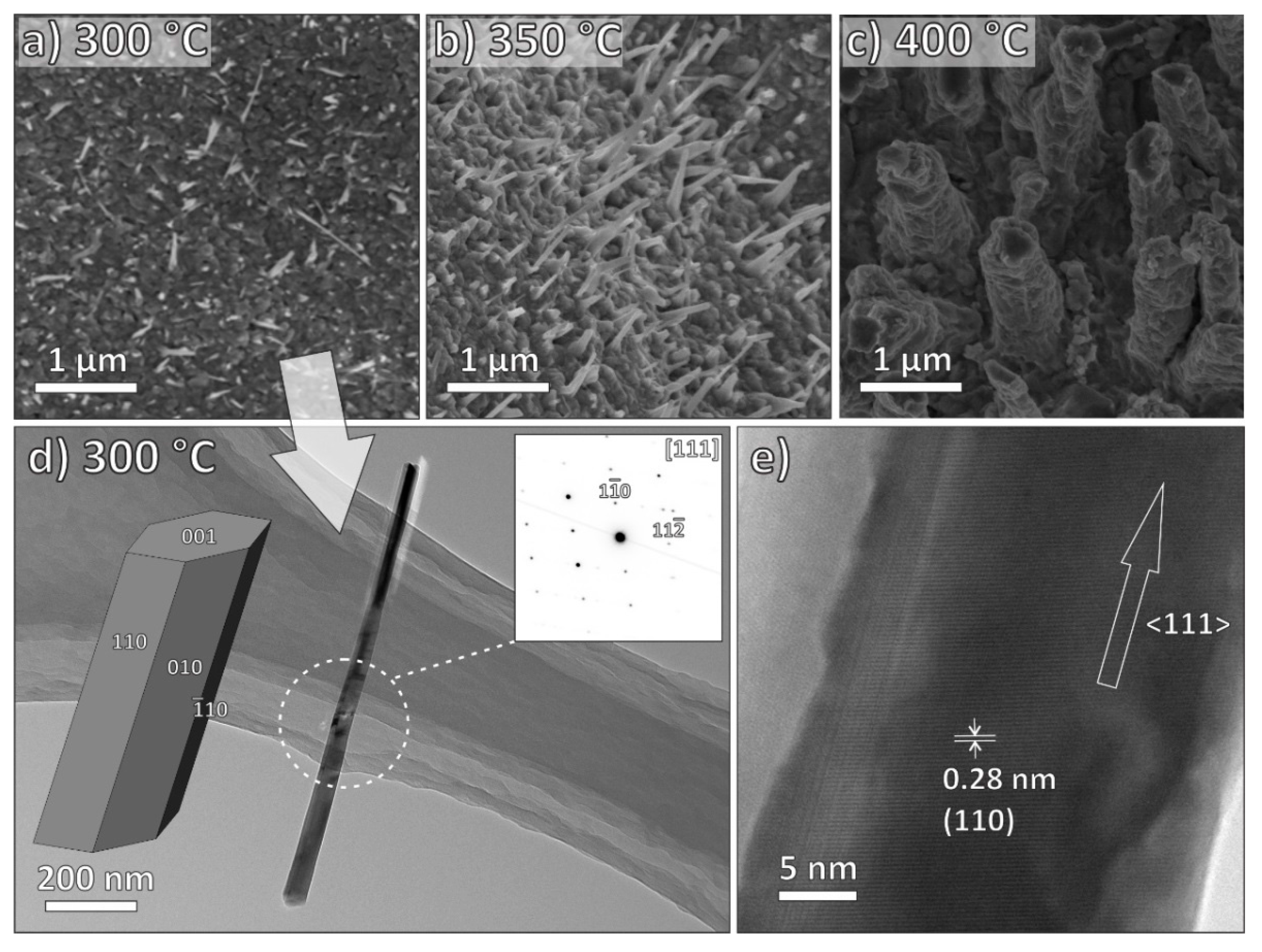
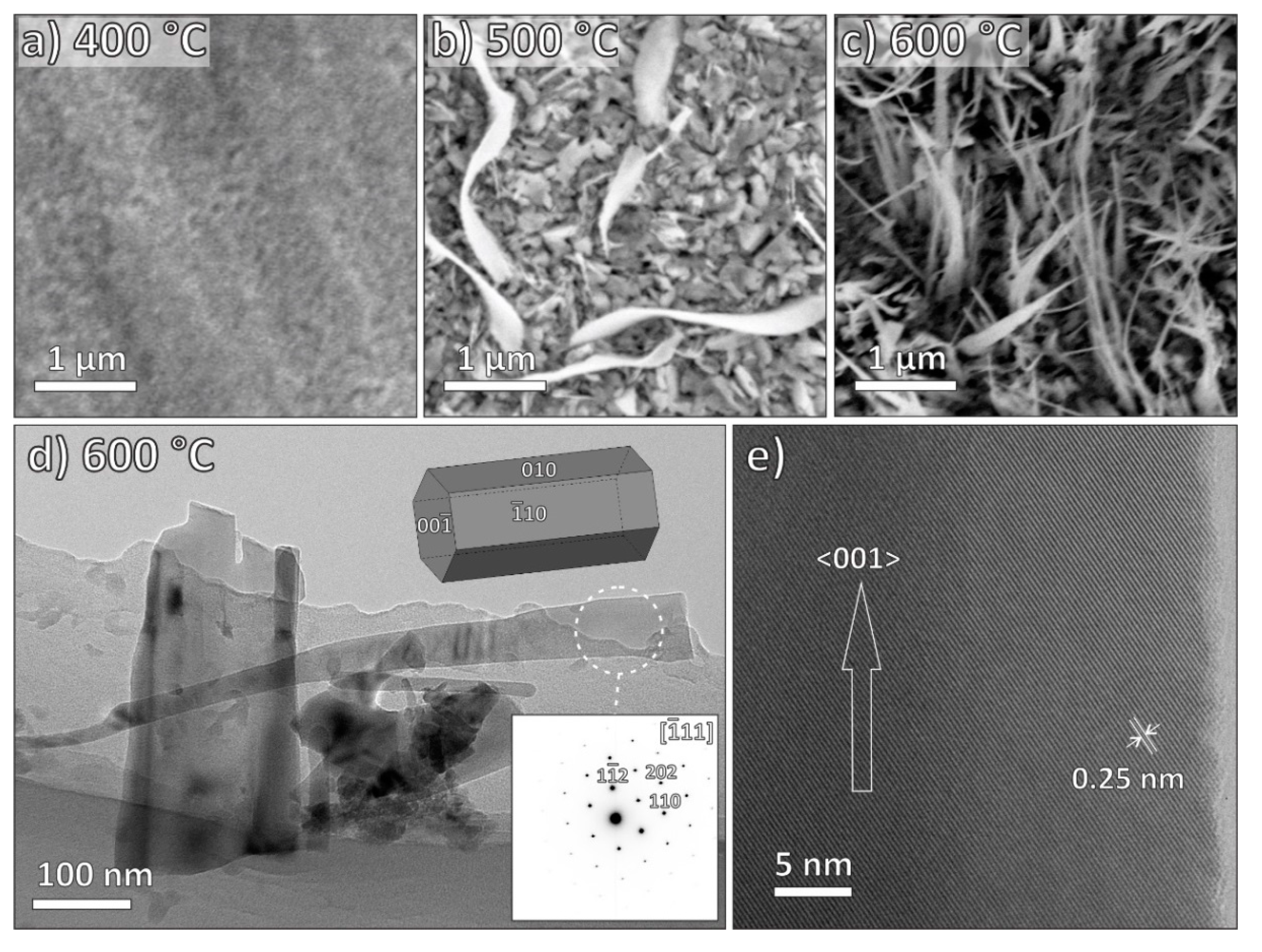
| MO | ZnO | CuO | Fe2O3 |
|---|---|---|---|
| Growth time | 30 min | 30 min | 30 min |
| Growth temperature | 300–500 °C | 300–500 °C | 400–600 °C |
| Diameter | 110–396 nm | 70–600 nm | / |
| Length | 0.4–1.8 μm | 0.8–1.4 μm | / |
| Growth direction | <0001> | <111> | <001> |
| Metal Oxide | Growth Temperature, °C | Experimental Value | Theoretical Value | ||
|---|---|---|---|---|---|
| Length, µm | Diameter, µm | Length, µm | Diameter, µm | ||
| ZnO | 400 | 0.4 | 0.396 | 0.374 | 0.377 |
| 450 | 1.4 | 0.110 | 1.267 | 0.106 | |
| 500 | 1.8 | 0.130 | 1.823 | 0.106 | |
| CuO | 300 | 0.8 | 0.070 | 0.763 | 0.075 |
| 350 | 0.9 | 0.075 | 0.873 | 0.075 | |
| 400 | 1.4 | 0.6 | 1.486 | 0.558 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; Košiček, M.; Fu, J.; Qu, Y.; Lin, G.; Baranov, O.; Zavašnik, J.; Cheng, Q.; Ostrikov, K.; Cvelbar, U. Single-Crystalline Metal Oxide Nanostructures Synthesized by Plasma-Enhanced Thermal Oxidation. Nanomaterials 2019, 9, 1405. https://doi.org/10.3390/nano9101405
Guo B, Košiček M, Fu J, Qu Y, Lin G, Baranov O, Zavašnik J, Cheng Q, Ostrikov K, Cvelbar U. Single-Crystalline Metal Oxide Nanostructures Synthesized by Plasma-Enhanced Thermal Oxidation. Nanomaterials. 2019; 9(10):1405. https://doi.org/10.3390/nano9101405
Chicago/Turabian StyleGuo, Bin, Martin Košiček, Junchi Fu, Yazhou Qu, Guanhua Lin, Oleg Baranov, Janez Zavašnik, Qijin Cheng, Kostya (Ken) Ostrikov, and Uroš Cvelbar. 2019. "Single-Crystalline Metal Oxide Nanostructures Synthesized by Plasma-Enhanced Thermal Oxidation" Nanomaterials 9, no. 10: 1405. https://doi.org/10.3390/nano9101405





