Facile Synthesis of GNPs@NixSy@MoS2 Composites with Hierarchical Structures for Microwave Absorption
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of GNPs@Ni Composite
2.3. Synthesis of GNPs@NixSy@MoS2 Composites
2.4. Characterization
3. Results and Discussion
3.1. XRD Analysis
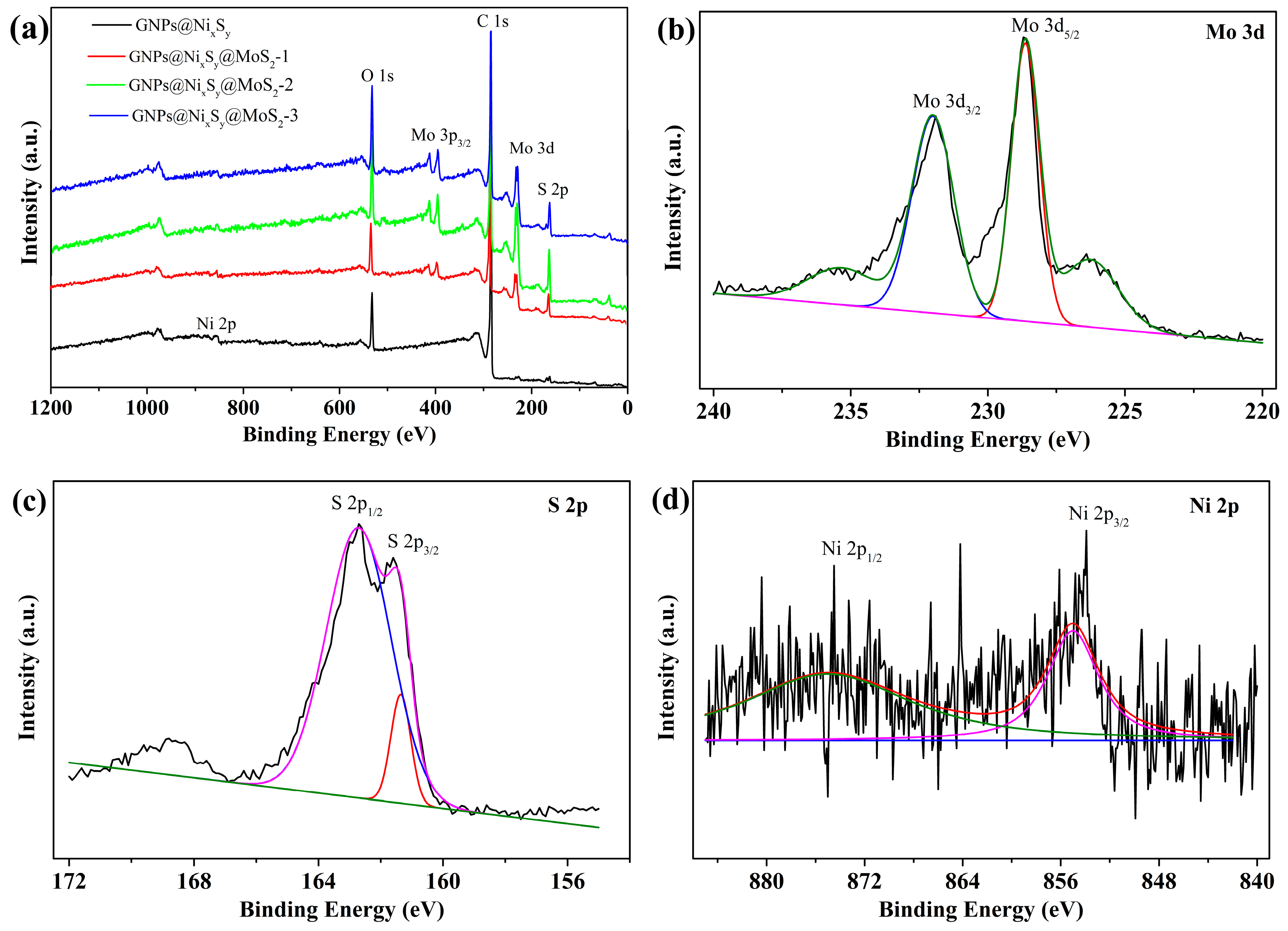
3.2. XPS Analysis
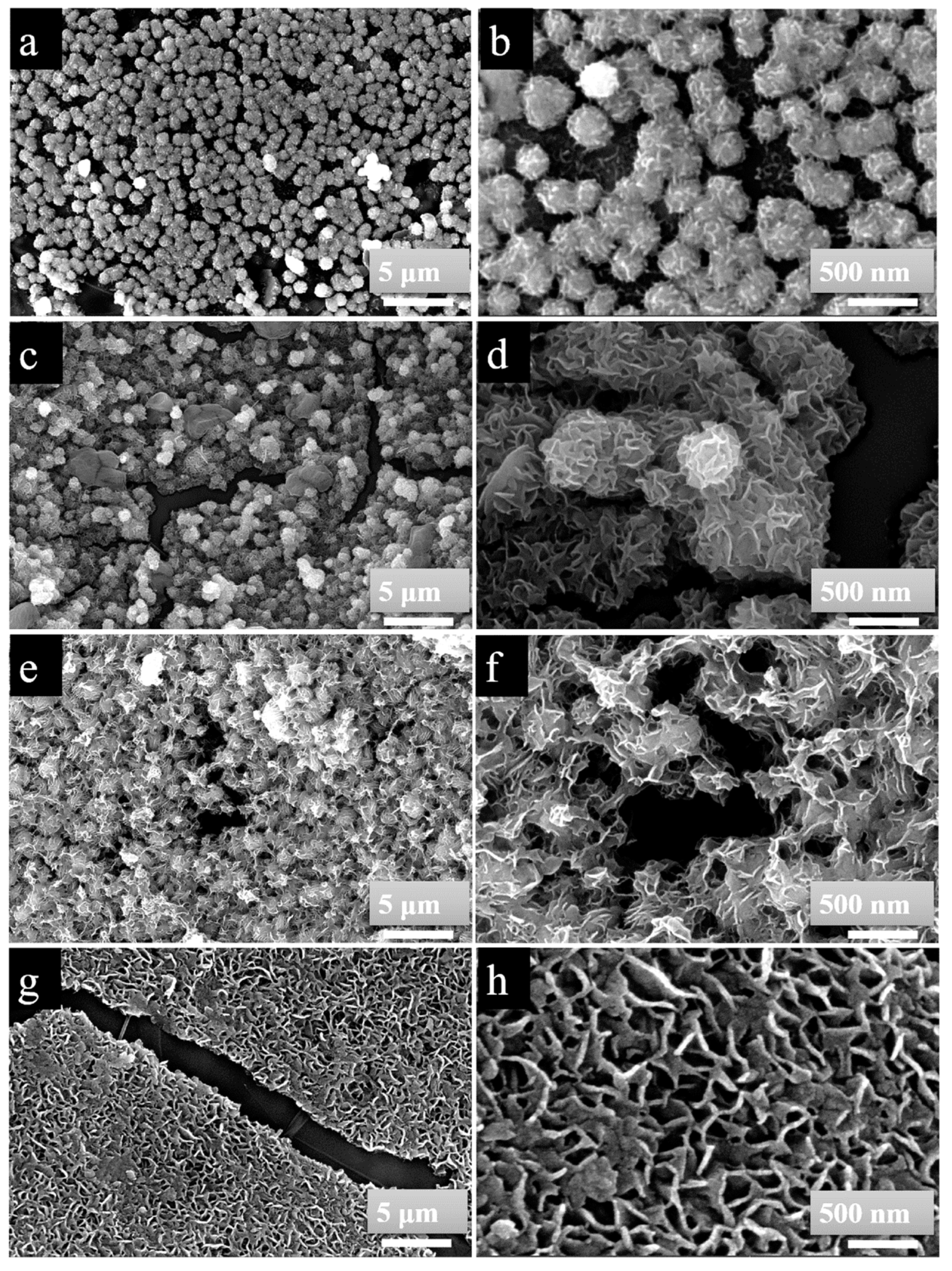
3.3. SEM Analysis
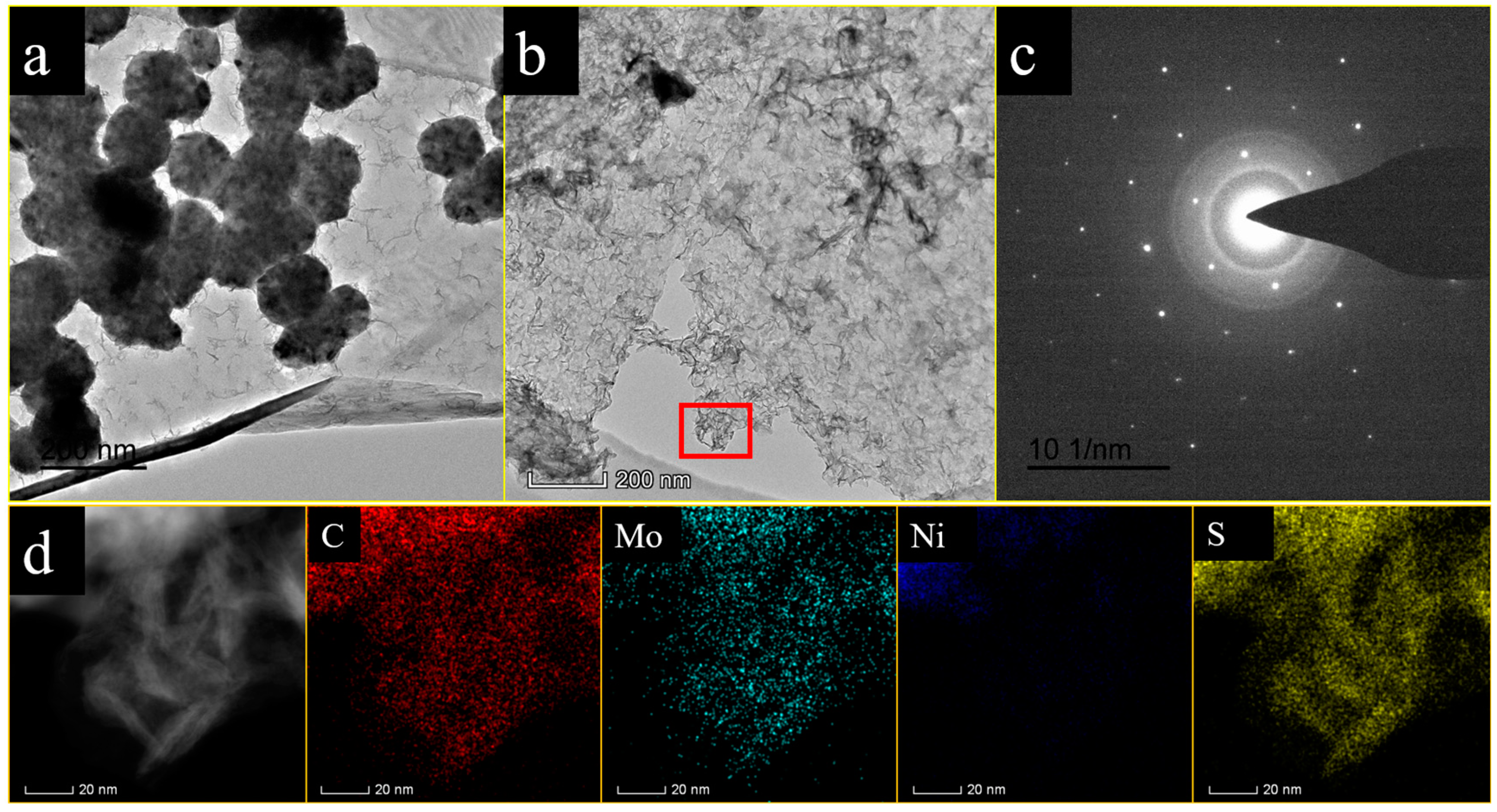
3.4. TEM Analysis
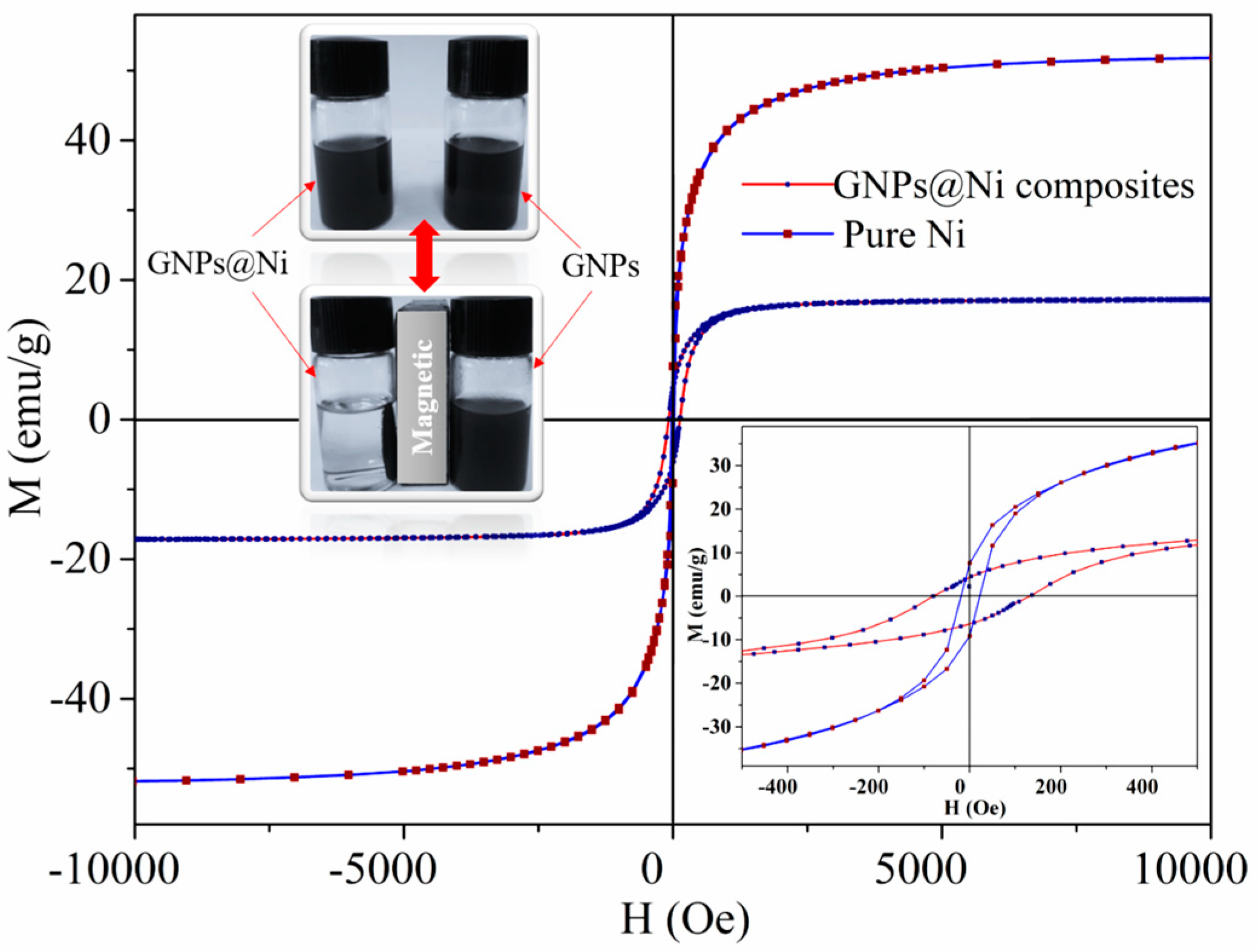
3.5. Magnetic Properties
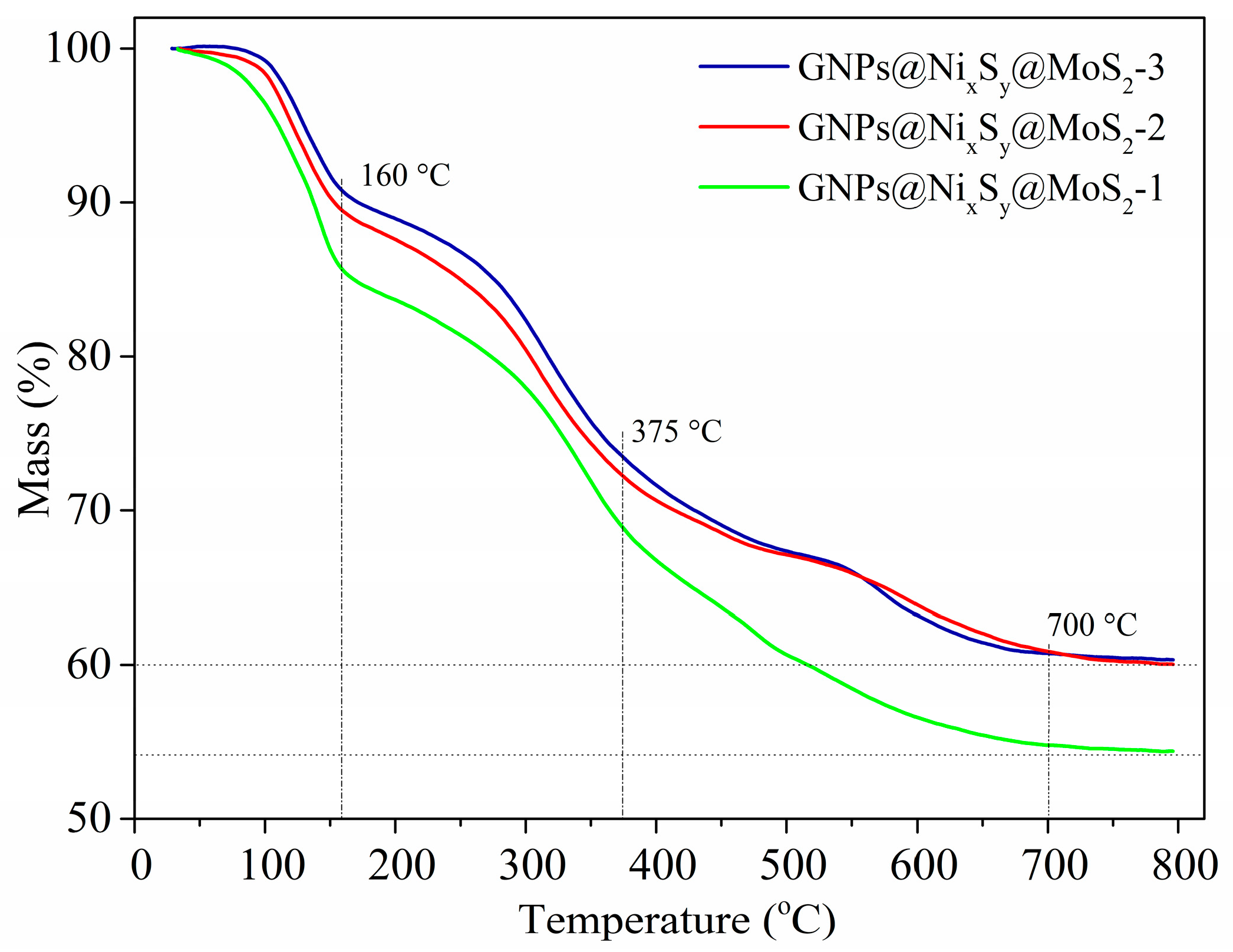
3.6. Thermogravimetric Analysis (TGA)
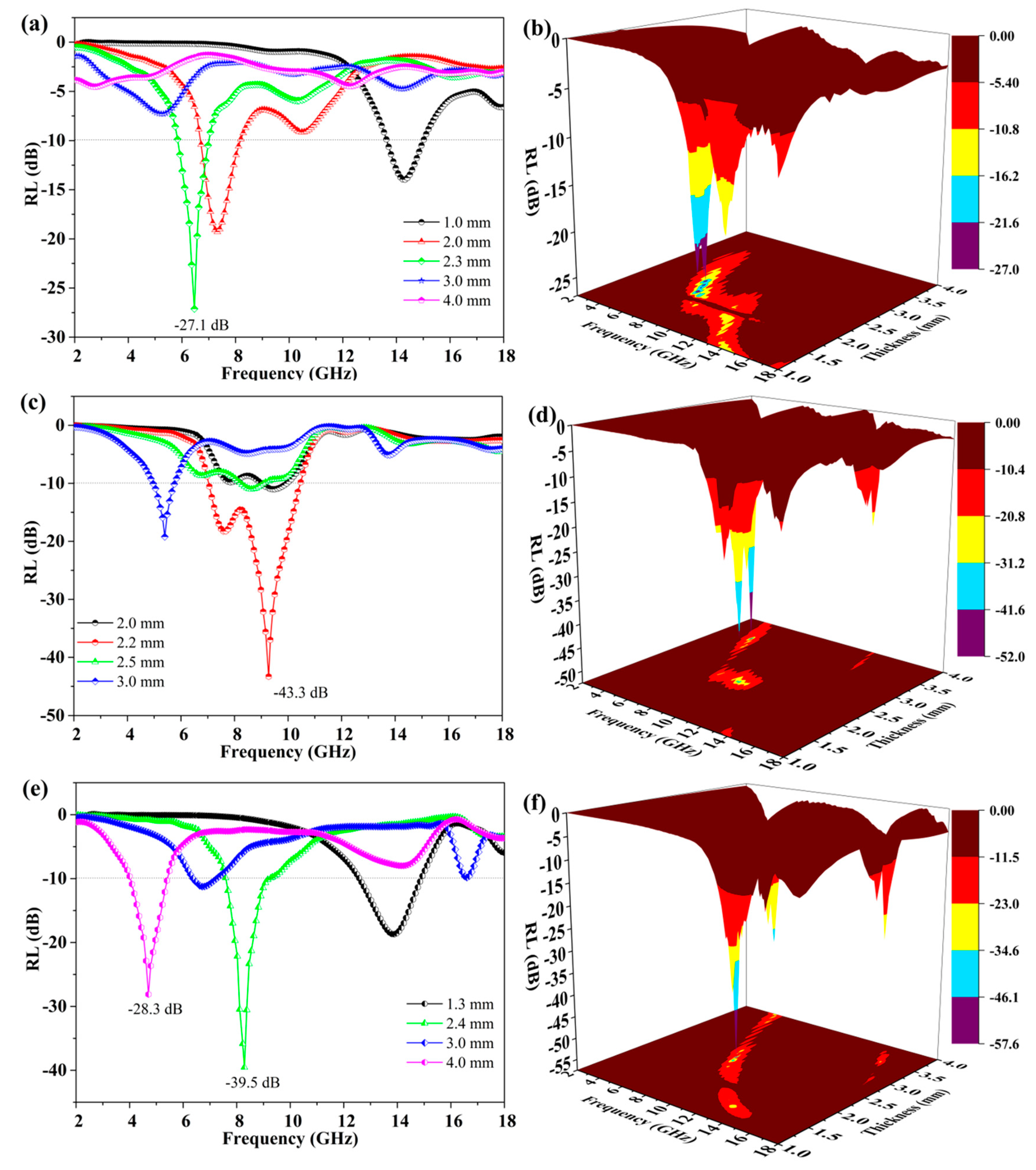
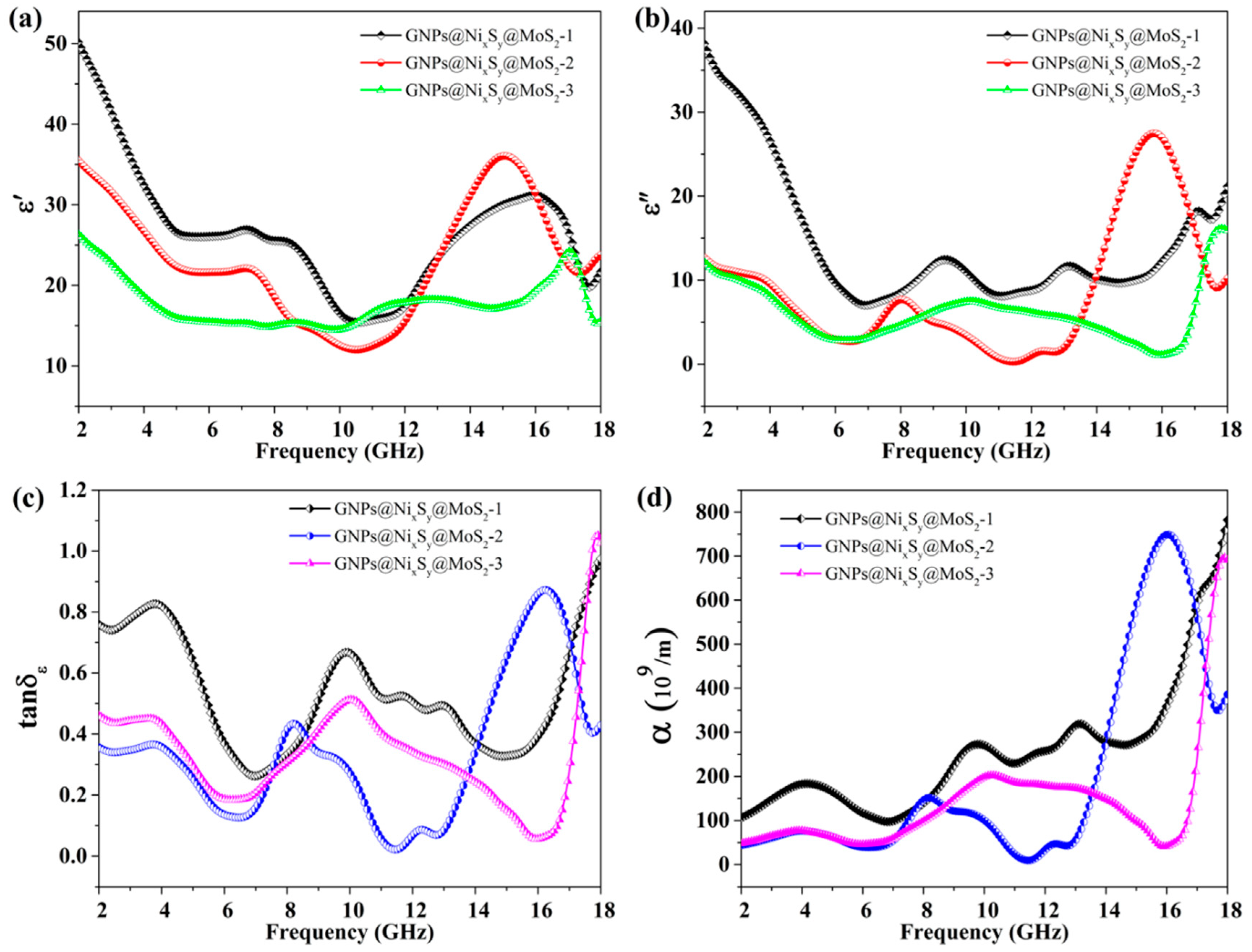
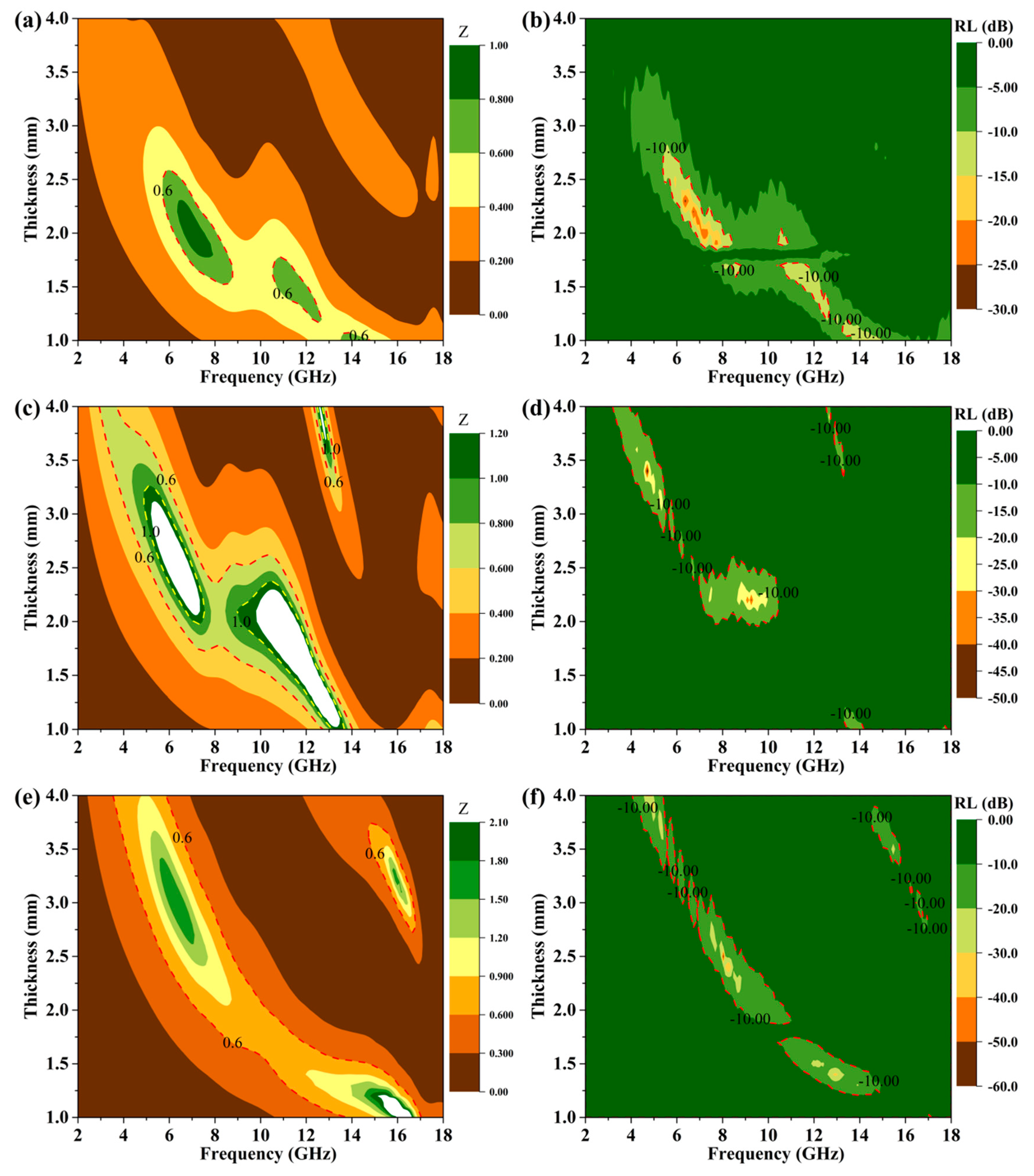
3.7. Microwave Absorption Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jia, K.; Zhao, R.; Zhong, J.; Liu, X. Preparation and microwave absorption properties of loose nanoscale Fe3O4 spheres. J. Magn. Magn. Mater. 2010, 322, 2167–2171. [Google Scholar] [CrossRef]
- Zhao, N.; Zou, T.; Shi, C.; Li, J.; Guo, W. Microwave absorbing properties of activated carbon-fiber felt screens (vertical-arranged carbon fibers)/epoxy resin composites. Mater. Sci. Eng. B 2006, 127, 207–211. [Google Scholar] [CrossRef]
- Deng, L.; Han, M. Microwave absorbing performances of multiwalled carbon nanotube composites with negative permeability. Appl. Phys. Lett. 2007, 91, 023119. [Google Scholar] [CrossRef]
- Oyharçabal, M.; Olinga, T.; Foulc, M.-P.; Lacomme, S.; Gontier, E.; Vigneras, V. Influence of the morphology of polyaniline on the microwave absorption properties of epoxy polyaniline composites. Compos. Sci. Technol. 2013, 74, 107–112. [Google Scholar] [CrossRef]
- Zhang, P.; Han, X.; Kang, L.; Qiang, R.; Liu, W.; Du, Y. Synthesis and characterization of polyaniline nanoparticles with enhanced microwave absorption. RSC Adv. 2013, 3, 12694. [Google Scholar] [CrossRef]
- Tian, X.; Meng, F.; Meng, F.; Chen, X.; Guo, Y.; Wang, Y.; Zhu, W.; Zhou, Z. Synergistic Enhancement of Microwave Absorption Using Hybridized Polyaniline@helical CNTs with Dual Chirality. ACS Appl. Mater. Interfaces 2017, 9, 15711–15718. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Li, Y.; Wang, J.; Liu, R.; Zhang, Y.; Wang, Z.; Yu, J.; Chen, W.; Shi, Z.; et al. Improved microwave absorbing properties by designing heterogeneous interfaces in Mo@2D-MoS2. J. Alloys Compd. 2018, 767, 1–6. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Zheng, Y.; Guo, C.; Yang, M.; Li, Z.; Wu, H.; Qiu, H.; Yan, H.; Qi, S. Preparation of Polyaniline@MoS2@Fe3O4 Nanowires with a Wide Band and Small Thickness toward Enhancement in Microwave Absorption. ACS Appl. Nano Mater. 2018, 1, 5865–5875. [Google Scholar] [CrossRef]
- Fang, X.-Y.; Yu, X.-X.; Zheng, H.-M.; Jin, H.-B.; Wang, L.; Cao, M.-S. Temperature-and thickness-dependent electrical conductivity of few-layer graphene and graphene nanosheets. Phys. Lett. A 2015, 379, 2245–2251. [Google Scholar] [CrossRef]
- Wang, X.; Yu, S.; Wu, Y.; Pang, H.; Yu, S.; Chen, Z.; Hou, J.; Alsaedi, A.; Hayat, T.; Wang, S. The synergistic elimination of uranium (VI) species from aqueous solution using bi-functional nanocomposite of carbon sphere and layered double hydroxide. Chem. Eng. J. 2018, 342, 321–330. [Google Scholar] [CrossRef]
- Ye, F.; Song, Q.; Zhang, Z.; Li, W.; Zhang, S.; Yin, X.; Zhou, Y.; Tao, H.; Liu, Y.; Cheng, L.; et al. Direct Growth of Edge-Rich Graphene with Tunable Dielectric Properties in Porous Si3N4 Ceramic for Broadband High-Performance Microwave Absorption. Adv. Funct. Mater. 2018, 28, 1707205. [Google Scholar] [CrossRef]
- Sun, G.; Wu, H.; Liao, Q.; Zhang, Y. Enhanced microwave absorption performance of highly dispersed CoNi nanostructures arrayed on graphene. Nano Res. 2018, 11, 2689–2704. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.; Zhu, W.; Cheng, B.; Qiu, H.; Qi, S. Core-shell nanostructured CS/MoS2: A promising material for microwave absorption. Appl. Surf. Sci. 2019, 463, 182–189. [Google Scholar] [CrossRef]
- Zhang, D.; Chai, J.; Cheng, J.; Jia, Y.; Yang, X.; Wang, H.; Zhao, Z.; Han, C.; Shan, G.; Zhang, W.; et al. Highly efficient microwave absorption properties and broadened absorption bandwidth of MoS2-iron oxide hybrids and MoS2-based reduced graphene oxide hybrids with Hetero-structures. Appl. Surf. Sci. 2018, 462, 872–882. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, X.; Liu, W.; Tang, D.; Zhang, B.; Ji, G. A simple hydrothermal process to grow MoS2 nanosheets with excellent dielectric loss and microwave absorption performance. J. Mater. Chem. C 2016, 4, 6816–6821. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Wu, H.; Yan, H.; Qi, S. Impact of morphology and dielectric property on the microwave absorbing performance of MoS2-based materials. J. Alloys Compd. 2018, 751, 34–42. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Li, S.; Wang, S.-W.; Yin, Z.-J.; Zhu, J.-Q.; Guo, A.-P.; Wang, G.-S.; Yin, P.-G.; Guo, L. Self-Supported Construction of Three-Dimensional MoS2 Hierarchical Nanospheres with Tunable High-Performance Microwave Absorption in Broadband. J. Phys. Chem. C 2016, 120, 22019–22027. [Google Scholar] [CrossRef]
- Wei, H.; Dong, J.; Fang, X.; Zheng, W.; Sun, Y.; Qian, Y.; Jiang, Z.; Huang, Y. Ti3C2Tx MXene/polyaniline (PANI) sandwich intercalation structure composites constructed for microwave absorption. Compos. Sci. Technol. 2019, 169, 52–59. [Google Scholar] [CrossRef]
- Cao, M.-S.; Cai, Y.-Z.; He, P.; Shu, J.-C.; Cao, W.-Q.; Yuan, J. 2D MXenes: Electromagnetic property for microwave absorption and electromagnetic interference shielding. Chem. Eng. J. 2019, 359, 1265–1302. [Google Scholar] [CrossRef]
- Li, X.; Yin, X.; Han, M.; Song, C.; Sun, X.; Xu, H.; Cheng, L.; Zhang, L. A controllable heterogeneous structure and electromagnetic wave absorption properties of Ti2CTx MXene. J. Mater. Chem. C 2017, 5, 7621–7628. [Google Scholar] [CrossRef]
- Liu, P.; Ng, V.M.H.; Yao, Z.; Zhou, J.; Kong, L.B. Ultrasmall Fe3O4 nanoparticles on MXenes with high microwave absorption performance. Mater. Lett. 2018, 229, 286–289. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, W.; Wang, Y.; Xu, X.; Wang, T.; Wu, L.; Du, Y. MoS2-Based Mixed-Dimensional van der Waals Heterostructures: A New Platform for Excellent and Controllable Microwave-Absorption Performance. ACS Appl. Mater. Interfaces 2017, 9, 34243–34255. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Song, J.; Wang, B.; Zhang, C.; Xiang, J.; Wen, F.; Liu, Z. Two-dimensional materials and one-dimensional carbon nanotube composites for microwave absorption. Nanotechnology 2017, 29, 025704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Wang, S.-W.; Wang, G.-S.; Li, Z.; Guo, A.-P.; Zhu, J.-Q.; Liu, D.-P.; Yin, P.-G. Facile synthesis of NiS2@MoS2 core–shell nanospheres for effective enhancement in microwave absorption. RSC Adv. 2017, 7, 22454–22460. [Google Scholar] [CrossRef]
- Wu, C.; Maier, J.; Yu, Y. Generalizable Synthesis of Metal-Sulfides/Carbon Hybrids with Multiscale, Hierarchically Ordered Structures as Advanced Electrodes for Lithium Storage. Adv. Mater. 2016, 28, 174–180. [Google Scholar] [CrossRef]
- Xiao, Y.; Lee, S.H.; Sun, Y.-K. The Application of Metal Sulfides in Sodium Ion Batteries. Adv. Energy Mater. 2017, 7, 1601329. [Google Scholar] [CrossRef]
- Zhao, B.; Guo, X.; Zhou, Y.; Su, T.; Ma, C.; Zhang, R. Constructing hierarchical hollow CuS microspheres via a galvanic replacement reaction and their use as wide-band microwave absorbers. CrystEngComm 2017, 19, 2178–2186. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Zhang, X.; Zhu, Q.; Zhu, W.; Wang, Y.; Qi, S. Structure and performance of Ni@Ni3S2 foam for microwave absorption. J. Phys. Appl. Phys. 2019. [Google Scholar] [CrossRef]
- Lv, J.; Cheng, Y.; Liu, W.; Quan, B.; Liang, X.; Ji, G.; Du, Y. Achieving better impedance matching by a sulfurization method through converting Ni into NiS/Ni3S4composites. J. Mater. Chem. C 2018, 6, 1822–1828. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Nagaura, T.; Ishikawa, A.; Chikyow, T.; Inoue, S. Evolution of Standing Mesochannels on Porous Anodic Alumina Substrates with Designed Conical Holes. J. Am. Chem. Soc. 2008, 130, 10165–10170. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Ishihara, S.; Leo, S.-Y.; Ariga, K.; Wu, K.C.-W.; Yamauchi, Y. Polymeric Micelle Assembly for Preparation of Large-Sized Mesoporous Metal Oxides with Various Compositions. Langmuir 2014, 30, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Nandi, M.; Mondal, J.; Sarkar, K.; Yamauchi, Y.; Bhaumik, A. Highly ordered acid functionalized SBA-15: A novel organocatalyst for the preparation of xanthenes. Chem. Commun. 2011, 47, 6677. [Google Scholar] [CrossRef] [PubMed]
- Malgras, V.; Henzie, J.; Takei, T.; Yamauchi, Y. Stable Blue Luminescent CsPbBr3 Perovskite Nanocrystals Confined in Mesoporous Thin Films. Angew. Chem. Int. Ed. 2018, 57, 8881–8885. [Google Scholar] [CrossRef]
- Wu, C.-W.; Yamauchi, Y.; Ohsuna, T.; Kuroda, K. Structural study of highly ordered mesoporous silica thin films and replicated Pt nanowires by high-resolution scanning electron microscopy (HRSEM). J. Mater. Chem. 2006, 16, 3091. [Google Scholar] [CrossRef]
- Huang, H.-S.; Chang, K.-H.; Suzuki, N.; Yamauchi, Y.; Hu, C.-C.; Wu, K.C.-W. Evaporation-Induced Coating of Hydrous Ruthenium Oxide on Mesoporous Silica Nanoparticles to Develop High-Performance Supercapacitors. Small 2013, 9, 2520–2526. [Google Scholar] [CrossRef] [PubMed]
- Ataee-Esfahani, H.; Liu, J.; Hu, M.; Miyamoto, N.; Tominaka, S.; Wu, K.C.W.; Yamauchi, Y. Mesoporous Metallic Cells: Design of Uniformly Sized Hollow Mesoporous Pt-Ru Particles with Tunable Shell Thicknesses. Small 2013, 9, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Qiao, Y.; Yan, H.; Qi, S. Covalently bonded GNPs-NH-PANI nanorod arrays modified by Fe3O4 nanoparticles as high-performance electromagnetic wave absorption materials. Mater. Lett. 2018, 216, 101–105. [Google Scholar] [CrossRef]
- Guan, R.; Wang, Y.; Zheng, S.; Su, N.; Ji, Z.; Liu, Z.; An, Y.; Chen, B. Fabrication of aluminum matrix composites reinforced with Ni-coated graphene nanosheets. Mater. Sci. Eng. A 2019, 754, 437–446. [Google Scholar] [CrossRef]
- Wang, X.; Wen, B.; Yang, X. Construction of core-shell structural nickel@graphite nanoplate functional particles with high electromagnetic shielding effectiveness. Compos. Part B Eng. 2019, 173, 106904. [Google Scholar] [CrossRef]
- Sun, D.; Ye, D.; Liu, P.; Tang, Y.; Guo, J.; Wang, L.; Wang, H. MoS2/Graphene Nanosheets from Commercial Bulky MoS2 and Graphite as Anode Materials for High Rate Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702383. [Google Scholar] [CrossRef]
- Xing, L.; Li, X.; Wu, Z.; Yu, X.; Liu, J.; Wang, L.; Cai, C.; You, W.; Chen, G.; Ding, J.; et al. 3D hierarchical local heterojunction of MoS2/FeS2 for enhanced microwave absorption. Chem. Eng. J. 2020, 379, 122241. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Yang, S.; Wang, F.; Zhuang, X.; Müllen, K.; Feng, X. Vertically Aligned MoS2 Nanosheets Patterned on Electrochemically Exfoliated Graphene for High-Performance Lithium and Sodium Storage. Adv. Energy Mater. 2018, 8, 1702254. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Zhang, X.; Xu, G.; Wang, D.; Lv, J.; Zheng, Z.; Wu, Y. NiS and MoS2 nanosheet co-modified graphitic C3N4 ternary heterostructure for high efficient visible light photodegradation of antibiotic. J. Hazard. Mater. 2018, 341, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-F.; Feng, T.; Liu, Z.-W.; Wu, D.-Y.; Yang, J. Laser-direct-writing of 3D self-supported NiS2/MoS2 heterostructures as an efficient electrocatalyst for hydrogen evolution reaction in alkaline and neutral electrolytes. Chin. J. Catal. 2019, 40, 1147–1152. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, C.; Chang, J.; Chen, L.; Wang, P.; Wu, D.; Xu, F.; Jiang, K. Porous Co3S4@Ni3S4 heterostructure arrays electrode with vertical electrons and ions channels for efficient hybrid supercapacitor. Chem. Eng. J. 2018, 343, 572–582. [Google Scholar] [CrossRef]
- Kuang, P.; He, M.; Zou, H.; Yu, J.; Fan, K. 0D/3D MoS2-NiS2/N-doped graphene foam composite for efficient overall water splitting. Appl. Catal. B Environ. 2019, 254, 15–25. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Zeng, W. Hydrothermal synthesis of hierarchical flower-like ZnO nanostructure and its enhanced ethanol gas-sensing properties. Appl. Surf. Sci. 2018, 427, 281–287. [Google Scholar] [CrossRef]
- Wang, Q.; Kou, X.; Liu, C.; Zhao, L.; Lin, T.; Liu, F.; Yang, X.; Lin, J.; Lu, G. Hydrothermal synthesis of hierarchical CoO/SnO2 nanostructures for ethanol gas sensor. J. Colloid Interface Sci. 2018, 513, 760–766. [Google Scholar] [CrossRef]
- Trpkov, D.; Panjan, M.; Kopanja, L.; Tadić, M. Hydrothermal synthesis, morphology, magnetic properties and self-assembly of hierarchical α-Fe2O3 (hematite) mushroom-, cube- and sphere-like superstructures. Appl. Surf. Sci. 2018, 457, 427–438. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Y.; Hu, H.; Li, X.; Liu, J.; Guan, L.; Tian, W.; Wang, X.; Li, Y.; Wu, M. 3D self-assembly synthesis of hierarchical porous carbon from petroleum asphalt for supercapacitors. Carbon 2018, 134, 345–353. [Google Scholar] [CrossRef]
- Zhang, W.; Li, L.; Zhu, W.; Yan, H.; Qi, S. Preparation and microwave absorbing performance of MoS2@Fe3O4@PANI composites. J. Mater. Sci. Mater. Electron. 2017, 28, 15488–15494. [Google Scholar] [CrossRef]
- Liu, P.; Ng, V.M.H.; Yao, Z.; Zhou, J.; Lei, Y.; Yang, Z.; Lv, H.; Kong, L.B. Facile Synthesis and Hierarchical Assembly of Flowerlike NiO Structures with Enhanced Dielectric and Microwave Absorption Properties. ACS Appl. Mater. Interfaces 2017, 9, 16404–16416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Zhang, W.; Luo, C.; Li, J.; Wang, Y. Fabrication of flower-like Ni0.5Co0.5(OH)2@PANI and its enhanced microwave absorption performances. Mater. Res. Bull. 2018, 98, 59–63. [Google Scholar] [CrossRef]
- Zheng, H.; Yao, W.; Sun, H.; Tong, G. Highly enhanced microwave absorption properties of CoFeBSiNb metallic glasses through corrosion. J. Magn. Magn. Mater. 2018, 468, 109–114. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, H.; Wang, N.; Zhou, S.; Xie, D.; Li, T. Annealing effects on the microstructure, magnetism and microwave-absorption properties of Fe/TiO2 nanocomposites. J. Magn. Magn. Mater. 2019, 471, 346–354. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, C.; Xiao, F.; Wang, J.; Su, X. Synthesis of nano-TiO2-decorated MoS2 nanosheets for lithium ion batteries. New J. Chem. 2015, 39, 683–688. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Zhang, Y.; Yu, Z.; Zhang, T.; Ge, W.; Zhang, L. NiS2/rGO/S capable of lithium polysulfide trapping as an enhanced cathode material for lithium sulfur batteries. J. Alloys Compd. 2018, 766, 804–812. [Google Scholar] [CrossRef]
- Cao, M.; Wang, X.; Cao, W.; Fang, X.; Wen, B.; Yuan, J. Thermally Driven Transport and Relaxation Switching Self-Powered Electromagnetic Energy Conversion. Small 2018, 14, 1800987. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Y.; Zhang, X.; Zhu, Q.; Yan, H.; Liotta, L.F.; Wu, H.; Qi, S. Synthesis and mechanism investigation of wide-bandwidth Ni@MnO2 NS foam microwave absorbent. J. Alloys Compd. 2019, 792, 945–952. [Google Scholar] [CrossRef]
- Zhang, X.; Rao, Y.; Guo, J.; Qin, G. Multiple-phase carbon-coated FeSn2/Sn nanocomposites for high-frequency microwave absorption. Carbon 2016, 96, 972–979. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Y.; Zhang, X.; Zhu, Q.; Tian, L.; Wu, H.; Yan, H.; Qi, S. Structure-microwave absorption performance correlations of GNPs/ZnO nanocomposite absorber: Synthesis, characteration and mechanism investigation. Ceram. Int. 2019, 45, 13376–13384. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, H.; Dong, J.; Du, Y.; Fang, X.; Zheng, W.; Sun, Y.; Jiang, Z. Fabrication of urchin-like ZnO-MXene nanocomposites for high-performance electromagnetic absorption. Ceram. Int. 2017, 43, 10757–10762. [Google Scholar] [CrossRef]
- Luo, H.; Feng, W.; Liao, C.; Deng, L.; Liu, S.; Zhang, H.; Xiao, P. Peaked dielectric responses in Ti3C2 MXene nanosheets enabled composites with efficient microwave absorption. J. Appl. Phys. 2018, 123, 104103. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.-B.; Xie, X.; Yang, R.; Liu, Z.; Liu, Y.; Yu, Z.-Z. Multifunctional, Superelastic, and Lightweight MXene/Polyimide Aerogels. Small 2018, 14, 1802479. [Google Scholar] [CrossRef] [PubMed]
- Quan, B.; Liang, X.; Ji, G.; Cheng, Y.; Liu, W.; Ma, J.; Zhang, Y.; Li, D.; Xu, G. Dielectric polarization in electromagnetic wave absorption: Review and perspective. J. Alloys Compd. 2017, 728, 1065–1075. [Google Scholar] [CrossRef]
- Cao, M.; Han, C.; Wang, X.; Zhang, M.; Zhang, Y.; Shu, J.; Yang, H.; Fang, X.; Yuan, J. Graphene nanohybrids: Excellent electromagnetic properties for the absorbing and shielding of electromagnetic waves. J. Mater. Chem. C 2018, 6, 4586–4602. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Zhang, L.; Zhang, W.; Zhang, F.; Li, Z.; Zhu, Q.; Qi, S. Facile Synthesis of GNPs@NixSy@MoS2 Composites with Hierarchical Structures for Microwave Absorption. Nanomaterials 2019, 9, 1403. https://doi.org/10.3390/nano9101403
Zhu W, Zhang L, Zhang W, Zhang F, Li Z, Zhu Q, Qi S. Facile Synthesis of GNPs@NixSy@MoS2 Composites with Hierarchical Structures for Microwave Absorption. Nanomaterials. 2019; 9(10):1403. https://doi.org/10.3390/nano9101403
Chicago/Turabian StyleZhu, Wenfeng, Li Zhang, Weidong Zhang, Fan Zhang, Zhao Li, Qing Zhu, and Shuhua Qi. 2019. "Facile Synthesis of GNPs@NixSy@MoS2 Composites with Hierarchical Structures for Microwave Absorption" Nanomaterials 9, no. 10: 1403. https://doi.org/10.3390/nano9101403




