Magnetic Nanoparticles Applications for Amyloidosis Study and Detection: A Review
Abstract
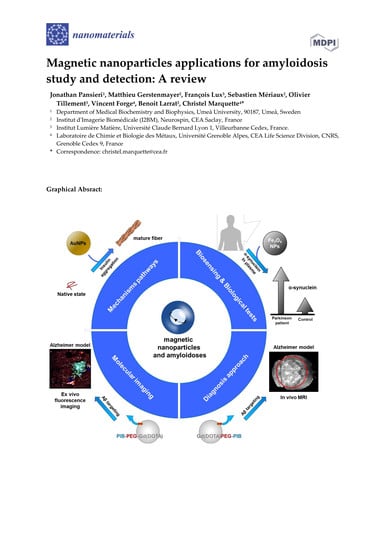
:1. Introduction
2. MNPs for the In Vitro Investigation of Amyloid Deposition Mechanisms
2.1. MNPs for the Understanding of Aβ Fibrillation
2.2. MNPs for the Control of Various Amyloidogenic Proteins’ Assembly
3. MNPs Functionalization for the Detection of Amyloid Aggregates
3.1. Congo Red and Thioflavin Derivatives Dyes
3.2. Peptides Derived from Amyloid Proteins
3.3. Antibodies and Nanobodies
3.4. MNPs for Other Amyloidosis Detection Tests
4. MNPs as In Vivo Diagnostic Probes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MNPs | magnetic nanoparticles |
| PIB | Pittsburgh compound B |
| AD | Alzheimer’s Disease |
| PD | Parkinson’s Disease |
| Aβ | amyloid beta protein |
| α-syn | α-synuclein |
| PrP | prion protein |
| PrPc | cellular prion protein |
| IAPP | islet amyloid polypeptide |
| TTR | transthyretin |
| BBB | blood–brain barrier |
| MRI | magnetic resonance imaging |
| PET | positron emission tomography |
| PEG | polyethylene glycol |
| USPIO | ultrasmall paramagnetic iron oxide |
| MION | magnetic iron oxide nanoparticles |
| SPION | supramagnetic iron oxide nanoparticles |
| ThT | Thioflavin T |
| i.v. | intravenous administration |
References
- Klein, W.L.; Krafft, G.A.; Finch, C.E. Targeting small Aβ oligomers: The solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001, 24, 219–224. [Google Scholar] [CrossRef]
- Sipe, J.D.; Benson, M.D.; Buxbaum, J.N.; Ikeda, S.; Merlini, G.; Saraiva, M.J.M.; Westermark, P. Amyloid fibril proteins and amyloidosis: Chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid 2016, 23, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Floege, J.; Ehlerding, G. Beta-2-Microglobulin-Associated Amyloidosis. Nephron 1996, 72, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Reches, M.; Porat, Y.; Gazit, E. Amyloid Fibril Formation by Pentapeptide and Tetrapeptide Fragments of Human Calcitonin. J. Biol. Chem. 2002, 277, 35475–35480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haataja, L.; Gurlo, T.; Huang, C.J.; Butler, P.C. Islet Amyloid in Type 2 Diabetes, and the Toxic Oligomer Hypothesis. Endocr. Rev. 2008, 29, 303–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatters, D.M.; Howlett, G.J. The structural basis for amyloid formation by plasma apolipoproteins: A review. Eur. Biophys. J. 2002, 31, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Teoh, C.L.; Griffin, M.D.W.; Howlett, G.J. Apolipoproteins and amyloid fibril formation in atherosclerosis. Protein Cell 2011, 2, 116–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef] [PubMed]
- World Alzheimer Report 2016—Improving Healthcare for People Living with Dementia: Coverage, Quality and Costs Now and in the Future; Alzheimer’s Disease International (ADI): London, UK, 2016; 140p.
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. Neurotherapeutics 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Boil. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, G.K.; Irudayaraj, J. Magnetic and Gold-Coated Magnetic Nanoparticles as a DNA Sensor. Anal. Chem. 2006, 78, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Ahmad, E.; Qadeer, A.; Rabbani, G.; Khan, R.H. Nanoparticles in relation to peptide and protein aggregation. Int. J. Nanomed. 2014, 9, 899–912. [Google Scholar] [CrossRef]
- Shaalan, M.; Saleh, M.; El-Mahdy, M.; El-Matbouli, M. Recent progress in applications of nanoparticles in fish medicine: A review. Nanomed. Nanotechnol. Boil. Med. 2016, 12, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, G.; Shen, Y.; Guo, N.; Ma, N. Circulating Tumor Cells: DNA-Templated Magnetic Nanoparticle-Quantum Dot Polymers for Ultrasensitive Capture and Detection of Circulating Tumor Cells. Adv. Funct. Mater. 2018, 28, 1870089. [Google Scholar] [CrossRef]
- Pansieri, J.; Plissonneau, M.; Stransky-Heilkron, N.; Dumoulin, M.; Heinrich-Balard, L.; Rivory, P.; Morfin, J.-F.; Toth, E.; Saraiva, M.J.; Allémann, E.; et al. Multimodal imaging Gd-nanoparticles functionalized with Pittsburgh compound B or a nanobody for amyloid plaques targeting. Nanomedicine 2017, 12, 1675–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Gan, N.; Li, T.; Zhou, H.; Li, X.; Cao, Y.; Wang, L.; Sang, W.; Hu, F. Ultratrace detection of C-reactive protein by a piezoelectric immunosensor based on Fe3O4@SiO2 magnetic capture nanoprobes and HRP-antibody co-immobilized nano gold as signal tags. Sens. Actuators B Chem. 2013, 178, 494–500. [Google Scholar] [CrossRef]
- Zhou, H.; Gan, N.; Li, T.; Cao, Y.; Zeng, S.; Zheng, L.; GuO, Z. The sandwich-type electroluminescence immunosensor for a-fetoprotein based on enrichment by Fe3O4-Au magnetic nano probes and signal amplification by CdS-Au composite nanoparticles labeled anti-AFP. Anal. Chim. Acta 2012, 746, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Yang, X.; Xie, D.; Wu, Y.; Wen, W. A disposable organophosphorus pesticides enzyme biosensor based on magnetic composite nano-particles modified screen printed carbon electrode. Sensors 2010, 10, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Zamfir, L.G.; Geana, I.; Bourigua, S.; Rotariu, L.; Bala, C.; Errachid, A.; Jaffrezic-Renault, N. Highly sensitive label-free immunosensor for ochratoxin A based on functionalized magnetic nanoparticles and EIS/SPR detection. Sens. Actuators B Chem. 2011, 159, 178–184. [Google Scholar] [CrossRef]
- Agrawal, S.; Paknikar, K.; Bodas, D. Development of immunosensor using magnetic nanoparticles and circular microchannels in PDMS. Microelectron. Eng. 2014, 115, 66–69. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Cui, Z.; Lockman, P.R.; Atwood, C.S.; Hsu, C.-H.; Gupte, A.; Allen, D.D.; Mumper, R.J. Novel d-penicillamine carrying nanoparticles for metal chelation therapy in Alzheimer’s and other CNS diseases. Eur. J. Pharm. Biopharm. 2005, 59, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Pedram, M.; Shamloo, A.; Alasty, A.; Ghafar-Zadeh, E.; Pedram, M.Z.; Shamloo, A.; Alasty, A.; Ghafar-Zadeh, E. Optimal Magnetic Field for Crossing Super-Para-Magnetic Nanoparticles through the Brain Blood Barrier: A Computational Approach. Biosensors 2016, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.-Z.; Watari, F. Current investigations into magnetic nanoparticles for biomedical applications. J. Biomed. Mater. Res. Part A 2016, 104, 1285–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, N.V.; Yang, Y.; Teranishi, T.; Thi, C.M.; Cao, Y.; Nogami, M. Biomedical Applications of Advanced Multifunctional Magnetic Nanoparticles. J. Nanoscie. Nanotechn. 2015, 15, 10091–10107. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Shubayev, V.I.; Pisanic, T.R.; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, F.A.; Hofrichter, J.; Eaton, W.A. Kinetics of sickle hemoglobin polymerization: II. A double nucleation mechanism. J. Mol. Boil. 1985, 183, 611–631. [Google Scholar] [CrossRef]
- O’Nuallain, B.; Williams, A.D.; Westermark, P.; Wetzel, R. Seeding Specificity in Amyloid Growth Induced by Heterologous Fibrils. J. Biol. Chem. 2004, 279, 17490–17499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Udgaonkar, J.B. Mechanisms of amyloid fibril formation by proteins. Curr. Sci. 2010, 98, 639–656. [Google Scholar]
- Kell, D.B.; Pretorius, E. Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: Lessons from and for blood clotting. Prog. Biophys. Mol. Boil. 2017, 123, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Meisl, G.; Samuel, L.R.; Cohen, A.I.; Pfammatter, M.; Sari, A.; Hellstrand, E.; Buell, A.K.; Aguzzi, A.; Linse, S.; Vendruscolo, M.; et al. Scaling behaviour and rate-determining steps in filamentous self-assembly. Chem. Sci. 2017, 8, 7087. [Google Scholar]
- Pansieri, J.; Halim, M.A.; Vendrely, C.; Dumoulin, M.; Legrand, F.; Moulin Sallanon, M.; Chierici, S.; Denti, S.; Dagany, X.; Dugourd, P.; et al. Mass and charge distributions of amyloid fibers involved in neurodegenerative diseases: Mapping heterogeneity and polymorphism. Chem. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Shan, L. Superparamagnetic iron oxide nanoparticles (SPION) stabilized by alginate. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Cheng, K.K.; Chan, P.S.; Fan, S.; Kwan, S.M.; Yeung, K.L.; Wáng, Y.-X.J.; Chow, A.H.L.; Wu, E.X.; Baum, L. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 2015, 44, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Mirsadeghi, S.; Shanehsazzadeh, S.; Atyabi, F.; Dinarvand, R. Effect of PEGylated superparamagnetic iron oxide nanoparticles (SPIONs) under magnetic field on amyloid beta fibrillation process. Mater. Sci. Eng. C 2016, 59, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Quinlan-Pluck, F.; Monopoli, M.P.; Sheibani, S.; Vali, H.; Dawson, K.A.; Lynch, I. Influence of the Physiochemical Properties of Superparamagnetic Iron Oxide Nanoparticles on Amyloid β Protein Fibrillation in Solution. ACS Chem. Neurosci. 2013, 4, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Kambli, P.; Borana, M.; Mohanpuria, N.; Ahmad, B.; Kelkar-Mane, V.; Ladiwala, U. Attenuation of lysozyme amyloid cytotoxicity by SPION-mediated modulation of amyloid aggregation. Int. J. Boil. Macromol. 2015, 74, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Kouyoumdjian, H.; Zhu, D.C.; Huang, X. Heparin nanoparticles for β amyloid binding and mitigation of β amyloid associated cytotoxicity. Carbohydr. Res. 2015, 405, 110–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Mao, X.; Yu, Y.; Wang, C.-X.; Yang, Y.-L.; Wang, C. Nanomaterials for Reducing Amyloid Cytotoxicity. Adv. Mater. 2013, 25, 3780–3801. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Shokrgozar, M.A.; Sardari, S.; Moghadam, M.K.; Vali, H.; Laurent, S.; Stroeve, P. Irreversible changes in protein conformation due to interaction with superparamagnetic iron oxide nanoparticles. Nanoscale 2011, 3, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Amani, S.; Naeem, A. Transition of transferrin from native to fibrillar state: An implication for amyloid-linked diseases. Biochem. Eng. J. 2014, 91, 120–128. [Google Scholar] [CrossRef]
- Wu, W.; Sun, X.; Yu, Y.; Hu, J.; Zhao, L.; Liu, Q.; Zhao, Y.; Li, Y. TiO2 nanoparticles promote β-amyloid fibrillation in vitro. Biochem. Biophys. Res. Commun. 2008, 373, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Elbassal, E.A.; Morris, C.; Kent, T.W.; Lantz, R.; Ojha, B.; Wojcikiewicz, E.P.; Du, D. Gold Nanoparticles as a Probe for Amyloid-β Oligomer and Amyloid Formation. J. Phys. Chem. C 2017, 121, 20007–20015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benilova, I.; Karran, E.; Strooper, B.D. The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Glabe, C.G. Conformation-Dependent Anti-Amyloid Oligomer Antibodies. Methods Enzymol. 2006, 413, 326–344. [Google Scholar] [PubMed]
- Hsieh, S.; Chang, C.; Chou, H. Gold nanoparticles as amyloid-like fibrillogenesis inhibitors. Colloids Surf. B Biointerfaces 2013, 112, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Dubey, K.; Anand, B.G.; Badhwar, R.; Bagler, G.; Navya, P.N.; Daima, H.K.; Kar, K. Tyrosine- and tryptophan-coated gold nanoparticles inhibit amyloid aggregation of insulin. Amino Acids 2015, 47, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, Y.D.; Fauerbach, J.A.; Pellegrotti, J.V.; Jovin, T.M.; Jares-Erijman, E.A.; Stefani, F.D. Influence of Gold Nanoparticles on the Kinetics of α-Synuclein Aggregation. Nano Lett. 2013, 13, 6156–6163. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Konar, S.; Pathak, A.; Dasgupta, S.; DasGupta, S. Effect of Functionalized Magnetic MnFe2O4 Nanoparticles on Fibrillation of Human Serum Albumin. J. Phys. Chem. B 2014, 118, 11667–11676. [Google Scholar] [CrossRef] [PubMed]
- Skaat, H.; Margel, S. Newly Designed Magnetic and Non-Magnetic Nanoparticles for Potential Diagnostics and Therapy of Alzheimer’s Disease. J. Biotechnol. Biomater. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Wang, J.-X.; Zhuo, Y.; Zhou, Y.; Wang, H.-J.; Yuan, R.; Chai, Y.-Q. Ceria Doped Zinc Oxide Nanoflowers Enhanced Luminol-Based Electrochemiluminescence Immunosensor for Amyloid-β Detection. ACS Appl. Mater. Interfaces 2016, 8, 12968–12975. [Google Scholar] [CrossRef] [PubMed]
- Tsolakis, A.C.; Halevas, E.; Vouroutzis, N.; Koliakos, G.G.; Salifoglou, A.; Litsardakis, G. Magnetic Fluorescent Nanoparticles Binding to Amyloid-Beta Peptide: Silica-Coated, Thioflavin-T Functionalized Iron Oxide. IEEE Trans. Magn. 2017, 53, 1–4. [Google Scholar] [CrossRef]
- Martins, A.F.; Morfin, J.-F.; Geraldes, C.F.G.C.; Tóth, É. Gd3+ complexes conjugated to Pittsburgh compound B: Potential MRI markers of β-amyloid plaques. J. Biol. Inorg. Chem. 2014, 19, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Rabinovici, G.D.; Furst, A.J.; O’Neil, J.P.; Racine, C.A.; Mormino, E.C.; Baker, S.L.; Chetty, S.; Patel, P.; Pagliaro, T.A.; Klunk, W.E.; et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology 2007, 68, 1205. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Pike, K.E.; Chételat, G.; Ellis, K.A.; Mulligan, R.S.; Bourgeat, P.; Ackermann, U.; Jones, G.; Szoeke, C.; Salvado, O.; et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann. Neurol. 2011, 69, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, A.F.; Dias, D.M.; Morfin, J.-F.; Lacerda, S.; Laurents, D.V.; Tóth, É.; Geraldes, C.F.G.C. Interaction of PiB-Derivative Metal Complexes with Beta-Amyloid Peptides: Selective Recognition of the Aggregated Forms. Chem. A Eur. J. 2015, 21, 5413–5422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadghiri, Y.Z.; Sigurdsson, E.M.; Sadowski, M.; Elliott, J.I.; Li, Y.; Scholtzova, H.; Tang, C.Y.; Aguinaldo, G.; Pappolla, M.; Duff, K.; et al. Detection of Alzheimer’s amyloid in transgenic mice using magnetic resonance microimaging. Magn. Reson. Med. 2003, 50, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Xiao, W.; Wang, X.; Marik, J.; Park, S.H.; Takada, Y.; Lam, K.S. Discovery of Targeting Ligands for Breast Cancer Cells Using the One-Bead One-Compound Combinatorial Method. J. Med. Chem. 2009, 52, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Delehanty, J.B.; Boeneman, K.; Bradburne, C.E.; Robertson, K.; Bongard, J.E.; Medintz, I.L. Peptides for specific intracellular delivery and targeting of nanoparticles: Implications for developing nanoparticle-mediated drug delivery. Ther. Deliv. 2010, 1, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, I.; Araya, E.; Sanz, F.; Medina, E.; Arbiol, J.; Toledo, P.; Álvarez-Lueje, A.; Giralt, E.; Kogan, M.J. How Changes in the Sequence of the Peptide CLPFFD-NH2 Can Modify the Conjugation and Stability of Gold Nanoparticles and Their Affinity for β-Amyloid Fibrils. Bioconjug. Chem. 2008, 19, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Skaat, H.; Shafir, G.; Margel, S. Acceleration and inhibition of amyloid-β fibril formation by peptide-conjugated fluorescent-maghemite nanoparticles. J. Nanopart. Res. 2011, 13, 3521–3534. [Google Scholar] [CrossRef]
- Plissonneau, M.; Pansieri, J.; Heinrich-Balard, L.; Morfin, J.-F.; Stransky-Heilkron, N.; Rivory, P.; Mowat, P.; Dumoulin, M.; Cohen, R.; Allémann, É.; et al. Gd-nanoparticles functionalization with specific peptides for ß-amyloid plaques targeting. J. Nanobiotechnol. 2016, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Wang, X.; Zhou, B.; Wu, Y.; Mao, W.; Liu, L. Electrochemical Detection of Amyloid-β Oligomers Based on the Signal Amplification of a Network of Silver Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 19303–19311. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Tokuda, T.; Kasai, T.; Ishigami, N.; Hidaka, H.; Kondo, M.; Allsop, D.; Nakagawa, M. High-molecular-weight β-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J. 2010, 24, 2716–2726. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Jiang, X.; Bi, W.; Chen, H.; Jin, L.; Zhang, S.; Wang, C.; Zhang, W. Sensitive electrochemical immunosensor for α-synuclein based on dual signal amplification using PAMAM dendrimer-encapsulated Au and enhanced gold nanoparticle labels. Biosens. Bioelectron. 2012, 32, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Chiu, M.-J.; Lin, C.-H.; Horng, H.-E.; Yang, C.-C.; Chieh, J.-J.; Chen, H.-H.; Liu, B.-H. Development of an ultra-high sensitive immunoassay with plasma biomarker for differentiating Parkinson disease dementia from Parkinson disease using antibody functionalized magnetic nanoparticles. J. Nanobiotechnol. 2016, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Poduslo, J.F.; Hultman, K.L.; Curran, G.L.; Preboske, G.M.; Chamberlain, R.; Marjańska, M.; Garwood, M.; Jack, C.R.; Wengenack, T.M. Targeting Vascular Amyloid in Arterioles of Alzheimer Disease Transgenic Mice with Amyloid β Protein Antibody-Coated Nanoparticles. J. Neuropathol. Exp. Neurol. 2011, 70, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Zameer, A.; Kasturirangan, S.; Emadi, S.; Nimmagadda, S.V.; Sierks, M.R. Anti-oligomeric Aβ Single-chain Variable Domain Antibody Blocks Aβ-induced Toxicity Against Human Neuroblastoma Cells. J. Mol. Boil. 2008, 384, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Domanska, K.; Vanderhaegen, S.; Srinivasan, V.; Pardon, E.; Dupeux, F.; Marquez, J.A.; Giorgetti, S.; Stoppini, M.; Wyns, L.; Bellotti, V.; et al. Atomic structure of a nanobody-trapped domain-swapped dimer of an amyloidogenic β2-microglobulin variant. Proc. Natl. Acad. Sci. USA 2011, 108, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. Single domain camel antibodies: Current status. Rev. Mol. Biotechnol. 2001, 74, 277–302. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural Single-Domain Antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Siontorou, C.G. Nanobodies as novel agents for disease diagnosis and therapy. Int. J. Nanomed. 2013, 8, 4215–4227. [Google Scholar] [CrossRef] [PubMed]
- Habicht, G.; Haupt, C.; Friedrich, R.P.; Hortschansky, P.; Sachse, C.; Meinhardt, J.; Wieligmann, K.; Gellermann, G.P.; Brodhun, M.; Götz, J.; et al. Directed selection of a conformational antibody domain that prevents mature amyloid fibril formation by stabilizing Aβ protofibrils. Proc. Natl. Acad. Sci. USA 2007, 104, 19232–19237. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.K.; Rashid, M.; Bisht, M. Multiplexed magnetic nanoparticle-antibody conjugates (MNPs-ABS) based prognostic detection of ovarian cancer biomarkers, CA-125, β-2M and ApoA1 using fluorescence spectroscopy with comparison of surface plasmon resonance (SPR) analysis. Biosens. Bioelectron. 2015, 73, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Kepe, V.; Moghbel, M.C.; Långström, B.; Zaidi, H.; Vinters, H.V.; Huang, S.-C.; Satyamurthy, N.; Doudet, D.; Mishani, E.; Cohen, R.M.; et al. Amyloid-β Positron Emission Tomography Imaging Probes: A Critical Review. J. Alzheimer’s Dis. 2013, 36, 613–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, I.-T.; Huang, C.-C.; Hsieh, C.-J.; Wey, S.-P.; Kung, M.-P.; Yen, T.-C.; Lin, K.-J. Perfusion-like template and standardized normalization-based brain image analysis using 18F-florbetapir (AV-45/Amyvid) PET. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 908–920. [Google Scholar] [CrossRef] [PubMed]
- De Lartigue, J. Flutemetamol (18F): A β-amyloid positron emission tomography tracer for Alzheimer’s and dementia diagnosis. Drugs Today 2014, 50, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Leuzy, A.; Zimmer, E.R.; Heurling, K.; Rosa-Neto, P.; Gauthier, S. Use of amyloid PET across the spectrum of Alzheimer’s disease: Clinical utility and associated ethical issues. Amyloid 2014, 21, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, K.; Yang, C.; Schneider, J.A.; Senjem, M.L.; Reyes, D.A.; Lowe, V.J.; Barnes, L.L.; Aggarwal, N.T.; Bennett, D.A.; Smith, G.E.; et al. Ante mortem amyloid imaging and β-amyloid pathology in a case with dementia with Lewy bodies. Neurobiol. Aging 2012, 33, 878–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuchi, M.; Iwata, N.; Matsuba, Y.; Sato, K.; Sasamoto, K.; Saido, T.C. 19F and 1H MRI detection of amyloid β plaques in vivo. Nat. Neurosci. 2005, 8, 527–533. [Google Scholar] [CrossRef] [PubMed]
- El Tayara, N.E.T.; Delatour, B.; Le Cudennec, C.; Guégan, M.; Volk, A.; Dhenain, M. Age-related evolution of amyloid burden, iron load, and MR relaxation times in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2006, 22, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, R.; Reyes, D.; Curran, G.L.; Marjanska, M.; Wengenack, T.M.; Poduslo, J.F.; Garwood, M.; Jack, C.R. Comparison of amyloid plaque contrast generated by T2-weighted, T1-weighted, and susceptibility-weighted imaging methods in transgenic mouse models of Alzheimer’s disease. Magn. Reson. Med. 2009, 61, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Dudeffant, C.; Vandesquille, M.; Herbert, K.; Garin, C.M.; Alves, S.; Blanchard, V.; Comoy, E.E.; Petit, F.; Dhenain, M. Contrast-enhanced MR microscopy of amyloid plaques in five mouse models of amyloidosis and in human Alzheimer’s disease brains. Sci. Rep. 2017, 7, 4955. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Wengenack, T.M.; Reyes, D.A.; Garwood, M.; Curran, G.L.; Borowski, B.J.; Lin, J.; Preboske, G.M.; Holasek, S.S.; Adriany, G.; et al. In vivo Magnetic Resonance Microimaging of Individual Amyloid Plaques in Alzheimer’s Transgenic Mice. J. Neurosci. 2005, 25, 10041–10048. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-P.; Falangola, M.F.; Nixon, R.A.; Duff, K.; Helpern, J.A. Visualization of β-amyloid plaques in a transgenic mouse model of Alzheimer’s disease using MR microscopy without contrast reagents. Magn. Reson. Med. 2004, 52, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.D.; Debeir, T.; Bridal, S.L.; Rooney, T.; Dhenain, M. Fast in vivo imaging of amyloid plaques using μ-MRI Gd-staining combined with ultrasound-induced blood–brain barrier opening. NeuroImage 2013, 79, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Wadghiri, Y.Z.; Li, J.; Wang, J.; Hoang, D.M.; Sun, Y.; Xu, H.; Tsui, W.; Li, Y.; Boutajangout, A.; Wang, A.; et al. Detection of Amyloid Plaques Targeted by Bifunctional USPIO in Alzheimer’s Disease Transgenic Mice Using Magnetic Resonance Microimaging. PLoS ONE 2013, 8, e57097. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zaim Wadghiri, Y.; Minh Hoang, D.; Tsui, W.; Sun, Y.; Chung, E.; Li, Y.; Wang, A.; de Leon, M.; Wisniewski, T. Detection of amyloid plaques targeted by USPIO-Aβ1–42 in Alzheimer’s disease transgenic mice using magnetic resonance microimaging. NeuroImage 2011, 55, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Sillerud, L.O.; Solberg, N.O.; Chamberlain, R.; Orlando, R.A.; Heidrich, J.E.; Brown, D.C.; Brady, C.I.; Vander Jagt, T.A.; Garwood, M.; Vander Jagt, D.L. SPION-Enhanced Magnetic Resonance Imaging of Alzheimer’s Disease Plaques in AβPP/PS-1 Transgenic Mouse Brain. J. Alzheimer’s Dis. 2013, 34, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Ha, T.L.; Im, G.H.; Yang, J.; Seo, S.W.; Lee, I.S.; Lee, J.H. Magnetic resonance imaging of amyloid plaques using hollow manganese oxide nanoparticles conjugated with antibody aβ1–40 in a transgenic mouse model. NeuroReport 2013, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Larbanoix, L.; Burtea, C.; Laurent, S.; Van Leuven, F.; Toubeau, G.; Elst, L.V.; Muller, R.N. Potential amyloid plaque-specific peptides for the diagnosis of Alzheimer’s disease. Neurobiol. Aging 2010, 31, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Ansciaux, E.; Burtea, C.; Laurent, S.; Crombez, D.; Nonclercq, D.; Elst, L.V.; Muller, R.N. In vitro and in vivo characterization of several functionalized ultrasmall particles of iron oxide, vectorized against amyloid plaques and potentially able to cross the blood–brain barrier: Toward earlier diagnosis of Alzheimer’s disease by molecular imaging. Contrast Media Mol. Imaging 2015, 10, 211–224. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Ansciaux, E.; Saidi, E.; Larbanoix, L.; Stanicki, D.; Nonclercq, D.; Vander Elst, L.; Laurent, S.; Muller, R.N.; Burtea, C. Validation by Magnetic Resonance Imaging of the Diagnostic Potential of a Heptapeptide-Functionalized Imaging Probe Targeted to Amyloid-β and Able to Cross the Blood-Brain Barrier. J. Alzheimer’s Dis. 2017, 60, 1547–1565. [Google Scholar] [CrossRef] [PubMed]
- Skaat, H.; Corem-Slakmon, E.; Grinberg, I.; Last, D.; Goez, D.; Mardor, Y.; Margel, S. Antibody-conjugated, dual-modal, near-infrared fluorescent iron oxide nanoparticles for antiamyloidgenic activity and specific detection of amyloid-β fibrils. Int. J. Nanomed. 2013, 8, 4063–4076. [Google Scholar] [CrossRef]
- Salerno, M.; Santo Domingo Porqueras, D. Alzheimer’s disease: The use of contrast agents for magnetic resonance imaging to detect amyloid beta peptide inside the brain. Coord. Chem. Rev. 2016, 327–328, 27–34. [Google Scholar] [CrossRef]
- Lam, T.; Pouliot, P.; Avti, P.K.; Lesage, F.; Kakkar, A.K. Superparamagnetic iron oxide based nanoprobes for imaging and theranostics. Adv. Colloid Interface Sci. 2013, 199–200, 95–113. [Google Scholar] [CrossRef] [PubMed]
- FDA Identifies No Harmful Effects to Date with Brain Retention of Gadolinium-Based Contrast Agents for MRIs; Review to Continue. FDA Drug Safety Communication. 27 July 2017. Available online: https://www.fda.gov/Drugs/DrugSafety/ucm559007.htm (accessed on 17 September 2018).
- Zhou, Z.; Lu, Z.-R. Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sancey, L.; Kotb, S.; Truillet, C.; Appaix, F.; Marais, A.; Thomas, E.; van der Sanden, B.; Klein, J.-P.; Laurent, B.; Cottier, M.; et al. Long-Term in vivo Clearance of Gadolinium-Based AGuIX Nanoparticles and Their Biocompatibility after Systemic Injection. ACS Nano 2015, 9, 2477–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poduslo, J.F.; Wengenack, T.M.; Curran, G.L.; Wisniewski, T.; Sigurdsson, E.M.; Macura, S.I.; Borowski, B.J.; Jack, C.R. Molecular Targeting of Alzheimer’s Amyloid Plaques for Contrast-Enhanced Magnetic Resonance Imaging. Neurobiol. Dis. 2002, 11, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Vithanarachchi, S.M.; Allen, M.J. A multimodal, β-amyloid-targeted contrast agent. Chem. Commun. 2013, 49, 4148–4150. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, M.; Wengenack, T.M.; Kandimalla, K.K.; Curran, G.L.; Gilles, E.J.; Ramirez-Alvarado, M.; Lin, J.; Garwood, M.; Jack, C.R., Jr.; Poduslo, J.F. Selective Contrast Enhancement of Individual Alzheimer’s Disease Amyloid Plaques Using a Polyamine and Gd-DOTA Conjugated Antibody Fragment Against Fibrillar Aβ42 for Magnetic Resonance Molecular Imaging. Pharm. Res. 2008, 25, 1861. [Google Scholar] [CrossRef] [PubMed]
- Lux, F.; Mignot, A.; Mowat, P.; Louis, C.; Dufort, S.; Bernhard, C.; Denat, F.; Boschetti, F.; Brunet, C.; Antoine, R.; et al. Ultrasmall Rigid Particles as Multimodal Probes for Medical Applications. Angew. Chem. Int. Ed. 2011, 50, 12299–12303. [Google Scholar] [CrossRef] [PubMed]
- LeDuc, G.; Roux, S.; Paruta-Tuarez, A.; Dufort, S.; Brauer, E.; Marais, A.; Truillet, C.; Sancey, L.; Perriat, P.; Lux, F.; et al. Advantages of gadolinium based ultrasmall nanoparticles vs molecular gadolinium chelates for radiotherapy guided by MRI for glioma treatment. Cancer Nanotechnol. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancey, L.; Lux, F.; Kotb, S.; Roux, S.; Dufort, S.; Bianchi, A.; Crémillieux, Y.; Fries, P.; Coll, J.L.; Rodriguez-Lafrasse, C.; et al. The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. BJR 2014, 87, 20140134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truillet, C.; Bouziotis, P.; Tsoukalas, C.; Brugière, J.; Martini, M.; Sancey, L.; Brichart, T.; Denat, F.; Boschetti, F.; Darbost, U.; et al. Ultrasmall particles for Gd-MRI and 68Ga-PET dual imaging. Contrast Media Mol. Imaging 2015, 10, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.J.; McCann, B.; Kauppinen, R.A.; Coulthard, E.J. Magnetic Resonance Imaging to Detect Early Molecular and Cellular Changes in Alzheimer’s Disease. Front. Aging Neurosci. 2016, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Moncelet, D.; Lux, F.; Plissonneau, M.; Rizzitelli, S.; Ribot, E.J.; Tassali, N.; Bouchaud, V.; Tillement, O.; Voisin, P.; et al. Orotracheal administration of contrast agents: A new protocol for brain tumor targeting. NMR Biomed. 2015, 28, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR Imaging-guided Focal Opening of the Blood-Brain Barrier in Rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef] [PubMed]
- McDannold, N.; Arvanitis, C.D.; Vykhodtseva, N.; Livingstone, M.S. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: Safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012. [Google Scholar] [CrossRef] [PubMed]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 2336. [Google Scholar] [CrossRef] [PubMed]
- Marty, B.; Larrat, B.; Van Landeghem, M.; Robic, C.; Robert, P.; Port, M.; Le Bihan, D.; Pernot, M.; Tanter, M.; Lethimonnier, F.; et al. Dynamic Study of Blood–Brain Barrier Closure after its Disruption using Ultrasound: A Quantitative Analysis. J. Cereb. Blood Flow Metab. 2012, 32, 1948–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borchelt, D.R.; Ratovitski, T.; van Lare, J.; Lee, M.K.; Gonzales, V.; Jenkins, N.A.; Copeland, N.G.; Price, D.L.; Sisodia, S.S. Accelerated Amyloid Deposition in the Brains of Transgenic Mice Coexpressing Mutant Presenilin 1 and Amyloid Precursor Proteins. Neuron 1997, 19, 939–945. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.G.; Wang, M.; Li, N.; Loo, J.A.; Nel, A.E. Use of Proteomics to Demonstrate a Hierarchical Oxidative Stress Response to Diesel Exhaust Particle Chemicals in a Macrophage Cell Line. J. Boil. Chem. 2003, 278, 50781–50790. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pansieri, J.; Gerstenmayer, M.; Lux, F.; Mériaux, S.; Tillement, O.; Forge, V.; Larrat, B.; Marquette, C. Magnetic Nanoparticles Applications for Amyloidosis Study and Detection: A Review. Nanomaterials 2018, 8, 740. https://doi.org/10.3390/nano8090740
Pansieri J, Gerstenmayer M, Lux F, Mériaux S, Tillement O, Forge V, Larrat B, Marquette C. Magnetic Nanoparticles Applications for Amyloidosis Study and Detection: A Review. Nanomaterials. 2018; 8(9):740. https://doi.org/10.3390/nano8090740
Chicago/Turabian StylePansieri, Jonathan, Matthieu Gerstenmayer, François Lux, Sebastien Mériaux, Olivier Tillement, Vincent Forge, Benoit Larrat, and Christel Marquette. 2018. "Magnetic Nanoparticles Applications for Amyloidosis Study and Detection: A Review" Nanomaterials 8, no. 9: 740. https://doi.org/10.3390/nano8090740