Bidisperse Magnetic Particles Coated with Gelatin and Graphite Oxide: Magnetorheology, Dispersion Stability, and the Nanoparticle-Enhancing Effect
Abstract
:1. Introduction
2. Materials and Methods
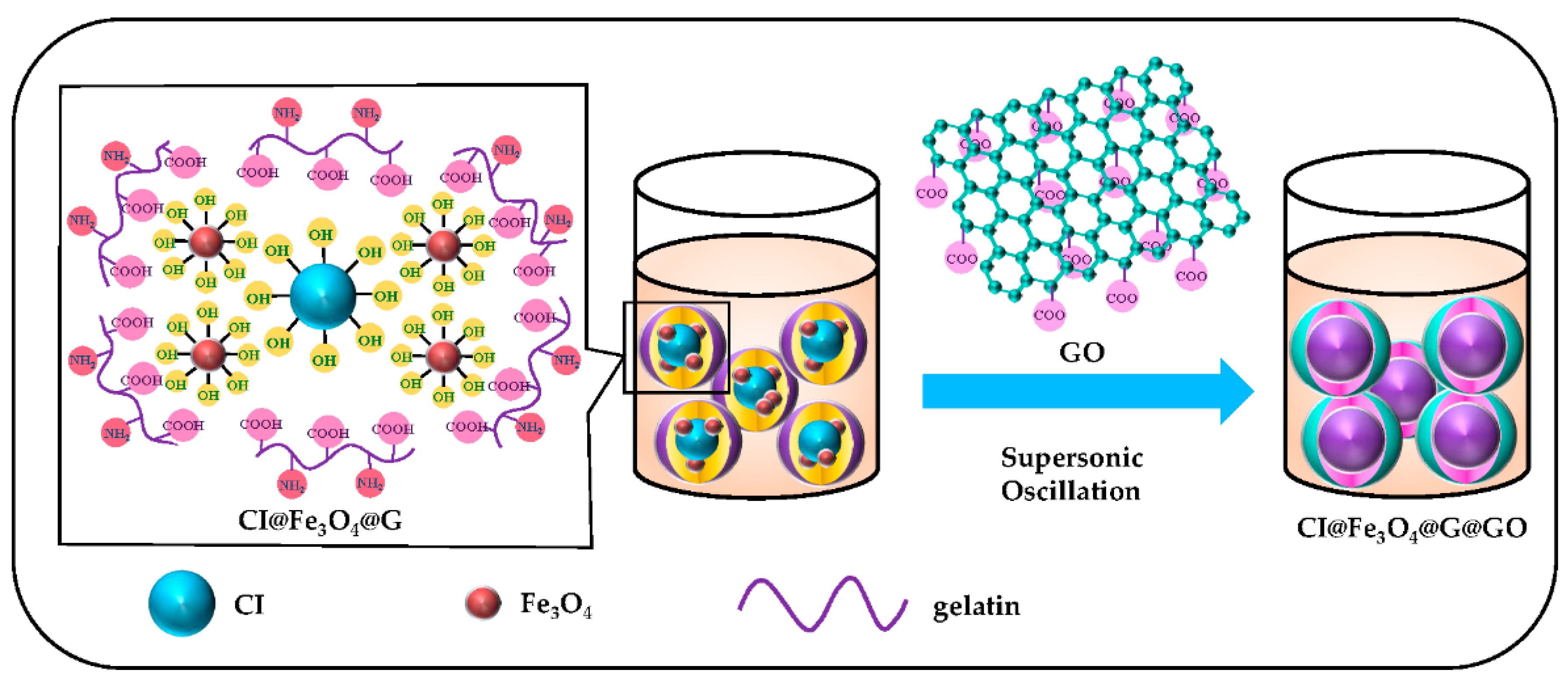
2.1. Synthesis of Gelatin Coated CI@Fe3O4 (CI@Fe3O4@G) Particles
2.2. Fabrication of the GO-Nest Wrapped CI@Fe3O4@G (CI@Fe3O4@G@GO) Particles
2.3. Characterization Methods
3. Results and Discussion
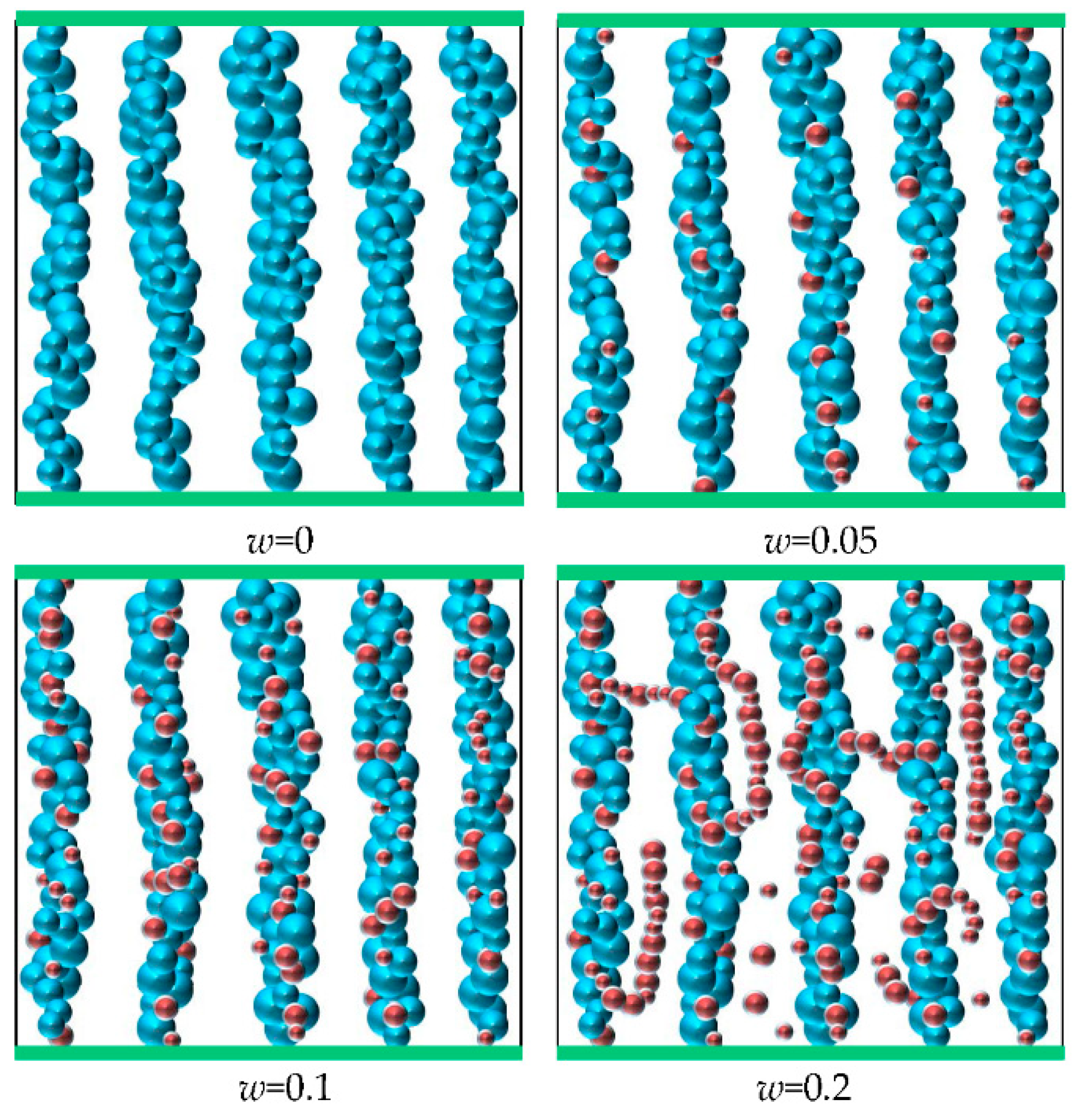
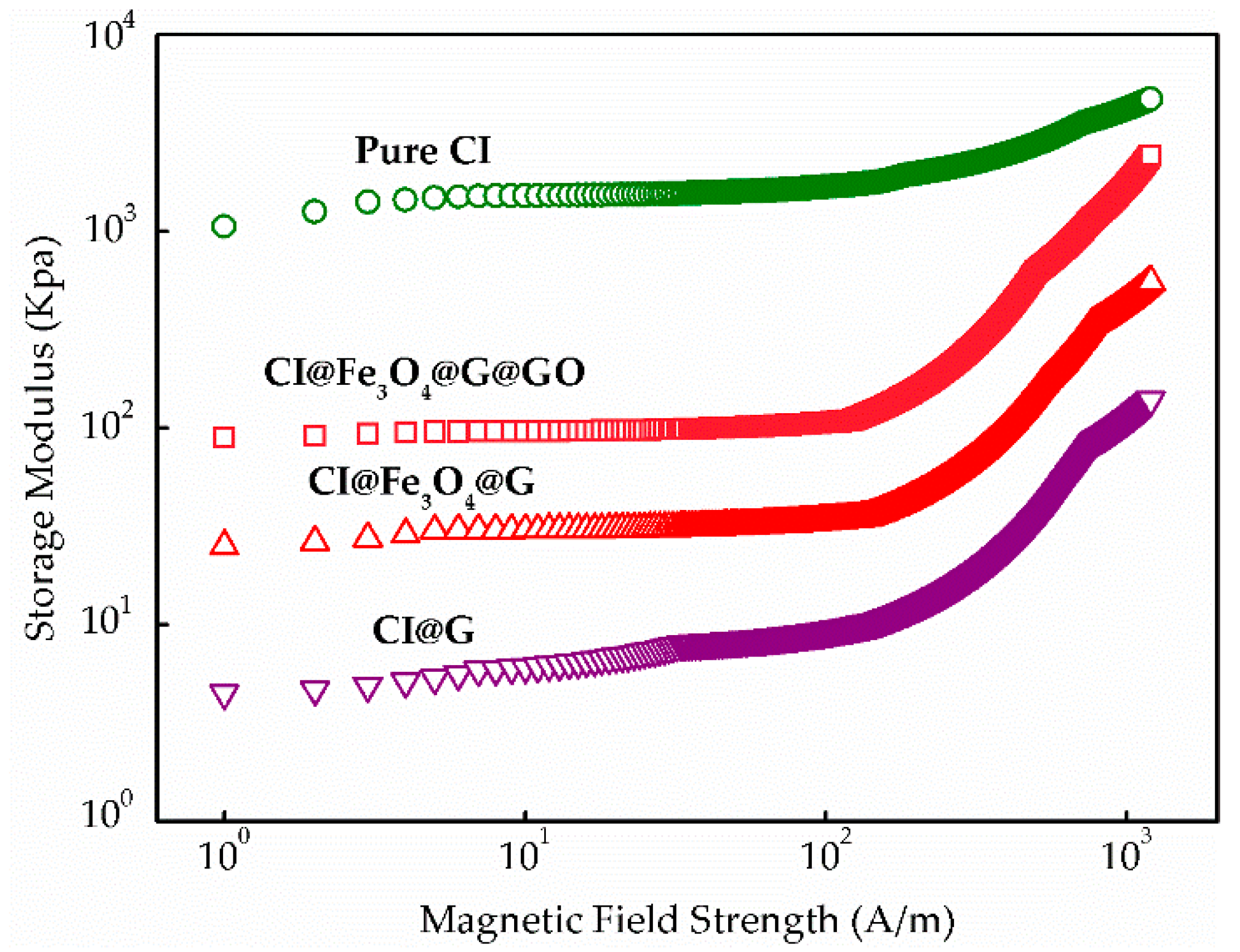
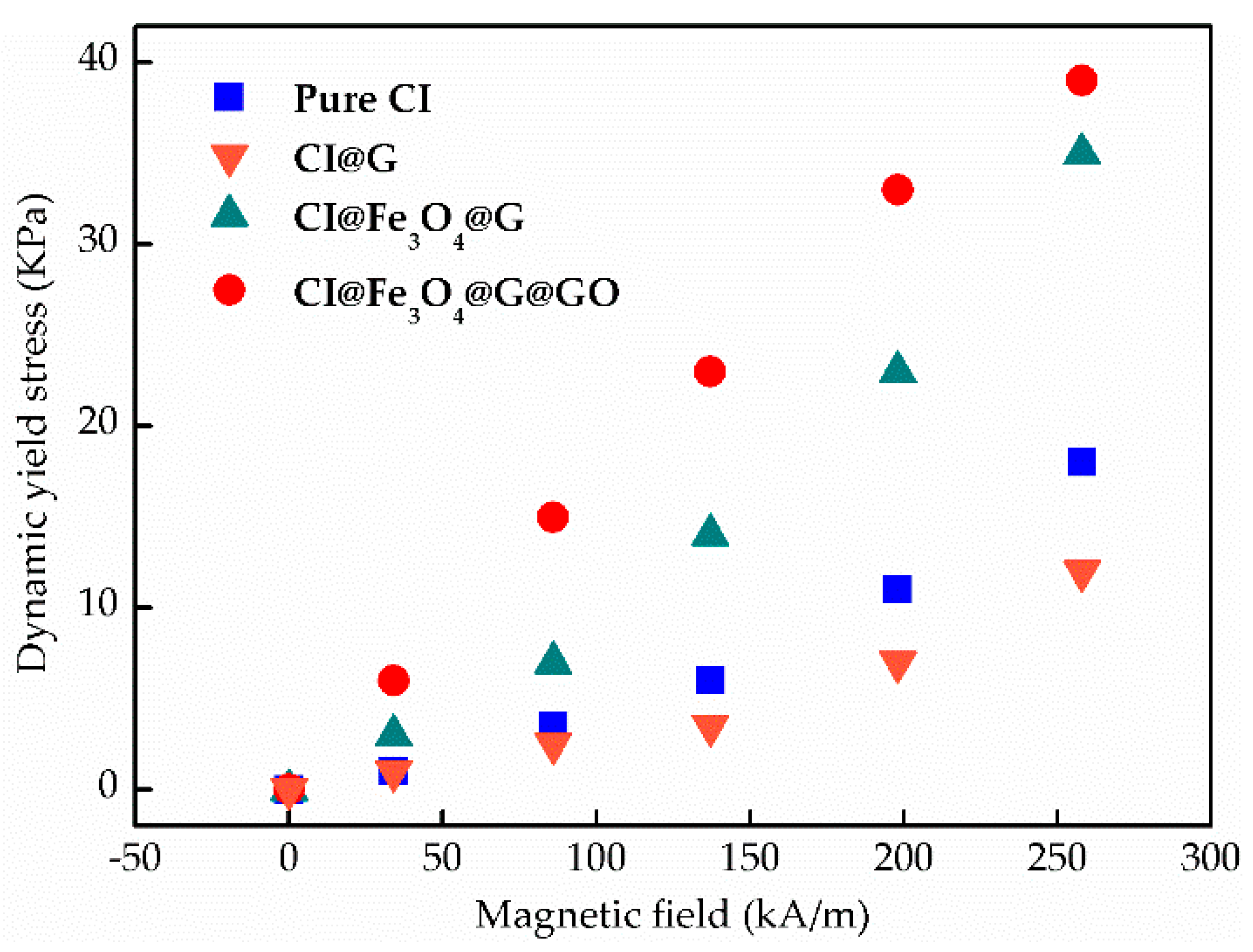
3.1. Nanoparticle-Enhancing Effect
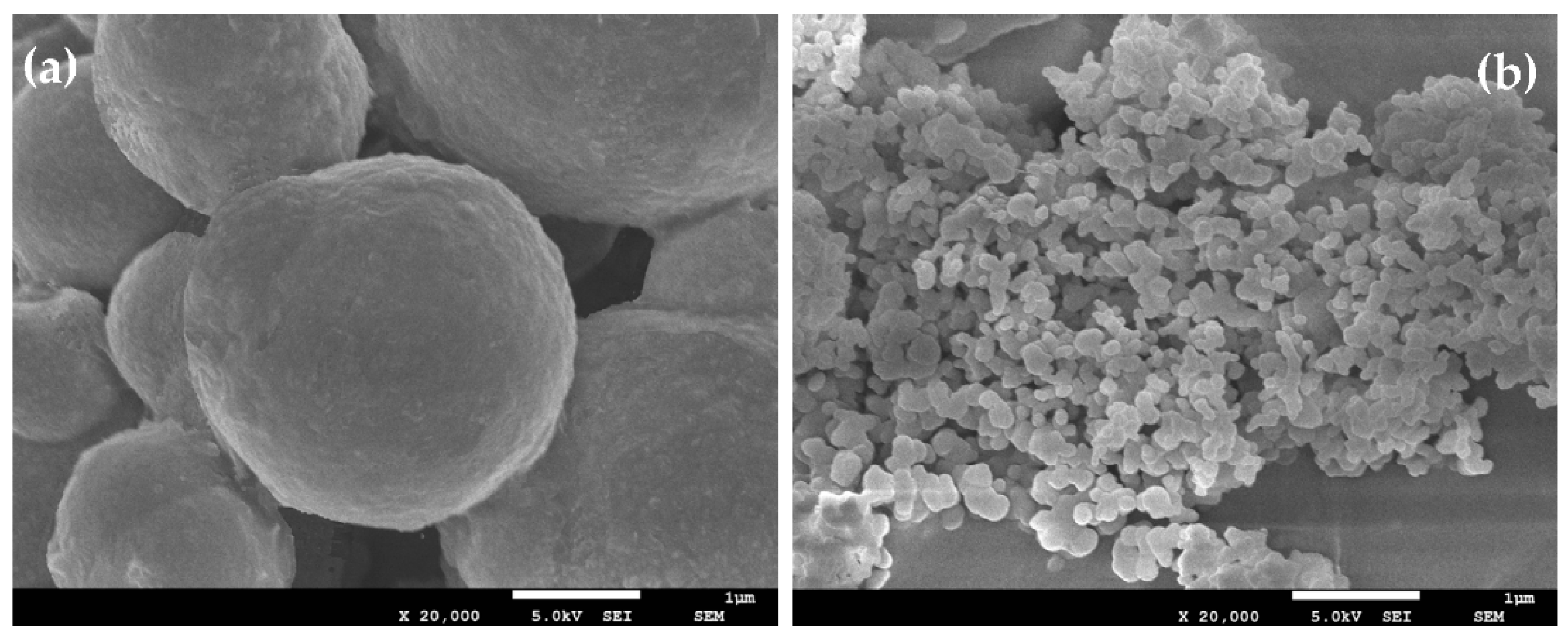
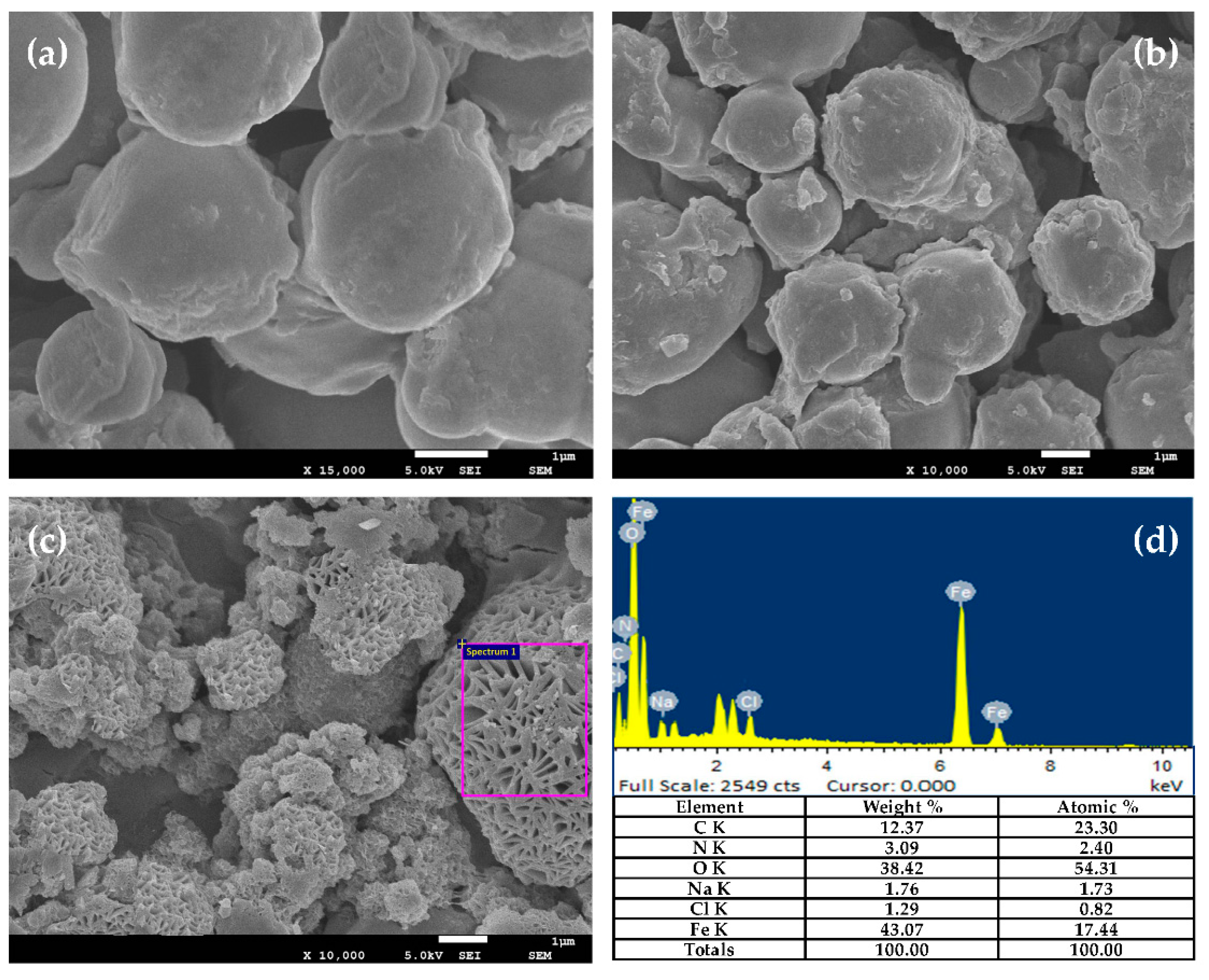
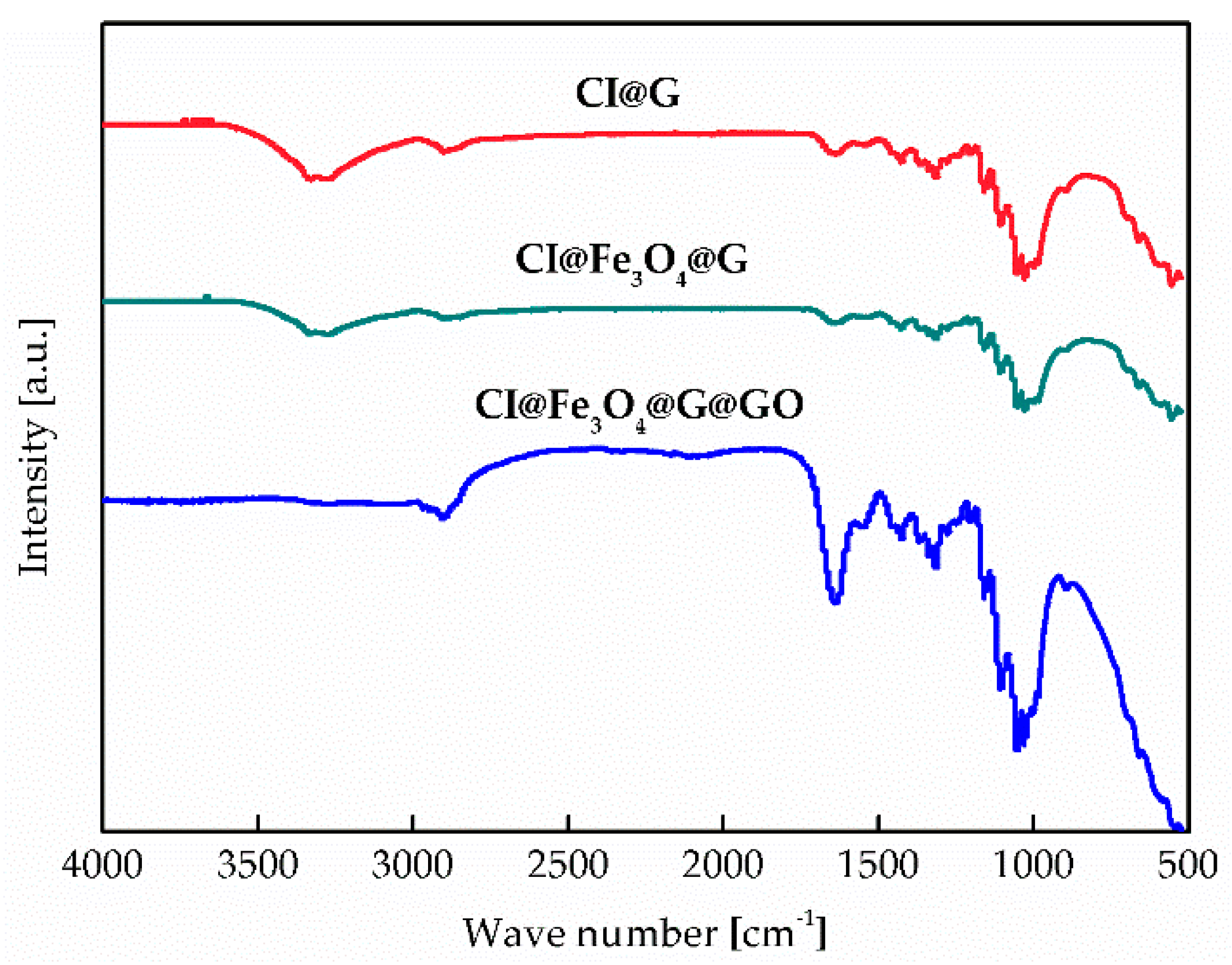
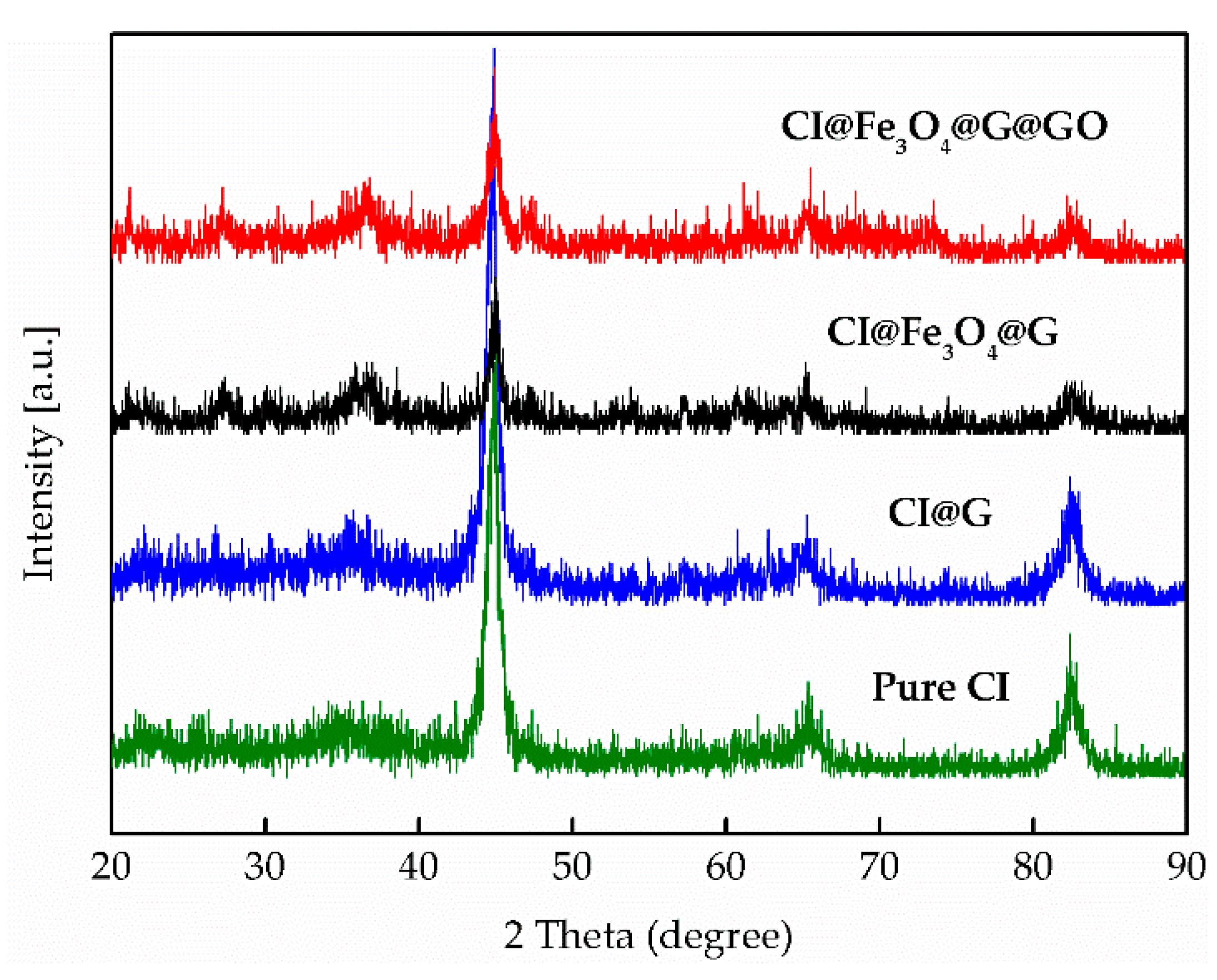
3.2. Particle Morphology and Chemical Composition
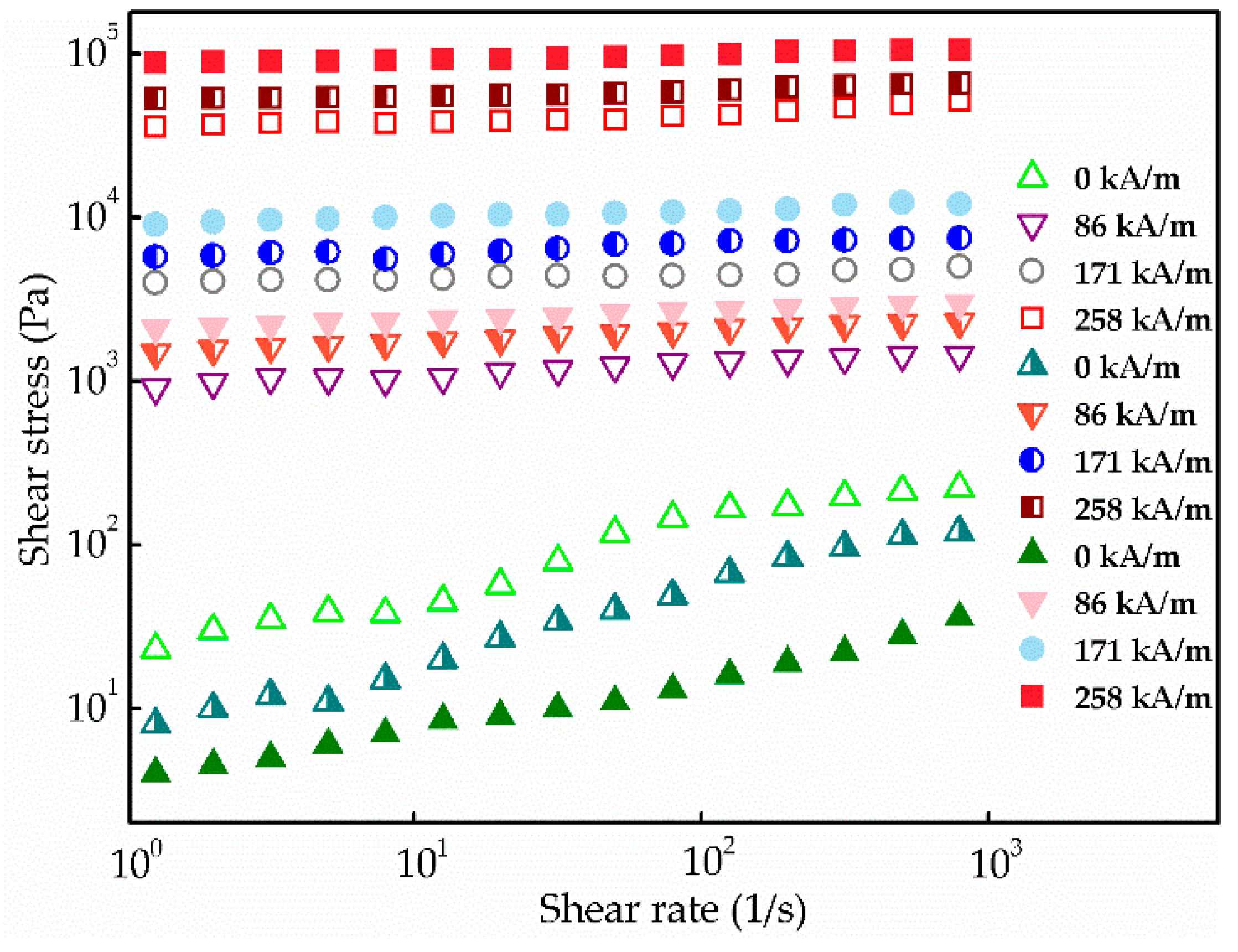
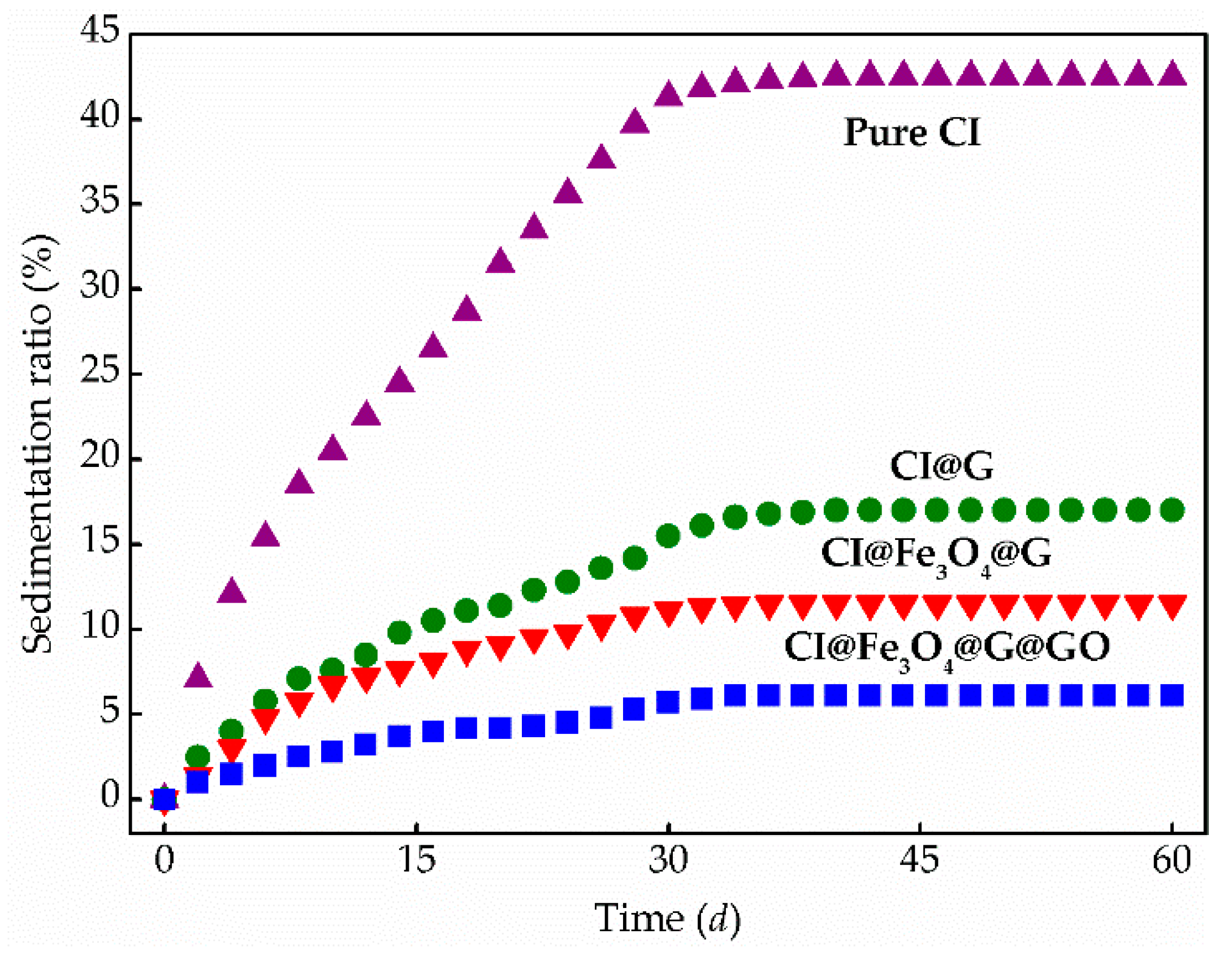
3.3. MR Behavior and Dispersion Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, B.J.; Fang, F.F.; Choi, H.J. Magnetorheology: Materials and application. Soft Matter 2010, 6, 5246–5253. [Google Scholar] [CrossRef]
- Liu, X.H.; Shi, Y.; Xu, J. Parameters tuning approach for proportion integration differentiation controller of magnetorheological fluids brake based on improved fruit fly optimization algorithm. Symmetry (Basel) 2017, 9, 12. [Google Scholar] [CrossRef]
- De Vicente, J.; Klingenberg, D.J.; Hidalgo-Alvarez, R. Magnetorheological fluids: A review. Soft Matter 2011, 7, 3701–3710. [Google Scholar] [CrossRef]
- Rwei, S.P.; Ranganathan, P.; Chiang, W.Y.; Wang, T.Y. The magnetorheological fluid of carbonyl iron suspension blended with grafted MWCNT or graphene. J. Magn. Magn. Mater. 2017, 443, 58–66. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Xuan, S.H.; Jiang, W.Q.; Gong, X.L. The normal stress of an electrorheological fluid in compression mode. RSC Adv. 2017, 7, 25855–25860. [Google Scholar] [CrossRef] [Green Version]
- Niranjan, M.; Jha, S.; Kotnala, R.K. Ball end magnetorheological finishing using bidisperse magnetorheological polishing fluid. Mater. Manuf. Process. 2014, 29, 487–492. [Google Scholar] [CrossRef]
- Pang, H.M.; Xuan, S.H.; Sun, C.L.; Gong, X.L. A novel energy absorber based on magnetorheological gel. Smart Mater. Struct. 2017, 26, 105017. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.L.; Ruan, X.H.; Xuan, S.H.; Yan, Q.F.; Deng, H.X. Magnetorheological damper working in squeeze mode. Adv. Mech. Eng. 2014, 6, 410158. [Google Scholar] [CrossRef]
- Liao, G.J.; Gong, X.L.; Xuan, S.H. Phase based stiffness tuning algorithm for a magnetorheological elastomer dynamic vibration absorber. Smart Mater. Struct. 2014, 23, 015016. [Google Scholar] [CrossRef]
- Ruan, X.H.; Wang, Y.; Xuan, S.H.; Gong, X.L. Magnetic field dependent electric conductivity of the magnetorheological fluids: The influence of oscillatory shear. Smart Mater. Struct. 2017, 26, 035067. [Google Scholar] [CrossRef]
- Zeinali, M.; Mazlan, S.A.; Abd Fatah, A.Y.; Zamzuri, H. A phenomenological dynamic model of a magnetorheological damper using a neuro-fuzzy system. Smart Mater. Struct. 2013, 22, 125013. [Google Scholar] [CrossRef]
- Rascol, E.; Daurat, M.; Da Silva, A.; Maynadier, M.; Dorandeu, C.; Charnay, C.; Garcia, M.; Lai-Kee-Him, J.; Bron, P.; Auffan, M.; et al. Biological fate of Fe3O4 core-shell mesoporous silica nanoparticles depending on particle surface chemistry. Nanomaterials 2017, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, F.; Sgarbossa, P.; Sieni, E.; Semenzato, A.; Dughiero, F.; Forzan, M.; Bertani, R.; Del Bianco, L. Synthesis of ferrofluids made of iron oxide nanoflowers: Interplay between carrier fluid and magnetic properties. Nanomaterials 2017, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilnezhad, E.; Choi, H.J.; Schaffie, M.; Gholizadeh, M.; Ranjbar, M.; Kwon, S.H. Rheological analysis of magnetite added carbonyl iron based magnetorheological fluid. J. Magn. Magn. Mater. 2017, 444, 161–167. [Google Scholar] [CrossRef]
- Liu, T.X.; Gong, X.L.; Xu, Y.G.; Xuan, S.H. Magneto-induced stress enhancing effect in a colloidal suspension of paramagnetic and superparamagnetic particles dispersed in a ferrofluid medium. Soft Matter 2014, 10, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Ketsko, V.A.; Beresnev, E.N.; Kop’eva, M.A.; Elesina, L.V.; Baranchikov, A.I.; Stognii, A.I.; Trukhanov, A.V.; Kuznetsov, N.T. Specifics of pyrohydrolytic and solid-phase syntheses of solid solutions in the (MgGa2O4)x (MgFe2O4)1−x system. Russ. J. Inorg. Chem. 2010, 55, 427–429. [Google Scholar] [CrossRef]
- Nipan, G.D.; Ketsko, V.A.; Kol’tsova, T.N.; Kop’eva, M.A.; Stognii, A.I.; Trukhanov, A.V. Subsolidus phase relations of Mg-Ga-Fe-O spinel solid solutions. Inorg. Mater. 2010, 46, 1019–1024. [Google Scholar] [CrossRef]
- Nipan, G.D.; Ketsko, V.A.; Stognij, A.I.; Trukhanov, A.V.; Kol’tsova, T.N.; Beresnev, E.N.; Kop’eva, M.A.; Elesina, L.V.; Kuznetsov, N.T. DMS solutions Mg(Fe1−xGax)2O4+delta. Doklady Phys. Chem. 2010, 430, 39–42. [Google Scholar] [CrossRef]
- Nipan, G.D.; Ketsko, V.A.; Stognij, A.I.; Trukhanov, A.V.; Kol’tsova, T.N.; Kop’eva, M.A.; Elesina, L.V.; Kuznetsov, N.T. Properties of Mg(Fe1−xGax)2O4+delta solid solutions in stable and metastable states. Inorg. Mater. 2010, 46, 429–433. [Google Scholar] [CrossRef]
- Stognij, A.I.; Trukhanov, A.V.; Ketsko, V.A.; Nipan, G.D. Properties of Mg(Fe0.8Ga0.2)2O4+delta Ceramics and Films. Inorg. Mater. 2011, 47, 204–207. [Google Scholar] [CrossRef]
- Leong, S.A.N.; Mazlan, S.A.; Samin, P.M.; Idris, A.; Ubaidillah. Performance of bidisperse magnetorheological fluids utilizing superparamagnetic maghemite nanoparticles. In Proceedings of the 6th Nanoscience and Nanotechnology Symposium (NNS), Surakarta, Indonesia, 4–5 November 2015. [Google Scholar]
- Wereley, N.M.; Chaudhuri, A.; Yoo, J.H.; John, S.; Kotha, S.; Suggs, A.; Radhakrishnan, R.; Love, B.J.; Sudarshan, T.S. Bidisperse magnetorheological fluids using Fe particles at nanometer and micron scale. J. Intell. Mater. Syst. Struct. 2006, 17, 393–401. [Google Scholar] [CrossRef]
- Patel, R. Mechanism of chain formation in nanofluid based MR fluids. J. Magn. Magn. Mater. 2011, 323, 1360–1363. [Google Scholar] [CrossRef]
- Chand, M.; Shankar, A.; Noorjahan; Jain, K.; Pant, R.P. Improved properties of bidispersed magnetorheological fluids. RSC Adv. 2014, 4, 53960–53966. [Google Scholar]
- Liu, Y.D.; Choi, H.J. Magnetorheology of core-shell typed dual-coated carbonyl iron particle fabricated by a sol-gel and self-assembly process. Mater. Res. Bull. 2015, 69, 92–97. [Google Scholar] [CrossRef]
- Fang, F.F.; Liu, Y.D.; Choi, H.J.; Seo, Y. Core-shell structured carbonyl iron microspheres prepared via dual-step functionality coatings and their magnetorheological response. ACS Appl. Mater. Interfaces 2011, 3, 3487–3495. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.F.; Choi, H.J.; Seo, Y. Sequential coating of magnetic carbonyliron particles with polystyrene and multiwalled carbon nanotubes and its effect on their magnetorheology. ACS Appl. Mater. Interfaces 2010, 2, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.F.; Choi, H.J. Fabrication of multiwalled carbon nanotube-wrapped magnetic carbonyl iron microspheres and their magnetorheology. Colloid Polym. Sci. 2010, 288, 79–84. [Google Scholar] [CrossRef]
- Wang, Z.W.; Holm, C. Structure and magnetic properties of polydisperse ferrofluids: A molecular dynamics study. Phys. Rev. E 2003, 68, 041401. [Google Scholar] [CrossRef] [PubMed]
- Rosaiah, P.; Zhu, J.H.; Hussain, O.M.; Liu, Z.G.; Qiu, Y.J. Well-dispersed rod-like LiFePO4 nanoparticles on reduced graphene oxide with excellent electrochemical performance for Li-ion batteries. J. Electroanal. Chem. 2018, 811, 1–7. [Google Scholar] [CrossRef]
- Rosaiah, P.; Zhu, J.H.; Hussain, O.M.; Qiu, Y.J. Graphenothermal reduction synthesis of MnO/RGO composite with excellent anodic behaviour in lithium ion batteries. Ceram. Int. 2018, 44, 3077–3084. [Google Scholar] [CrossRef]
- Kim, M.M.; Zydney, A.L. Effect of electrostatic, hydrodynamic, and Brownian forces on particle trajectories and sieving in normal flow filtration. J. Colloid Interface Sci. 2004, 269, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Satoh, A. 3D Monte Carlo simulations on the aggregate structures of a suspension composed of cubic hematite particles. Mol. Phys. 2018, 116, 2300–2309. [Google Scholar] [CrossRef]
- Liu, H.M.; Chan, T.L. Two-component aerosol dynamic simulation using differentially weighted operator splitting Monte Carlo method. Appl. Math. Model. 2018, 62, 237–253. [Google Scholar] [CrossRef]
- Liu, X.H.; Wang, L.F.; Lu, H.; Wang, D.D.; Chen, Q.Q.; Wang, Z.B. A study of the effect of nanometer Fe3O4 addition on the properties of silicone oil-based magnetorheological fluids. Mater. Manuf. Process. 2015, 30, 204–209. [Google Scholar] [CrossRef]
- Ahn, W.J.; Jung, H.S.; Kwon, S.H.; Hong, C.H.; Choi, H.J. Effect of surface treatment on magnetorheological characteristics of core-shell structured soft magnetic carbonyl iron particles. Colloid Polym. Sci. 2015, 293, 2647–2654. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Yao, J.; Zhao, H.; Zhao, G.; Wan, Z.; Qiu, Y. Bidisperse Magnetic Particles Coated with Gelatin and Graphite Oxide: Magnetorheology, Dispersion Stability, and the Nanoparticle-Enhancing Effect. Nanomaterials 2018, 8, 714. https://doi.org/10.3390/nano8090714
Fu Y, Yao J, Zhao H, Zhao G, Wan Z, Qiu Y. Bidisperse Magnetic Particles Coated with Gelatin and Graphite Oxide: Magnetorheology, Dispersion Stability, and the Nanoparticle-Enhancing Effect. Nanomaterials. 2018; 8(9):714. https://doi.org/10.3390/nano8090714
Chicago/Turabian StyleFu, Yu, Jianjun Yao, Honghao Zhao, Gang Zhao, Zhenshuai Wan, and Ying Qiu. 2018. "Bidisperse Magnetic Particles Coated with Gelatin and Graphite Oxide: Magnetorheology, Dispersion Stability, and the Nanoparticle-Enhancing Effect" Nanomaterials 8, no. 9: 714. https://doi.org/10.3390/nano8090714




