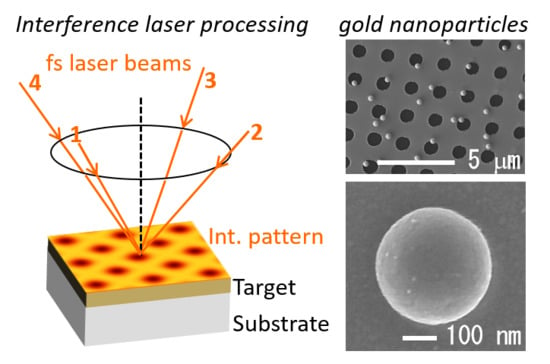
Local Melting of Gold Thin Films by Femtosecond Laser-Interference Processing to Generate Nanoparticles on a Source Target
Abstract
:1. Introduction
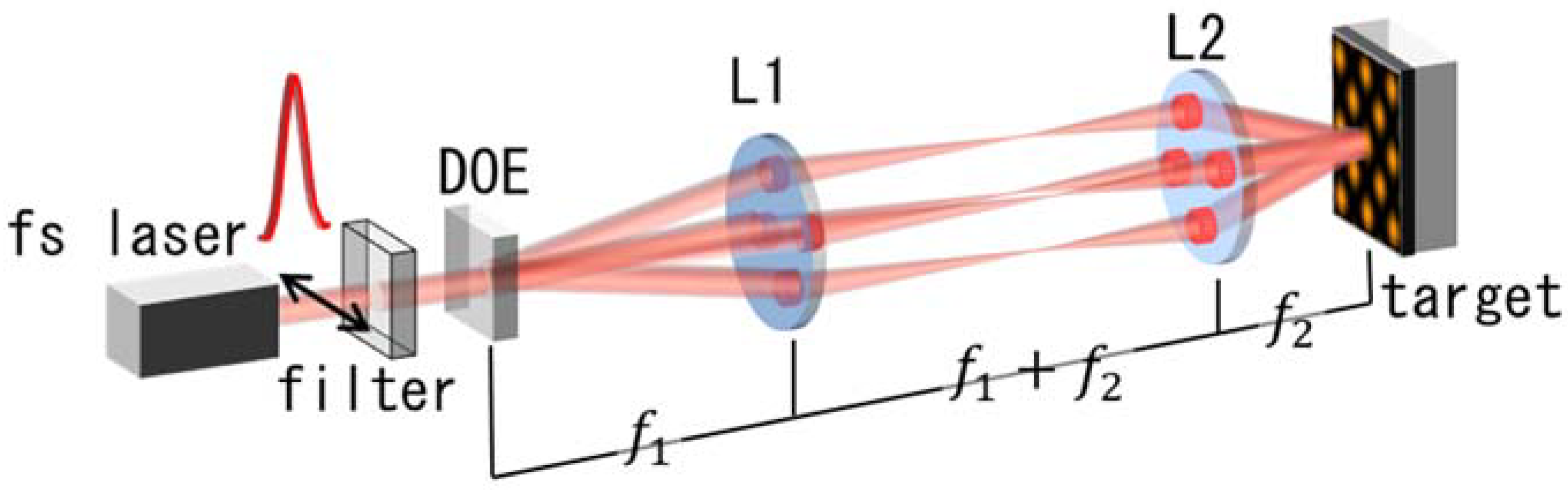
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet Chemical Synthesis of High Aspect Ratio Cylindrical Gold Nanorods. J. Phys. Chem. B 2001, 105, 4065–4067. [Google Scholar] [CrossRef]
- Silver, P.; Pietrobon, B.; Mceachran, M.; Kitaev, V. Synthesis of Size-Controlled Faceted Tunable Plasmonic Properties and Self- Assembly of These Nanorods. ACS Nano 2009, 3, 21–26. [Google Scholar] [CrossRef]
- Okumura, M.; Tsubota, S.; Iwamoto, M.; Haruta, M. Chemical Vapor Deposition of Gold Nanoparticles on MCM-41 and Their Catalytic Activities for the Low-temperature Oxidation of CO and of H2. Chem. Lett. 1998, 27, 315–316. [Google Scholar] [CrossRef]
- Vieu, C.; Carcenac, F.; Pepin, A.; Chen, Y.; Mejias, M.; Lebib, A.; Manin-Ferlazzo, L.; Couraud, L.; Launois, H. Electron beam lithography: Resolution limits and applications. Appl. Surf. Sci. 2000, 164, 111–117. [Google Scholar] [CrossRef]
- Guczi, L.; Horváth, D.; Pászti, Z.; Tóth, L.; Horváth, Z.E.; Karacs, A.; Petõ, G. Modeling Gold Nanoparticles: Morphology, Electron Structure, and Catalytic Activity in CO Oxidation. J. Phys. Chem. B 2000, 104, 3183–3193. [Google Scholar] [CrossRef]
- Muramoto, J.; Sakamoto, I.; Nakata, Y.; Okada, T.; Maeda, M. Influence of electric field on the behavior of Si nanoparticles generated by laser ablation. Appl. Phys. Lett. 1999, 75, 751–753. [Google Scholar] [CrossRef]
- Uetsuhara, H.; Goto, S.; Nakata, Y.; Vasa, N.; Okada, T.; Maeda, M. Fabrication of a Ti:sapphire planar waveguide by Pulsed Laser Deposition. Appl. Phys. A Mater. Sci. Process. 1999, 69, S719–S722. [Google Scholar] [CrossRef]
- Henley, S.J.; Carey, J.D.; Silva, S.R.P. Pulsed-laser-induced nanoscale island formation in thin metal-on-oxide films. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 72, 195408. [Google Scholar] [CrossRef]
- Mäder, M.; Höche, T.; Gerlach, J.W.; Böhme, R.; Zimmer, K.; Rauschenbach, B. Large area metal dot matrices made by diffraction mask projection laser ablation. Phys. Status Solidi Rapid Res. Lett. 2008, 2, 34–36. [Google Scholar] [CrossRef]
- Höche, T.; Böhme, R.; Gerlach, J.W.; Rauschenbach, B.; Syrowatka, F. Nanoscale laser patterning of thin gold films. Philos. Mag. Lett. 2006, 86, 661–667. [Google Scholar] [CrossRef]
- Nakata, Y.; Okada, T.; Maeda, M. Holographic fabrication of micron structures using interfered femtosecond laser beams split by diffractive optics. Proc. SPIE Int. Soc. Opt. Eng. 2003, 4977. [Google Scholar] [CrossRef]
- Nakata, Y.; Okada, T.; Maeda, M. Nano-sized hollow bump array generated by single femtosecond laser pulse. Jpn. J. Appl. Phys. 2003, 42, L1452–L1454. [Google Scholar] [CrossRef]
- Nakata, Y.; Momoo, K.; Hiromoto, T.; Miyanaga, N. Generation of superfine structure smaller than 10 nm by interfering femtosecond laser processing. In Proceedings of the SPIE the International Society for Optical Engineering, San Francisco, CA, USA, 22–27 January 2011; Volume 7920. [Google Scholar]
- Nakata, Y.; Tsuchida, K.; Miyanaga, N.; Furusho, H. Liquidly process in femtosecond laser processing. Appl. Surf. Sci. 2009, 255, 9761–9763. [Google Scholar] [CrossRef]
- Nakata, Y. Frozen water drops in the nanoworld. SPIE Newsroom 2009, 2, 1–2. [Google Scholar] [CrossRef]
- Nakata, Y.; Miyanaga, N.; Momoo, K.; Hiromoto, T. Solid-liquid-solid process for forming free-standing gold nanowhisker superlattice by interfering femtosecond laser irradiation. Appl. Surf. Sci. 2013, 274, 27–32. [Google Scholar] [CrossRef]
- Nakata, Y.; Miyanaga, N.; Momoo, K.; Hiromoto, T. Template free synthesis of free-standing silver nanowhisker and nanocrown superlattice by interfering femtosecond laser irradiation Template free synthesis of free-standing silver nanowhisker and nanocrown superlattice by interfering femtosecond laser irradiation. Jpn. J. Appl. Phys. 2014, 53, 096701. [Google Scholar]
- Wagner, R.S.; Ellis, W.C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Narazaki, A.; Sato, T.; Kurosaki, R.; Kawaguchi, Y.; Niino, H. Nano- and microdot array formation of FeSi2 by nanosecond excimer laser-induced forward transfer. Appl. Phys. Express 2008, 1, 0570011–0570013. [Google Scholar] [CrossRef]
- Narazaki, A.; Sato, T.; Kurosaki, R.; Kawaguchi, Y.; Niino, H. Nano- and microdot array formation by laser-induced dot transfer. Appl. Surf. Sci. 2009, 255, 9703–9706. [Google Scholar] [CrossRef]
- Kuznetsov, A.I.; Evlyukhin, A.B.; Reinhardt, C.; Seidel, A.; Kiyan, R.; Cheng, W.; Ovsianikov, A.; Chichkov, B.N. Laser-induced transfer of metallic nanodroplets for plasmonics and metamaterial applications. J. Opt. Soc. Am. B 2009, 26, B130. [Google Scholar] [CrossRef]
- Willis, D.A.; Grosu, V. Microdroplet deposition by laser-induced forward transfer. Appl. Phys. Lett. 2005, 86, 1–3. [Google Scholar] [CrossRef]
- Levene, M.L.; Scott, R.D.; Siryj, B.W. Material Transfer Recording. Appl. Opt. 1970, 9, 2260. [Google Scholar] [CrossRef] [PubMed]
- Bohandy, J. Metal deposition from a supported metal film using an excimer laser. J. Appl. Phys. 1986, 60, 10–12. [Google Scholar] [CrossRef]
- Nakata, Y.; Okada, T. Time-resolved microscopic imaging of the laser-induced forward transfer process. Appl. Phys. A Mater. Sci. Process. 1999, 69, S275–S278. [Google Scholar] [CrossRef]
- Jain, P.; Lee, K.; El-Sayed, I.; El-Sayed, M. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhigilei, L.V. Nanocrystalline and Polyicosahedral Structure of a Nanospike Generated on Metal Surface Irradiated by a Single Femtosecond Laser Pulse. J. Phys. Chem. C 2016, 120, 4438–4447. [Google Scholar] [CrossRef]
- Ivanov, D.S.; Lin, Z.; Rethfeld, B.; O’Connor, G.M.; Glynn, T.J.; Zhigilei, L.V. Nanocrystalline structure of nanobump generated by localized photoexcitation of metal film. J. Appl. Phys. 2010, 107. [Google Scholar] [CrossRef]
- Clasen, C.; Bico, J.; Entov, V.M.; McKinley, G.H. “Gobbling drops”: The jettingdripping transition in flows of polymer solutions. J. Fluid Mech. 2009, 636, 5–40. [Google Scholar] [CrossRef]
- Zhigunov, D.M.; Evlyukhin, A.B.; Shalin, A.S.; Zywietz, U.; Chichkov, B.N. Femtosecond Laser Printing of Single Ge and SiGe Nanoparticles with Electric and Magnetic Optical Resonances. ACS Photonics 2018, 5, 977–983. [Google Scholar] [CrossRef]

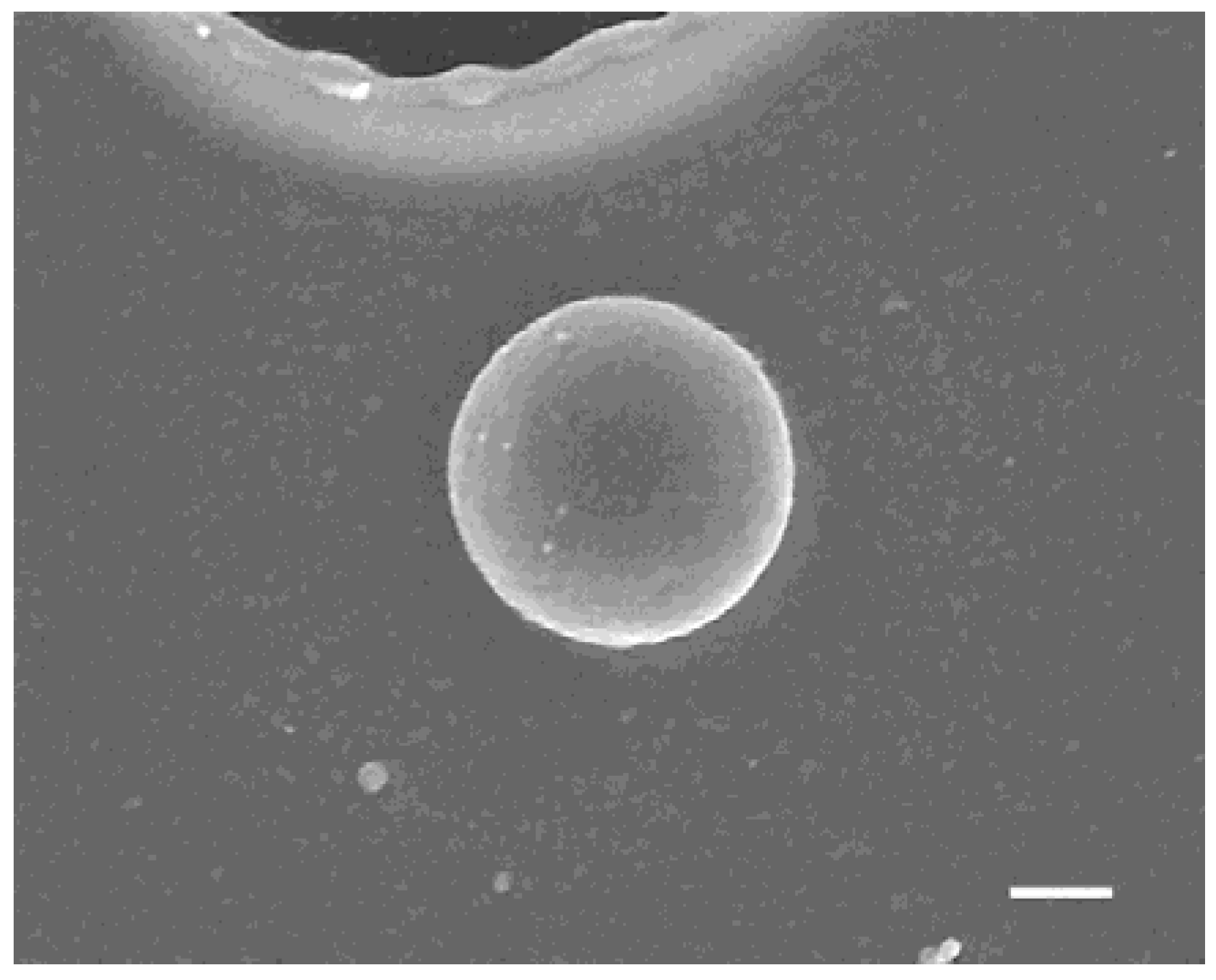
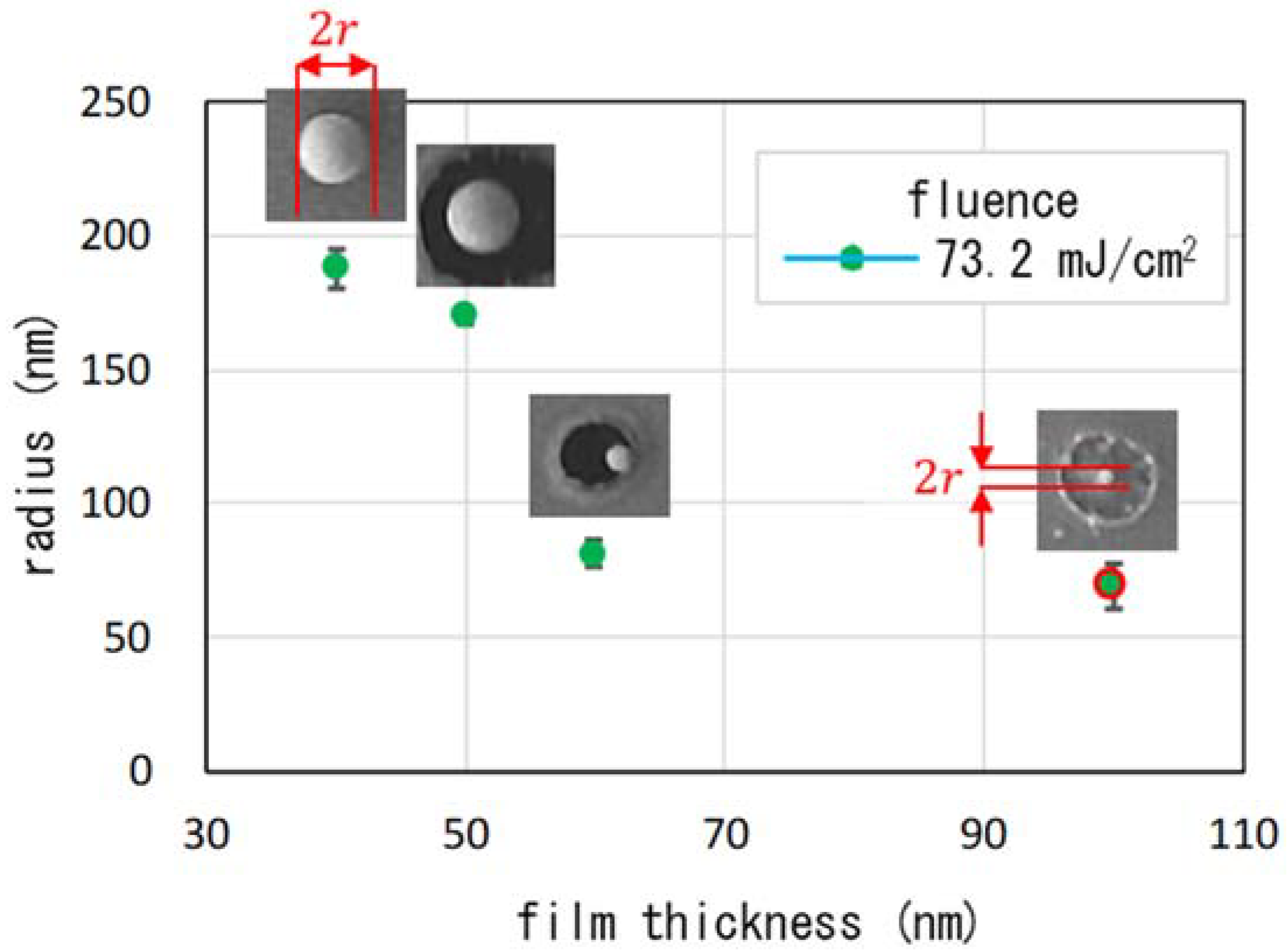
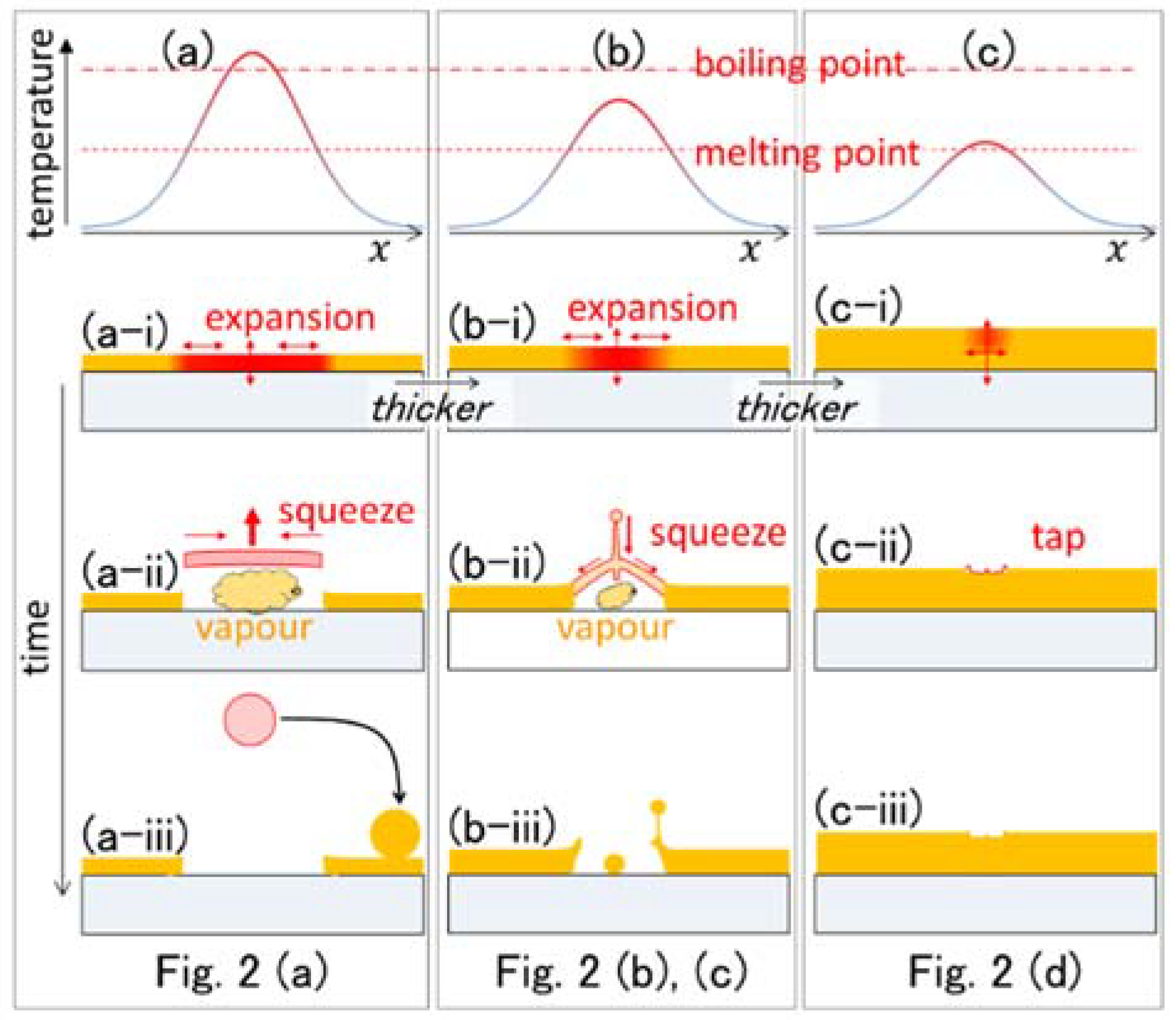

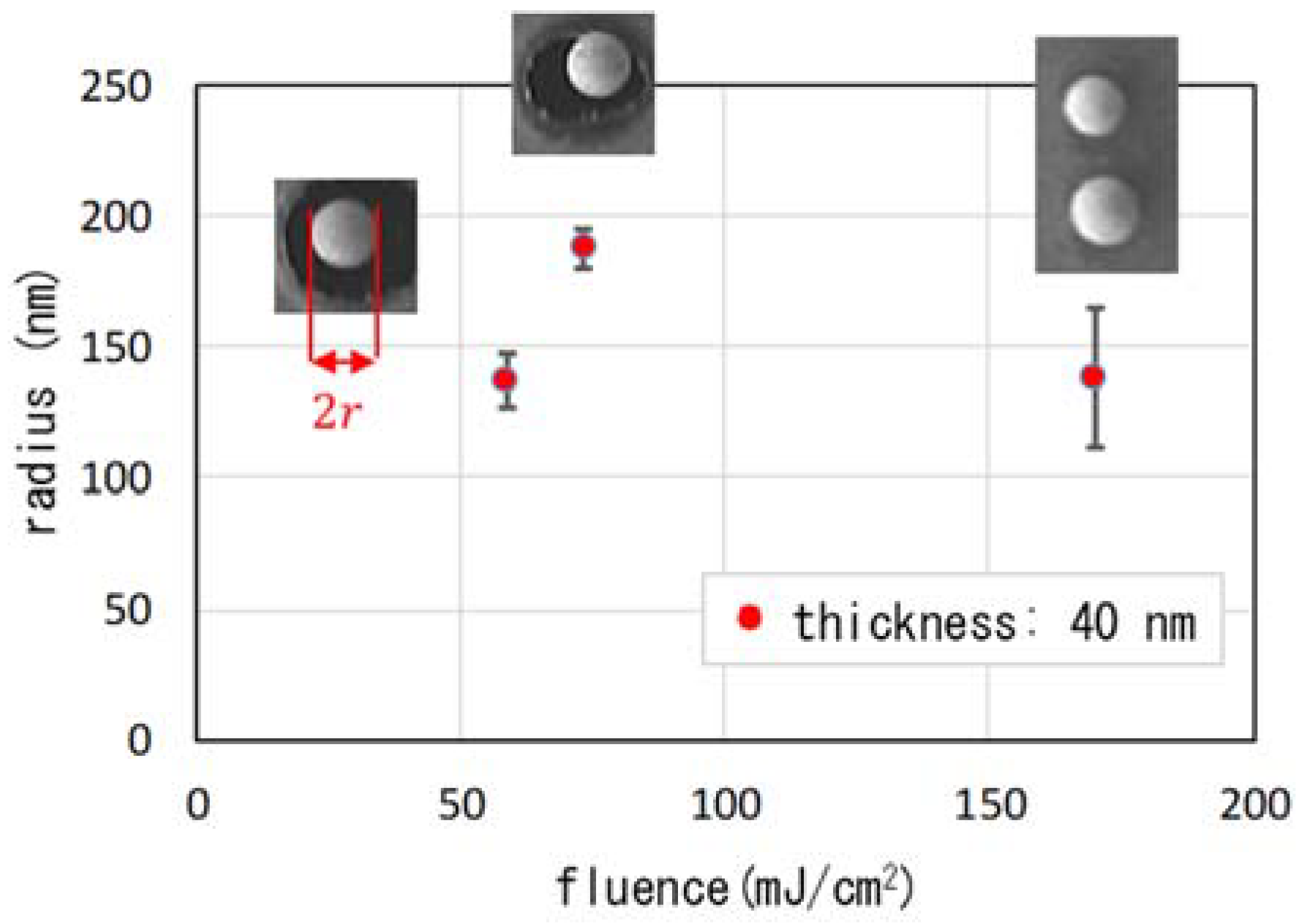
| Parameter | Figure 2(a-1,a-2), Figure 3 | Figure 2(b-1,b-2) | Figure 2(c-1,c-2) | Figure 2(d-1,d-2) | Figure 6(a-1,a-2) | Figure 6(b-1,b-2) | Figure 6(c-1,c-2) |
|---|---|---|---|---|---|---|---|
| film thickness (nm) | 40 | 50 | 60 | 100 | 30 | 40 | 40 |
| fluence (mJ/cm2) | 73.2 | 73.2 | 73.2 | 73.2 | 58.6 | 58.6 | 169.9 |
| averaged radius (nm) | 188 | 170 | 82 | 69 | n.d. | 137 | 138 |
| standard d. (nm) | 7 | 3 | 5 | 9 | n.d. | 10 | 27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakata, Y.; Murakawa, K.; Miyanaga, N.; Narazaki, A.; Shoji, T.; Tsuboi, Y. Local Melting of Gold Thin Films by Femtosecond Laser-Interference Processing to Generate Nanoparticles on a Source Target. Nanomaterials 2018, 8, 477. https://doi.org/10.3390/nano8070477
Nakata Y, Murakawa K, Miyanaga N, Narazaki A, Shoji T, Tsuboi Y. Local Melting of Gold Thin Films by Femtosecond Laser-Interference Processing to Generate Nanoparticles on a Source Target. Nanomaterials. 2018; 8(7):477. https://doi.org/10.3390/nano8070477
Chicago/Turabian StyleNakata, Yoshiki, Keiichi Murakawa, Noriaki Miyanaga, Aiko Narazaki, Tatsuya Shoji, and Yasuyuki Tsuboi. 2018. "Local Melting of Gold Thin Films by Femtosecond Laser-Interference Processing to Generate Nanoparticles on a Source Target" Nanomaterials 8, no. 7: 477. https://doi.org/10.3390/nano8070477