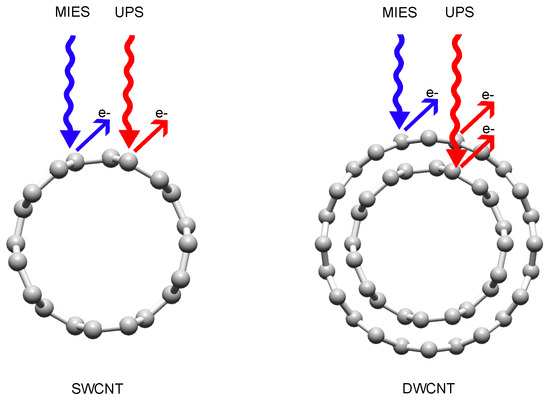
Measuring the Density of States of the Inner and Outer Wall of Double-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
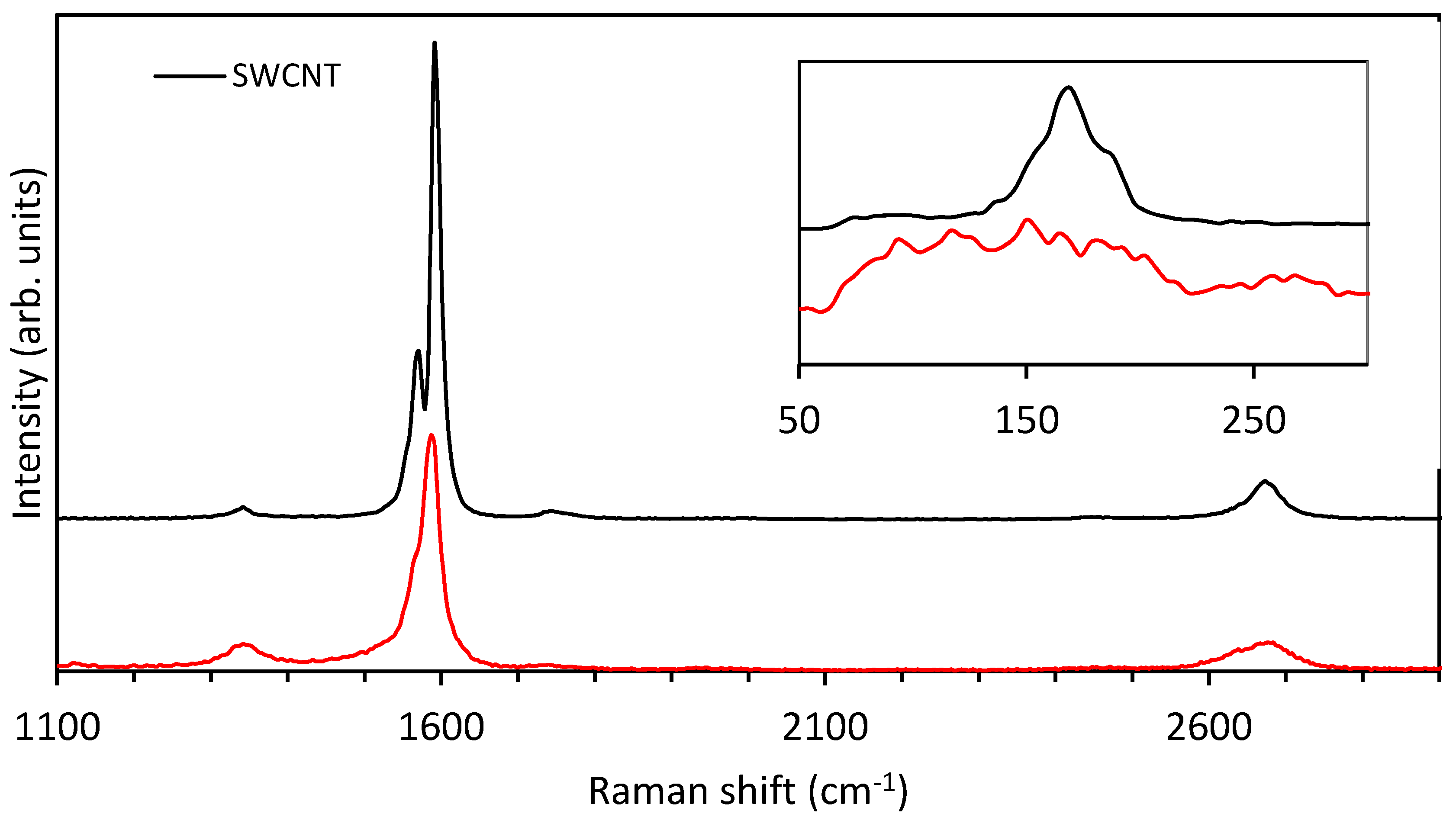
3.1. Raman Spectroscopy
3.2. Analysis of Ultraviolet Photoelectron (UP) and Metastable Helium Induced Electron (MIE) Spectra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boehm, H.P. The first observation of carbon nanotubes. Carbon 1997, 35, 581–584. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Ando, T. The electronic properties of graphene and carbon nanotubes. NPG Asia Mater. 2009, 1, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Brukh, R.; Mitra, S. Mechanism of carbon nanotube growth by CVD. Chem. Phys. Lett. 2006, 424, 126–132. [Google Scholar] [CrossRef]
- Reddy, N.K.; Meunier, J.-L.; Coulombe, S. Growth of carbon nanotubes directly on a nickel surface by thermal CVD. Mater. Lett. 2006, 60, 3761–3765. [Google Scholar] [CrossRef]
- Baddour, C.E.; Fadlallah, F.; Nasuhoglu, D.; Mitra, R.; Vandsburger, L.; Meunier, J.-L. A simple thermal CVD method for carbon nanotube synthesis on stainless steel 304 without the addition of an external catalyst. Carbon 2009, 47, 313–318. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Chrzanowska, J.; Hoffman, J.; Małolepszy, A.; Mazurkiewicz, M.; Kowalewski, T.A.; Szymanski, Z.; Stobinski, L. Synthesis of carbon nanotubes by the laser ablation method: Effect of laser wavelength. Phys. Status Solidi B 2015, 252, 1860–1867. [Google Scholar] [CrossRef] [Green Version]
- Muramatsu, H.; Kim, Y.A.; Hayashi, T.; Endo, M.; Yonemoto, A.; Arikai, H.; Okino, F.; Touhara, H. Fluorination of double-walled carbon nanotubes. Chem. Commun. 2005, 2002–2004. [Google Scholar] [CrossRef] [PubMed]
- Marcolongo, G.; Ruaro, G.; Gobbo, M.; Meneghetti, M. Amino acid functionalization of double-wall carbon nanotubes studied by Raman spectroscopy. Chem. Commun. 2007, 4925–4927. [Google Scholar] [CrossRef]
- Colomer, J.F.; Marega, R.; Traboulsi, H.; Meneghetti, M.; van Tendeloo, G.; Bonifazi, D. Microwave-Assisted Bromination of Double-Walled Carbon Nanotubes. Chem. Mater. 2009, 21, 4747–4749. [Google Scholar] [CrossRef]
- Hayashi, T.; Shimamoto, D.; Kim, Y.A.; Muramatsu, H.; Okino, F.; Touhara, H.; Shimada, T.; Miyauchi, Y.; Maruyama, S.; Terrones, M.; et al. Selective Optical Property Modification of Double-Walled Carbon Nanotubes by Fluorination. ACS Nano 2008, 2, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Bulusheva, L.G.; Okotrub, A.V.; Flahaut, E.; Asanov, I.P.; Gevko, P.N.; Koroteev, V.O.; Fedoseeva, Y.V.; Yaya, A.; Ewels, C.P. Bromination of Double-Walled Carbon Nanotubes. Chem. Mater. 2012, 24, 2708–2715. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yamamoto, S.; Mukai, K.; Yoshinobu, J.; Harada, Y.; Tokushima, T.; Takeuchi, T.; Takata, Y.; Shin, S.; Akagi, K.; et al. Direct observation of site-specific valence electronic structure at the SiO2/Si interface. Phys. Rev. B 2006, 73, 045336. [Google Scholar] [CrossRef]
- Chandra, A.; Andersona, G.; Melkote, S.; Gao, W.; Haitjema, H.; Wegener, K. Role of surfaces and interfaces in solar cell manufacturing. CIRP Ann. 2014, 63, 797–819. [Google Scholar] [CrossRef]
- Llinas, J.P.; Fairbrother, A.; Barin, G.B.; Shi, W.; Lee, K.; Wu, S.; Choi, B.Y.; Braganza, R.; Lear, J.; Kau, N.; et al. Short-channel field-effect transistors with 9-atom and 13-atom wide graphene nanoribbons. Nat. Commun. 2017, 8, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; Li, J.; Feng, Y. Carbon Nanotubes for Supercapacitor. Nanoscale Res. Lett. 2010, 5, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Morgner, H. The quantitative characterization of liquid and solid surfaces with metastable helium atoms. AIP Conf. Proc. 2000, 500, 687–698. [Google Scholar]
- Ayala, P.; Miyata, Y.; de Blauwe, K.; Shiozawa, H.; Feng, Y.; Yanagi, K.; Kramberger, C.; Silva, S.R.P.; Follath, R.; Kataura, H.; et al. Disentanglement of the electronic properties of metallicity-selected single-walled carbon nanotubes. Phys. Rev. B 2009, 80, 205427. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Sauer, M.; Saito, T.; Sato, Y.; Suenaga, K.; Pichler, T.; Shiozawa, H. Doping of single-walled carbon nanotubes controlled via chemical transformation of encapsulated nickelocene. Nanoscale 2015, 7, 1383–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-Q.; Sherwood, P.M.A. Studies of Carbon Nanotubes and Fluorinated Nanotubes by X-ray and Ultraviolet Photoelectron Spectroscopy. Chem. Mater. 2004, 16, 5427–5436. [Google Scholar] [CrossRef]
- Seah, M.P.; Dench, W.A. Quantitative electron spectroscopy of surfaces: A standard data base for electron inelastic mean free paths in solids. Surf. Interface Anal. 1979, 1, 2–11. [Google Scholar] [CrossRef]
- Itkis, M.E.; Perea, D.E.; Niyogi, S.; Rickard, S.M.; Hamon, M.A.; Hu, H.; Zhao, B.; Haddon, R.C. Purity Evaluation of As-Prepared Single-Walled Carbon Nanotube Soot by Use of Solution-Phase Near-IR Spectroscopy. Nano Lett. 2003, 3, 309–314. [Google Scholar] [CrossRef]
- Chambers, B.A.; Neumann, C.; Turchanin, A.; Gibson, C.T.; Andersson, G.G. The direct measurement of the electronic density of states of graphene using metastable induced electron spectroscopy. 2D Mater. 2017, 4, 025068. [Google Scholar] [CrossRef]
- Hagstrum, H.D. Excited-Atom Deexcitation Spectroscopy Using Incident Ions. Phys. Rev. Lett. 1979, 43, 1050–1053. [Google Scholar] [CrossRef]
- Ellis, A.V.; Al-deen, A.; Dalal, H.; Andersson, G.G. Structural Determination of Thermally and Hydrazine Treated Graphene Oxide Using Electron Spectroscopic Analysis. J. Phys. Chem. C 2013, 117, 21312–21319. [Google Scholar] [CrossRef]
- Morgner, H. The Characterization of Liquid and Solid Surfaces with Metastable Helium Atoms. In Advances in Atomic, Molecular, and Optical Physics; Benjamin, B., Herbert, W., Eds.; Academic Press: Cambridge, MA, USA, 2000; pp. 387–488. [Google Scholar]
- Chambers, B.A.; Notarianni, M.; Liu, J.; Motta, N.; Andersson, G.G. Examining the electrical and chemical properties of reduced graphene oxide with varying annealing temperatures in argon atmosphere. Appl. Surf. Sci. 2015, 356, 719–725. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Saito, R. Characterizing Graphene, Graphite, and Carbon Nanotubes by Raman Spectroscopy. Annu. Rev. Condens. Matter Phys. 2010, 1, 89–108. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Cui, J.; Pötschke, P.; Voit, B. Dispersion of pristine single-walled carbon nanotubes using pyrene-capped polystyrene and its application for preparation of polystyrene matrix composites. Carbon 2010, 48, 2603–2612. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Araujo, P.T.; Maciel, I.O.; Pesce, P.B.C.; Pimenta, M.A.; Doorn, S.K.; Qian, H.; Hartschuh, A.; Steiner, M.; Grigorian, L.; Hata, K.; et al. Nature of the constant factor in the relation between radial breathing mode frequency and tube diameter for single-wall carbon nanotubes. Phys. Rev. B 2008, 77, 241403. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A.; Filho, A.G.S.; Saito, R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- Pfeiffer, R.; Kuzmany, H.; Simon, F.; Bokova, S.N.; Obraztsova, E. Resonance Raman scattering from phonon overtones in double-wall carbon nanotubes. Phys. Rev. B 2005, 71, 155409. [Google Scholar] [CrossRef]
- Souza Filho, A.G.; Jorio, A.; Samsonidze, G.G.; Dresselhaus, G.; Pimenta, M.A.; Dresselhaus, M.S.; Swan, A.K.; Ünlü, M.S.; Goldberg, B.B.; Saito, R. Competing spring constant versus double resonance effects on the properties of dispersive modes in isolated single-wall carbon nanotubes. Phys. Rev. B 2003, 67, 035427. [Google Scholar] [CrossRef]
- Jorio, A.; Fantini, C.; Dantas, M.S.S.; Pimenta, M.A.; Filho, A.G.S.; Samsonidze, G.G.; Brar, V.W.; Dresselhaus, G.; Dresselhaus, M.S.; Swan, A.K.; et al. Linewidth of the Raman features of individual single-wall carbon nanotubes. Phys. Rev. B 2002, 66, 115411. [Google Scholar] [CrossRef]
- Moore, K.E.; Flavel, B.S.; Ellis, A.V.; Shapter, J.G. Comparison of double-walled with single-walled carbon nanotube electrodes by electrochemistry. Carbon 2011, 49, 2639–2647. [Google Scholar] [CrossRef]
- Luo, Z.; Shang, J.; Lim, S.; Li, D.; Xiong, Q.; Shen, Z.; Lin, J.; Yu, T. Modulating the electronic structures of graphene by controllable hydrogenation. Appl. Phys. Lett. 2010, 97, 233111. [Google Scholar] [CrossRef]
- Bianconi, A.; Hagström, S.B.M.; Bachrach, R.Z. Photoemission studies of graphite high-energy conduction-band and valence-band states using soft-X-ray synchrotron radiation excitation. Phys. Rev. B 1977, 16, 5543–5548. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chambers, B.A.; Shearer, C.J.; Yu, L.; Gibson, C.T.; Andersson, G.G. Measuring the Density of States of the Inner and Outer Wall of Double-Walled Carbon Nanotubes. Nanomaterials 2018, 8, 448. https://doi.org/10.3390/nano8060448
Chambers BA, Shearer CJ, Yu L, Gibson CT, Andersson GG. Measuring the Density of States of the Inner and Outer Wall of Double-Walled Carbon Nanotubes. Nanomaterials. 2018; 8(6):448. https://doi.org/10.3390/nano8060448
Chicago/Turabian StyleChambers, Benjamin A., Cameron J. Shearer, LePing Yu, Christopher T. Gibson, and Gunther G. Andersson. 2018. "Measuring the Density of States of the Inner and Outer Wall of Double-Walled Carbon Nanotubes" Nanomaterials 8, no. 6: 448. https://doi.org/10.3390/nano8060448