3.1. Morphological Properties of Al2O3-FTO Nanocomposites
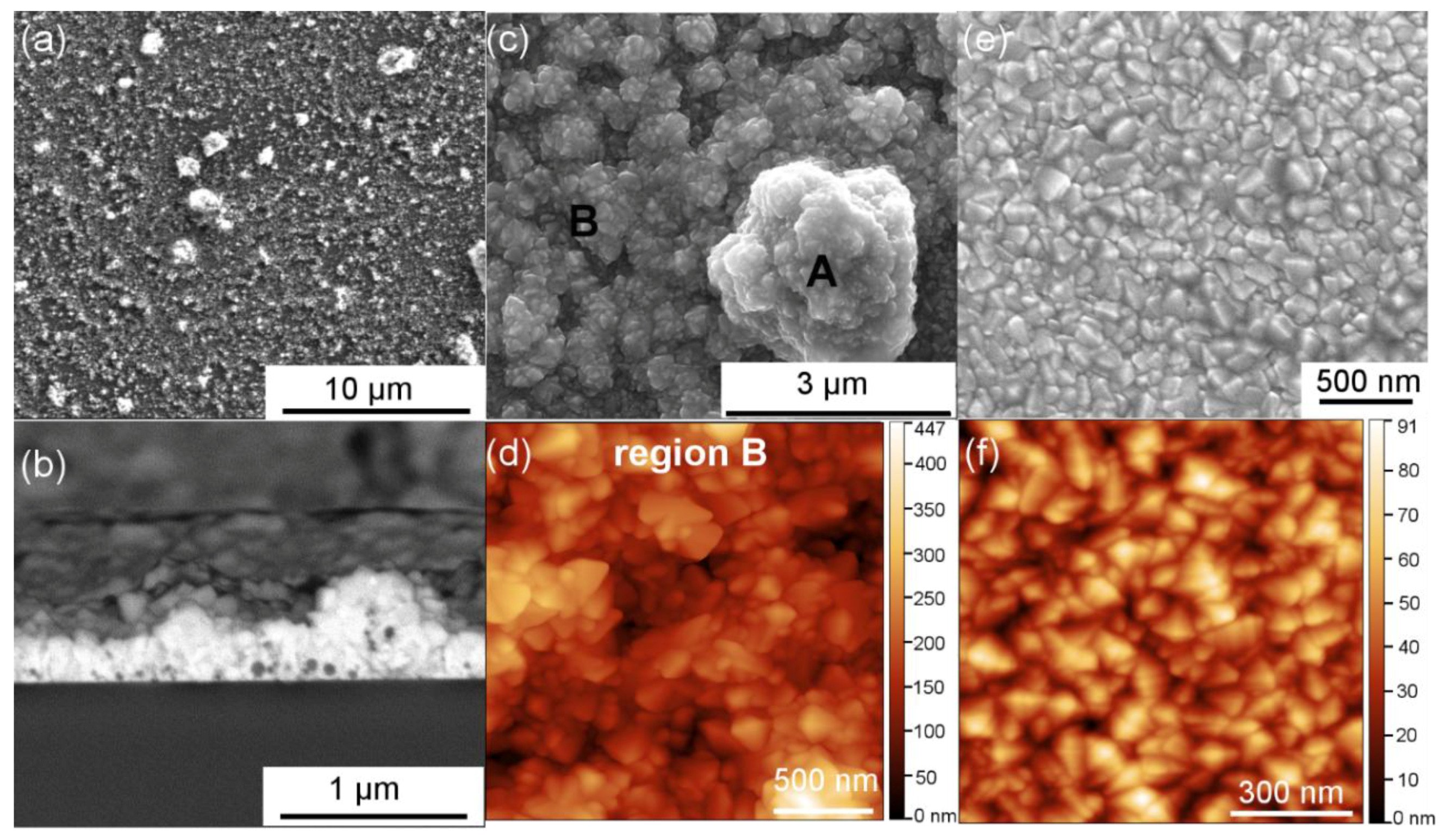
As one sees in
Figure 1a, after the first step where the Al
2O
3 nanoparticle suspension was spin-coated on the glass substrate. The nanoparticles (appearing in bright contrast) self-assembled into agglomerates of random sizes and did not cover the entire glass surface. Then in the second step, a thin film of FTO with a thickness of ~300 nm was deposited using ultrasonic spray pyrolysis, uniformly covering the nanoparticle agglomerates, as evidenced in
Figure 1b. The surface of Al
2O
3-FTO nanocomposites is fairly rough, as one sees in
Figure 1c. Contrarily, the surface of a reference FTO (i.e., FTO film deposited directly on the bare glass substrate without any nanoparticles) shown in
Figure 1e,f appears smooth and flat. The main structural and electrical properties of flat FTOs have already been investigated and discussed in previous articles [
16,
17,
18]. Similar to S:TiO
2-FTO nanocomposites reported in [
11], Al
2O
3-FTO nanocomposites also show two regions: rougher region A where the FTO film was deposited on larger nanoparticle agglomerates and semi-flat region B where small nanoparticle agglomerates are present, thus resulting in higher surface roughness than a reference flat FTO (cf. compare
Figure 1d,f with particular focus on the z-scale).
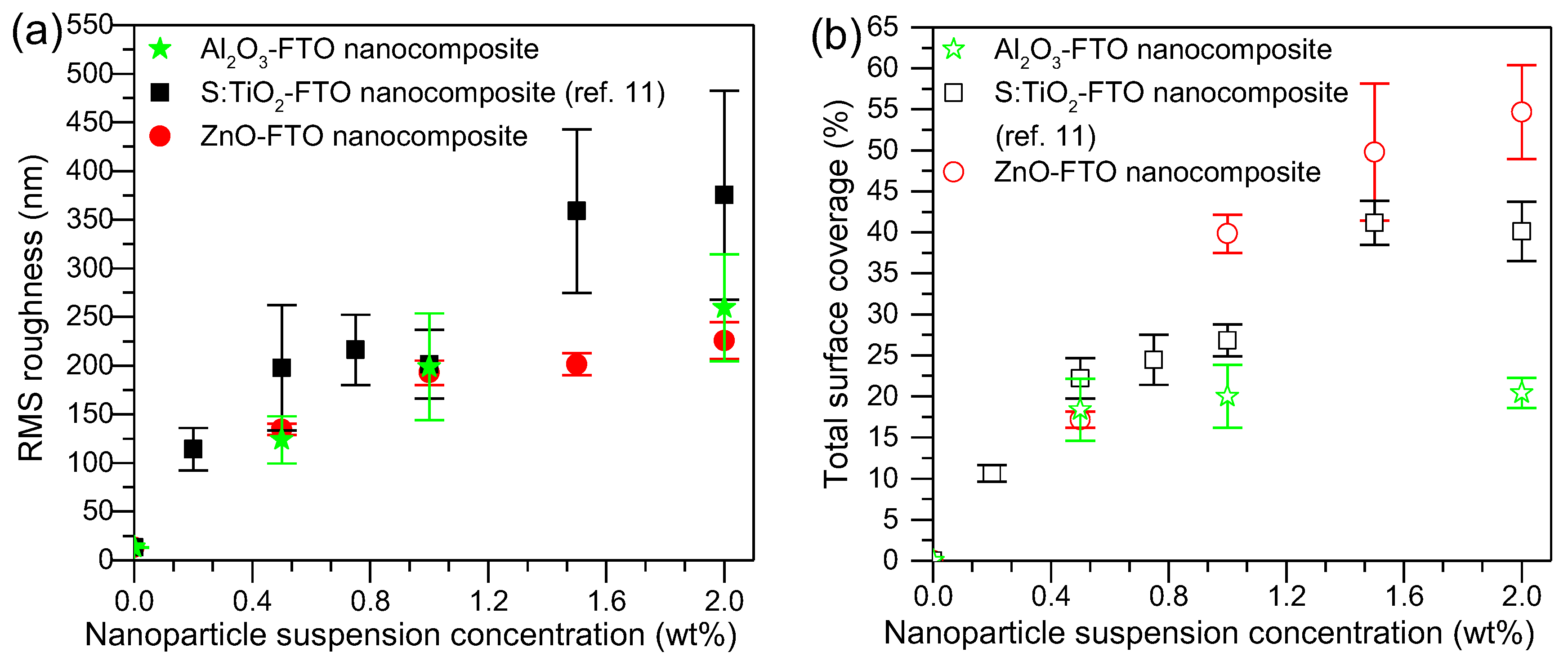
Figure 2a shows the root-mean-square (RMS) roughness of Al
2O
3-FTO nanocomposites, which was calculated from AFM images (40 × 40 μm
2 with resolution of 40 nm per pixel) in the same manner as in [
11]. The roughness of S:TiO
2-FTO nanocomposites (as in [
11]) and ZnO-FTO (recalculated in the same way as in this study to enable a fair comparison, and thus different from that of [
10] are also plotted for comparison. With increasing nanoparticle suspension concentration, the RMS roughness of Al
2O
3-FTO nanocomposites increases, being comparable with that of ZnO-FTO nanocomposites and lower than that of S:TiO
2-FTO nanocomposites.
In addition, another important parameter that can be obtained from AFM images is the total surface coverage, defined as the percentage of the area occupied by all nanoparticle agglomerates divided by the total image area. As seen in
Figure 2b, with increasing nanoparticle suspension concentration, the total surface coverage of Al
2O
3-FTO reaches a plateau near 20%, as opposed to what was observed for ZnO-FTO and S:TiO
2-FTO nanocomposites, for which total surface coverage increases constantly with nanoparticle suspension concentration. In order to get an in-depth understanding of surface morphologies characteristic of Al
2O
3-FTO nanocomposites, the size distribution of the nanoparticle agglomerates was statistically analyzed and compared to that of ZnO-FTO and S:TiO
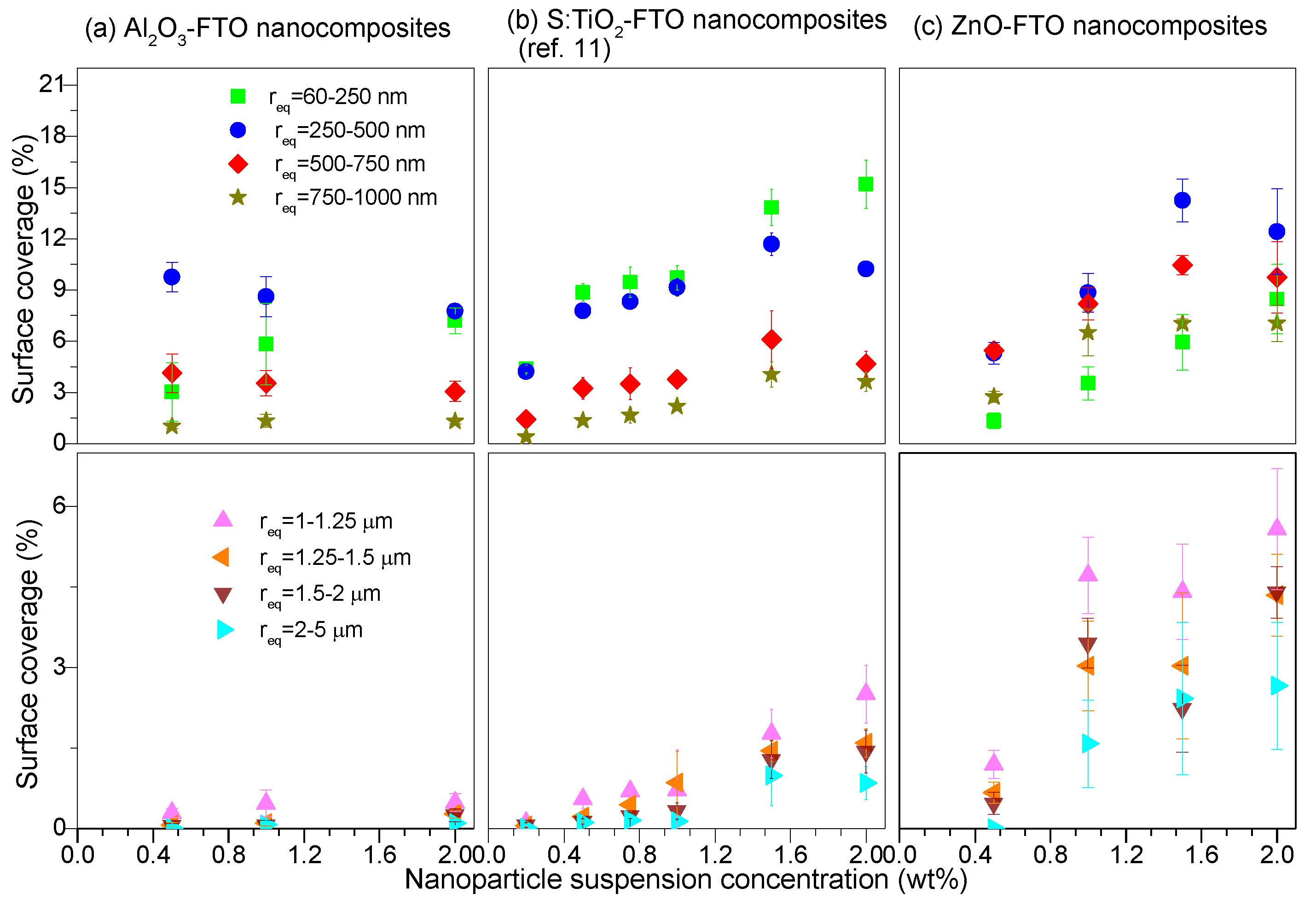
2-FTO nanocomposites in
Figure 3. The nanoparticle agglomerates are divided into eight groups based on their equivalent radius r
eq (defined as the effective radius of a circle whose area is equivalent to the projected area of the nanoparticle agglomerate): 60–250 nm, 250–500 nm, 500–750 nm, 750–1000 nm, 1–1.25 μm, 1.25–1.5 μm, 1.5–2 μm, and 2–5 μm. The surface coverage associated to each group can then be deduced as plotted in
Figure 3.
For the sake of clarity, the latter four groups corresponding to larger nanoparticle agglomerates (r
eq > 1 μm) are drawn separately from the former four groups (smaller nanoparticle agglomerates with r
eq < 1 μm). It should be noted that we have not taken into account the nanoparticle agglomerates with r
eq < 60 nm as they are out of the resolution of the AFM images analyzed here. Generally speaking, all three series of FTO nanocomposites tend to form nanoparticle agglomerates with r
eq less than 1 μm. However, S:TiO
2-FTO, and particularly ZnO-FTO nanocomposites also show a fairly high proportion of agglomerates with r
eq > 1 μm, the surface coverages of which significantly increase with increasing nanoparticle suspension concentration. For a target application in solar cells, it is well known that large feature sizes can generate technical problems for cell processing [
19,
20] (since it can for instance induce a local shunt). In comparison, the portion of large-sized nanoparticle agglomerates in Al
2O
3-FTO nanocomposites is almost negligible. Further, in Al
2O
3-FTO nanocomposites about 90% of agglomerates show r
eq < 750 nm while about 80% in ZnO-FTO and S:TiO
2-FTO nanocomposites show r
eq < 1.25 μm and r
eq < 1 μm, respectively. In this regard, among the three series of nanocomposites, Al
2O
3-FTO nanocomposites appear most favorable to be integrated in solar devices that are highly sensitive to TCO roughness and large feature sizes.
3.2. Structural and Electrical Properties of Al2O3-FTO Nanocomposites
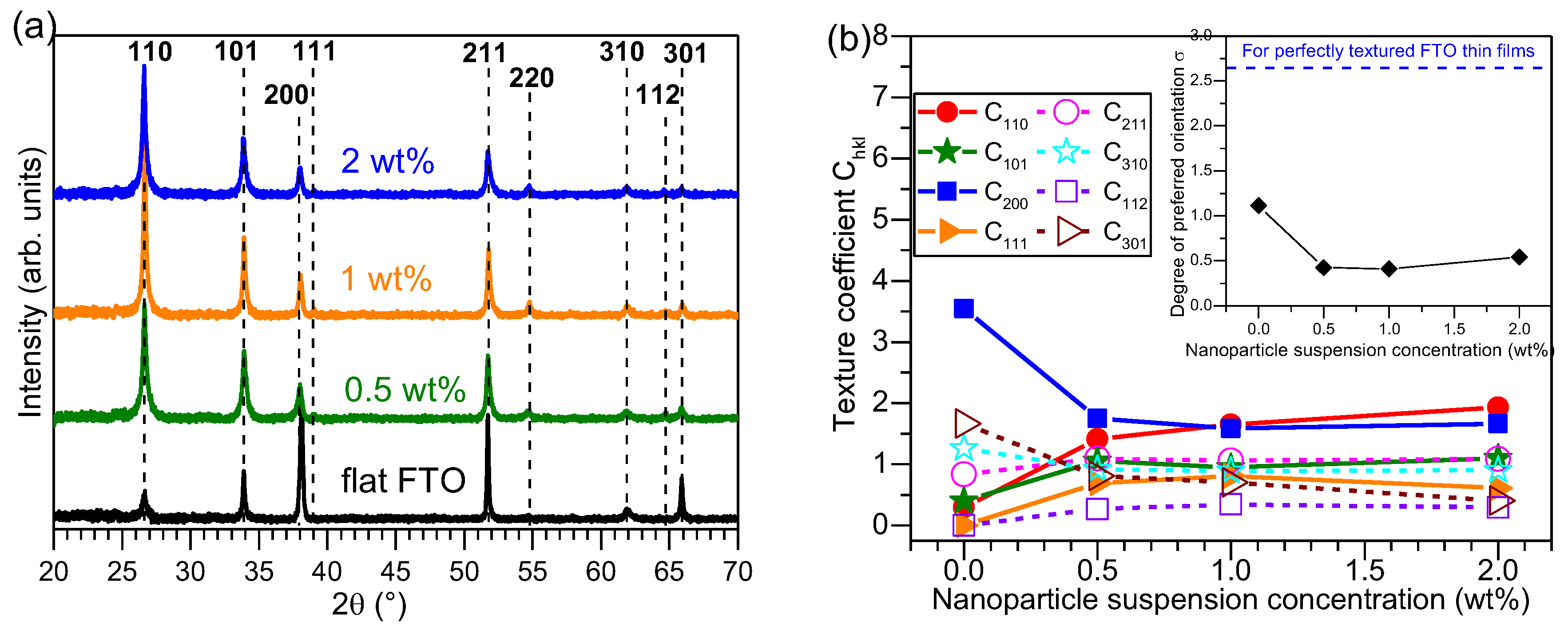
The X-ray diffraction (XRD) patterns of Al
2O
3-FTO nanocomposites are presented in
Figure 4a where the diffraction peaks corresponding to FTO are marked with dashed lines. No visible reflection corresponding to Al
2O
3 nanoparticles were observed, indicating the amorphous nature of the Al
2O
3 nanoparticles. The texture coefficient C
hkl for (110), (101), (200), (111), (211), (310), (112), and (301) planes of FTO are calculated according to Harris’s method [
21]:
where I
hkl and I
0,hkl represent the experimental and reference (taken from the powder diffraction files) diffraction intensity, respectively; while N equals the total number of diffraction peaks. The C
hkl for each plane is plotted as a function of nanoparticle suspension concentration in
Figure 4b, where the inset shows the evolution of the degree of preferred orientation σ obtained as:
When growing on top of Al
2O
3 nanoparticles, FTO grains do not align in the same orientations as those grown on bare glass surface. Thus, these FTO grains expose different crystal planes to the incident X-rays compared to the reference flat FTO. Consequently, as one sees in
Figure 4a,b, the texture of Al
2O
3-FTO nanocomposites is different from that of reference flat FTO. Furthermore, due to the presence of Al
2O
3 nanoparticles, the FTO grains are more randomly aligned. In other words, Al
2O
3-FTO nanocomposites structurally behave more like powder samples with random orientations. So the C
hkl of all diffraction peaks for Al
2O
3-FTO nanocomposites evolve towards 1 and also the degree of preferred orientation drops. The structural texture of Al
2O
3-FTO nanocomposites remains almost constant with varying nanoparticle suspension concentration. Unlike the local epitaxy observed between S:TiO
2 nanoparticles and FTO in [
11], the Al
2O
3 nanoparticles does not seem to impose any restrictions on the growth of subsequent FTO thin films, resulting in a more random orientation of the FTO grains.
The geometrical randomness imposed by the presence of nanoparticles also results in an increase of sheet resistance (R
s) in Al
2O
3-FTO nanocomposites, as seen in
Figure 5. To a first approximation, if one considers solely the FTO film in the nanocomposites, its cross section can be assumed constant (since the FTO film is conformally coated on top of nanoparticle agglomerates). Therefore, one expects the total volume of FTO film to be larger in nanocomposites than in the reference flat FTO. That is to say, according to the Pouillet’s law, the electrical carriers in FTO nanocomposites should travel a longer effective length: the higher the nanoparticle suspension concentration, the rougher the nanocomposite and the longer the effective length. Unlike the S:TiO
2 nanoparticles reported in [
11], the Al
2O
3 nanoparticles used in this work are not doped and are thus electrically insulating. When an electrical current is injected into Al
2O
3-FTO nanocomposites, the current is expected to flow only through the FTO film, encountering a larger resistance due to increased length. The large roughness is also expected to increase electrical recombination losses at the surface/interfaces, thus contributing to the increased resistance. Consequently, R
s of Al
2O
3-FTO nanocomposite is larger than that of reference flat FTO and increases further with increasing nanoparticle suspension concentration. The largest increase in R
s is observed in the 2 wt % Al
2O
3-FTO nanocomposite by 42%, which is nevertheless smaller than the increase of 57% observed in ZnO-FTO nanocomposite in [
10]. It should be mentioned that despite the relative increase with respect to the reference flat FTO, R
s of Al
2O
3-FTO still falls into the suitable range for PV applications [
22].
3.3. Optical Properties of Al2O3-FTO Nanocomposites
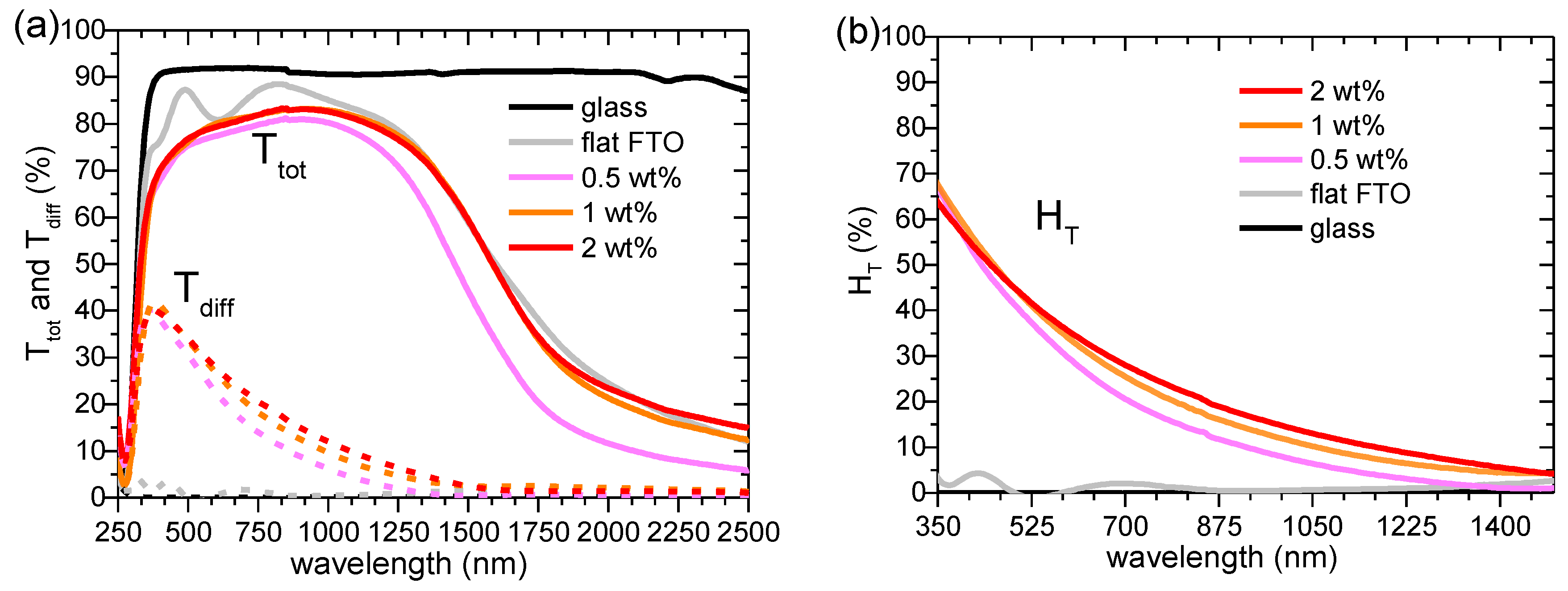
The total (T
tot) and diffuse (T
diff) transmittance in 250–2500 nm range for Al
2O
3-FTO nanocomposites is shown in
Figure 6a and the respective haze factor in transmittance (H
T) in 350–1500 nm is shown in
Figure 6b. The haze factor in transmittance H
T is defined as:
where T
tot equals to the sum of the specular transmittance (T
spec) and T
diff. Note that H
T, T
tot, and T
diff are wavelength dependent.
The reference flat FTO, as expected, shows essentially zero H
T at all wavelengths. Upon increasing the nanoparticle suspension concentration, H
T of Al
2O
3-FTO nanocomposites appears to increase only slightly, as opposed to the drastic change observed in ZnO-FTO (reported in [
10]) and S:TiO
2-FTO nanocomposites (reported in [
11]. To render a detailed comparison,
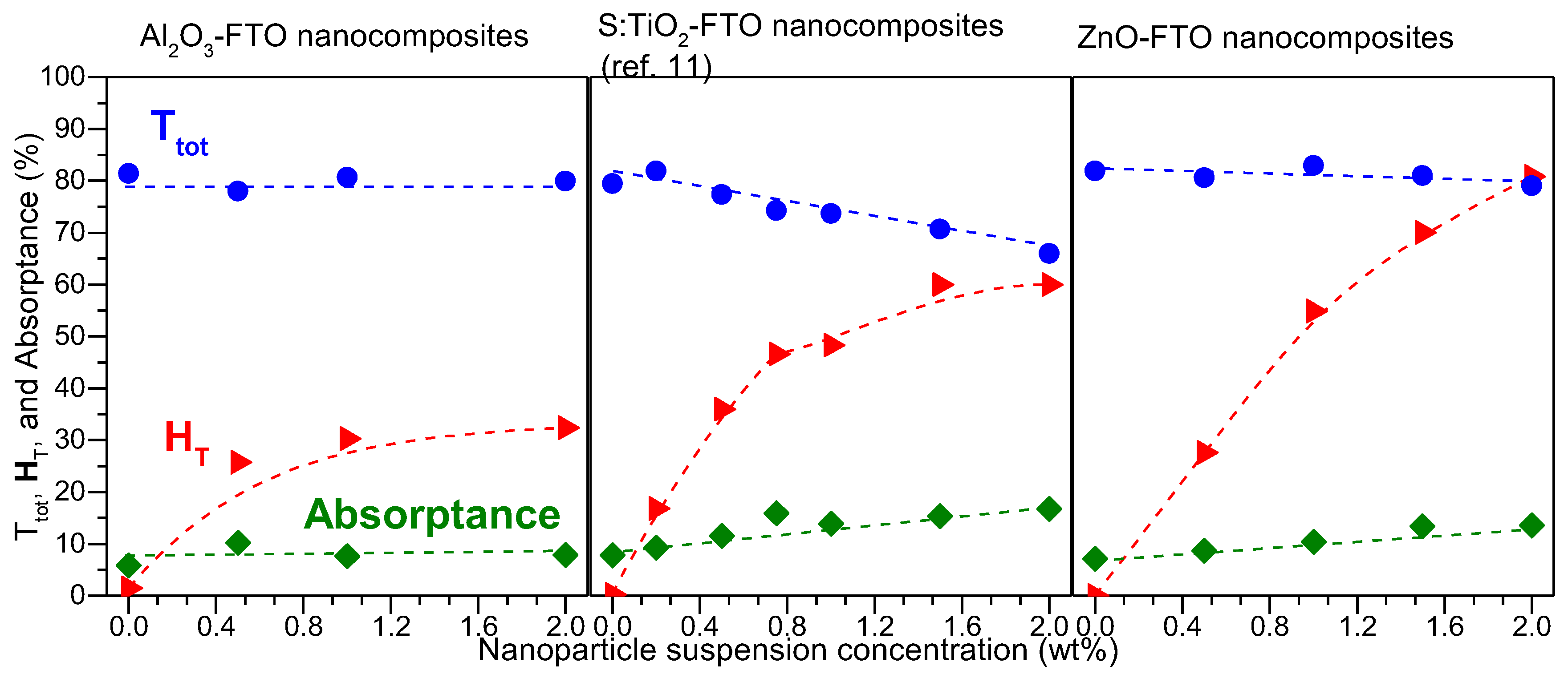
Figure 7 plots T
tot, H
T, and absorptance for Al
2O
3-FTO, S:TiO
2-FTO, and ZnO-FTO nanocomposites as a function of nanoparticle suspension concentration at a single visible wavelength, 635 nm in our case (since the visible range is of most interest for optoelectronic applications). The slight differences between the respective references flat FTOs for the three series of nanocomposites originate from the batch-to-batch differences. On the one hand, Al
2O
3-FTO nanocomposites exhibit a modest H
T of 32.3% compared to ZnO-FTO nanocomposite (highest H
T of 80.9%) and S:TiO
2-FTO nanocomposite (highest H
T of 60.0%). On the other hand, T
tot in Al
2O
3-FTO nanocomposite is almost invariant with a negligible loss of 1.8%. Whereas in ZnO-FTO nanocomposites a slightly higher drop in T
tot by 3.5% is observed and S:TiO
2-FTO nanocomposites show the highest loss in T
tot (up to 17%). For all three series of nanocomposites, the reduction in T
tot is accompanied by a simultaneous increase in absorptance, suggesting that the loss in transmittance is caused by an enhanced absorption. When passing through the FTO film deposited on top of the nanoparticle agglomerates, light essentially experiences a longer path compared to passing through the flat FTO film. Despite their roughness comparable with ZnO-FTO nanocomposites, Al
2O
3-FTO nanocomposites have a much smaller portion of large-sized nanoparticle agglomerates and thus experience the least absorption. As for S:TiO
2-FTO nanocomposites, the higher absorption in S:TiO
2-FTO is attributed to the additional absorption by S:TiO
2 nanoparticles due to the defect levels induced by S-doping as reported in [
11], and thus T
tot experiences the most severe reduction.
For the Al
2O
3-FTO and related FTO nanocomposites studied here, one clearly sees that depending on the nanoparticle selected, different tradeoffs between H
T, T
tot, and R
s would result, which offers a great flexibility to design and fabricate FTO and other TCO nanocomposites with optimized properties orientated for specific target application. For example, despite their medium H
T (~30%), Al
2O
3-FTO nanocomposites nevertheless present small feature sizes and thus should appear suitable for applications such as (planar) organic solar cells which are highly sensitive to TCO surface roughness [
23]; while ZnO-FTO and S:TiO
2-FTO nanocomposites are better used in applications such as dye-sensitized solar cells (DSSCs) which appear less sensitive to large feature sizes but require high H
T of TCO materials.
3.4. Modeling of Light Scattering by a Single Nanoparticle Agglomerate
Effectively, for the Al
2O
3-FTO and related FTO nanocomposites investigated here, the nanoparticle agglomerates exhibit a broad size distribution: some agglomerates appear to exhibit a size much larger (see
Figure 3) than the FTO film thickness (~300 nm). Consequently, it is less proper to consider these FTO nanocomposites as a “homogenous media” as usually considered in literature for other textured TCOs like the Asahi type-U or W-textured FTO [
24,
25]. Instead, here it is more reasonable to treat these nanoparticle agglomerates as “grains”, each functioning as an individual scattering center. Therefore, the optical scattering of the resultant nanocomposites is related to the overall scattering by all the “grains” present. Thus, understanding the optical scattering of an FTO nanocomposite containing a single “grain” is of key importance. To do so, we propose here a simple optical model in which we treat the “grain” (i.e., nanoparticle agglomerate) as a phase object φ:
where z(x,y) corresponds to the height of the top surface of the nanoparticle agglomerate in question (z varies as a function of the calculation coordinates x and y); λ refers to the wavelength and n is the refractive index of the nanoparticle. The transmittance can then be calculated as [
26]:
Here T0 stands for the transmittance of a perfectly flat surface. The influence of the surface roughness δ, including the interface between the nanoparticle agglomerates and FTO, as well as that of FTO and air, is expressed in the exponential term; The parameter of wave factor α is defined as: α/2π = ncosθ/λ (n being the refractive index of respective optical media), which in this case can essentially be simplified to α = 2πn/λ with incidence angle being 0°. The two subscripts “i” and “t” refer to the incident and transmitted light, respectively.
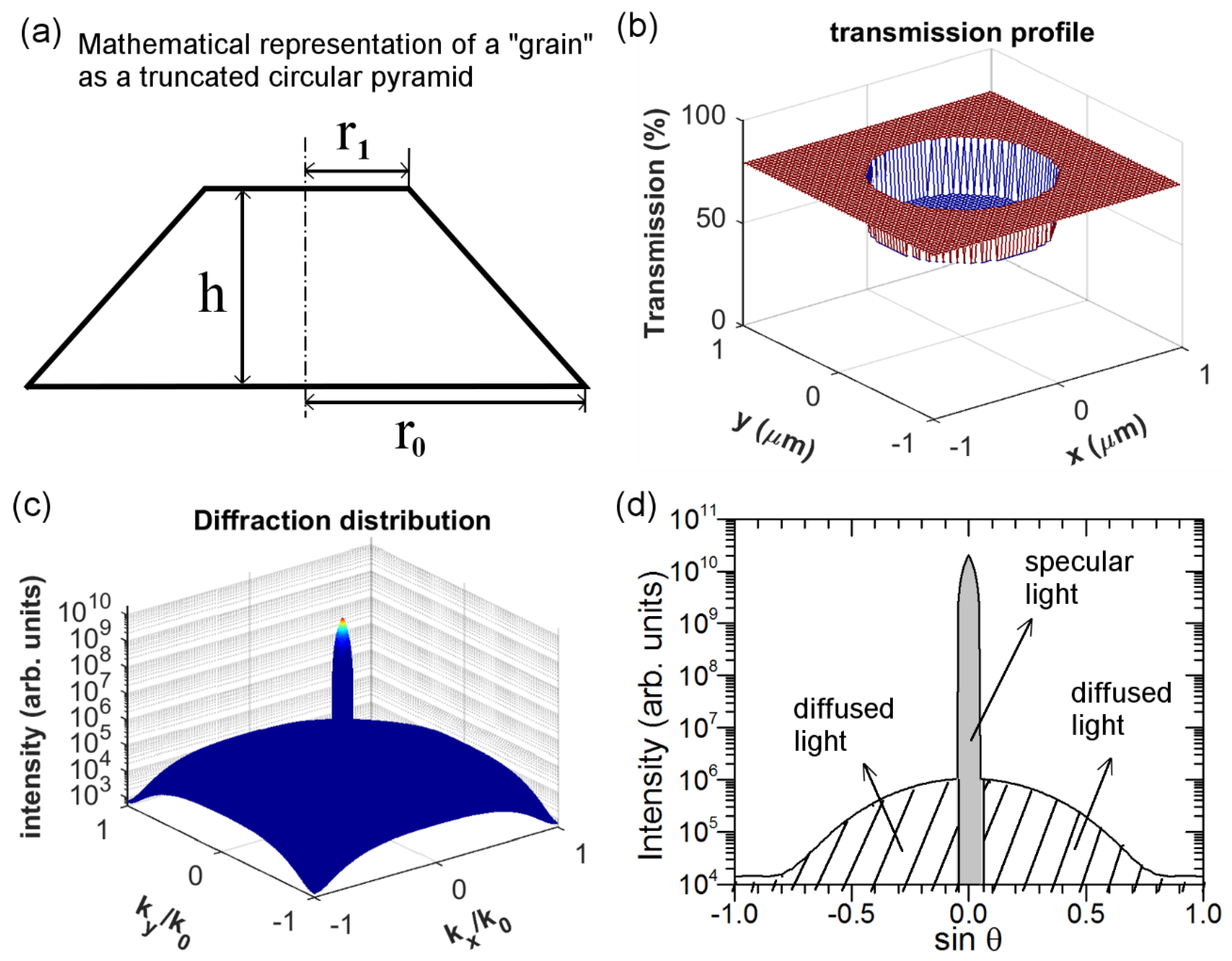
In a first approximation, the cross section of a single nanoparticle agglomerate (i.e., “grain”) can be mathematically represented by a truncated circular pyramid defined by three variables: the bottom radius r
0; the top radius r
1; and finally the height h as in
Figure 8a. The cross-section SEM image of a single agglomerate in
Figure S2 and height profiles of several grains examined in AFM images in
Figure S3 support such assumption. As detailed previously, compared to Al
2O
3-FTO and S:TiO
2-FTO nanocomposites, ZnO-FTO nanocomposites show the widest size distribution of “grains” thus are able to provide sufficient sample “grains” for screening purpose in the model. Therefore, the illustration of the optical model is exemplified with ZnO-FTO nanocomposite in the following discussion. As an example,
Figure 8b–d simulates the optical scattering of FTO nanocomposite containing a single ZnO nanoparticle agglomerate at λ = 635 nm. The size of the ZnO nanoparticle agglomerate is randomly selected, in this example we have chosen r
0, r
1, and h to be 600 nm, 200 nm, and 150 nm, respectively. In passing through such a ZnO nanoparticle agglomerate, the total transmission can be then calculated from Equation (4) and is plotted in
Figure 8b. The transmission, as expected, appears lower in the region where the ZnO nanoparticle agglomerate is located (one can note that the central part sinks in
Figure 8b). By performing the Fourier transform of the data reported in
Figure 8b [
27], one can calculate the transmitted light intensity in k-space, as shown in
Figure 8c where both axes correspond to the sine of the diffraction angle (θ), i.e., k
x/k
0 = k
y/k
0 = sinθ.
Figure 8d draws a 2D-plot of the transmitted light intensity versus sinθ to better show the transmitted light for different angles, which essentially corresponds to the cross section of
Figure 8c for k
y/k
0 = 0 (or equivalently, k
x/k
0 = 0). As shown in
Figure 8d, the specular light (here in this example corresponding to about 3°) appears much more intense than the diffused light. This means that light is mainly scattered in the specular direction by such a ZnO nanoparticle agglomerate. By performing proper integration, one obtains the light flux/power corresponding to specularly (P
spec) and totally (P
tot) transmitted light, whose difference gives the diffusely transmitted light power (P
diff): P
diff = P
tot − P
spec. Consequently, the haze factor of light passing through FTO nanocomposite containing a single nanoparticle agglomerate is: H
T = P
diff/P
tot.
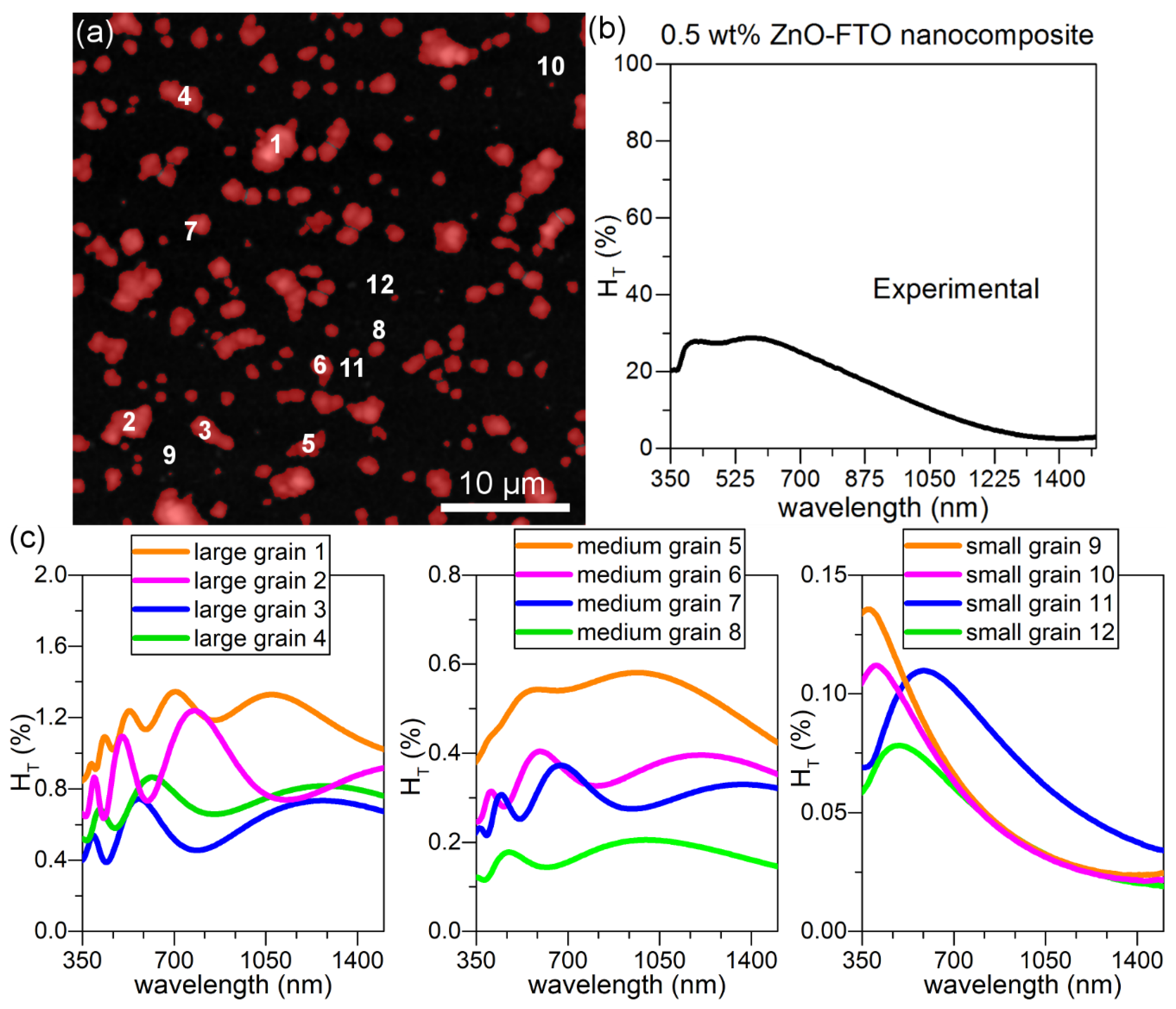
This model is then used to simulate real individual nanoparticle agglomerates present in a 0.5 wt % ZnO-FTO as reported in
Figure 9a where the nanoparticle agglomerates are counted as “grains” and colored in red for the sake of clarity. It is seen that the grain size distribution is rather broad and roughly they can be classified within three groups as shown by the randomly selected twelve grains: large grains 1–4 and medium grains 5–8, as well as small grains 9–12. As a reference, the r
eq (as defined previously) of grains 1–5 are superior to 1 μm while that of grains 6–12 are inferior to 1 μm. Detailed r
eq values of grain 1–12 are tabulated in
Table S1. The H
T(λ) for each of these 12 grains is simulated in
Figure 9c. For simplicity, the central one pixel is considered as the specular light. The large grains (grains 1–4) exhibit the largest H
T(λ) value; moreover, significant interference fringes can be seen. With decreasing grain size, the fringes gradually disappear (from grains 5–8 to grains 9–12), and the H
T(λ) is also observed to decrease accordingly (one notes that the scale of H
T changes in
Figure 9c), which suggests that among all grains the larger grains should have a more significant contribution to scattering light in such type of FTO nanocomposites. In order to simplify the calculation without altering the physical significance, it is most ideal to choose a small grain as grains 9–12 which does not show fringes and also best resembles the shape of H
T(λ) curve of the real nanocomposite (
Figure 9b).
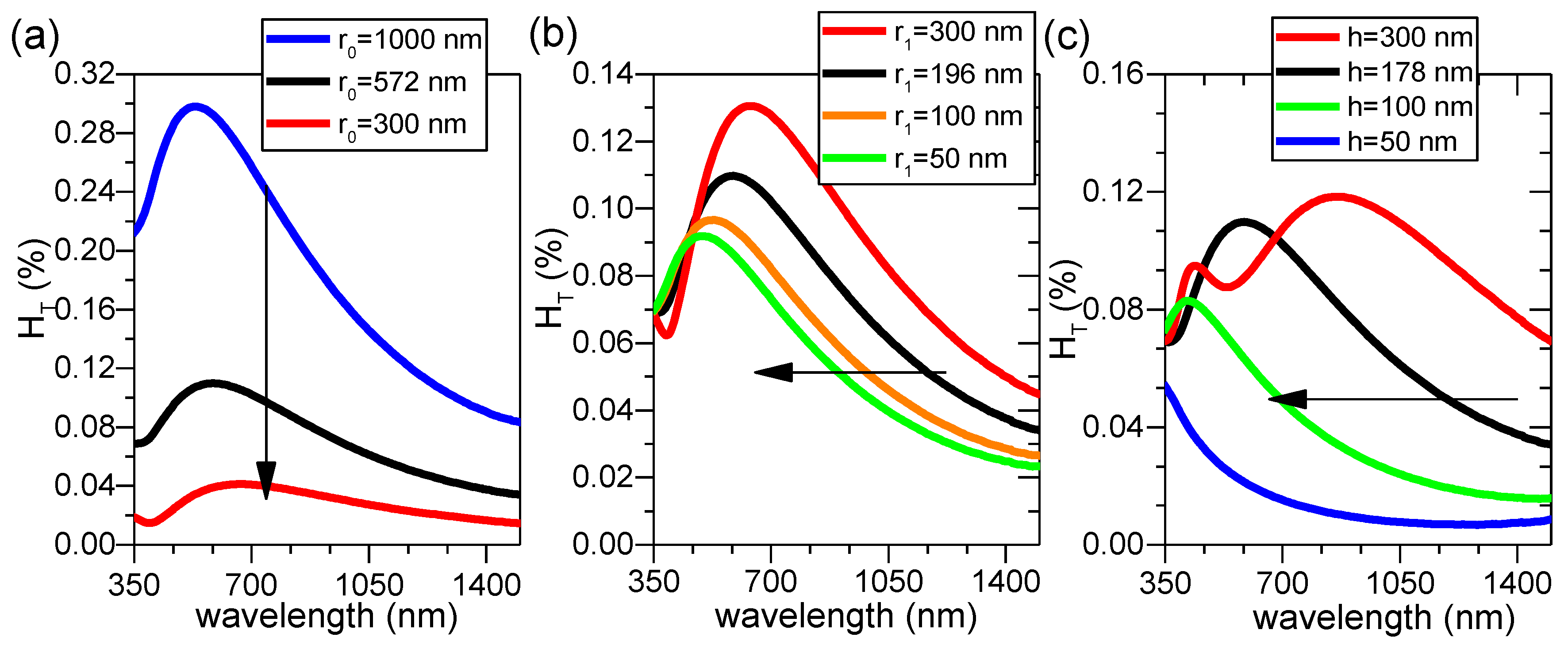
Hence, a grain with r
0, r
1, and h of 572, 196, and 178 nm (same as that of grain 11) is exemplified in
Figure 10 in order to study the individual influence of bottom and top radius (r
0 and r
1), as well as height (h), on H
T(λ). In
Figure 10a, with fixed r
1 and h values, when r
0 is decreased, H
T(λ) value shifts down to lower amplitude while the same shape remains. In
Figure 10b, with fixed r
0 and h, when r
1 is decreased, H
T(λ) maintains more or less the same shape but shifts to the left with the amplitude slightly lowered. Finally if r
0 and r
1 are fixed as in
Figure 10c, with decreasing h, the shape of H
T(λ) appears to change relatively significantly. This is effectively due to the extreme sensitivity of optical fringes on height. As is well known for thin film materials, the slight change in film thickness would immediately result in the change of optical fringes; consequently, the wavelengths corresponding to peaks/valleys of fringes also change.
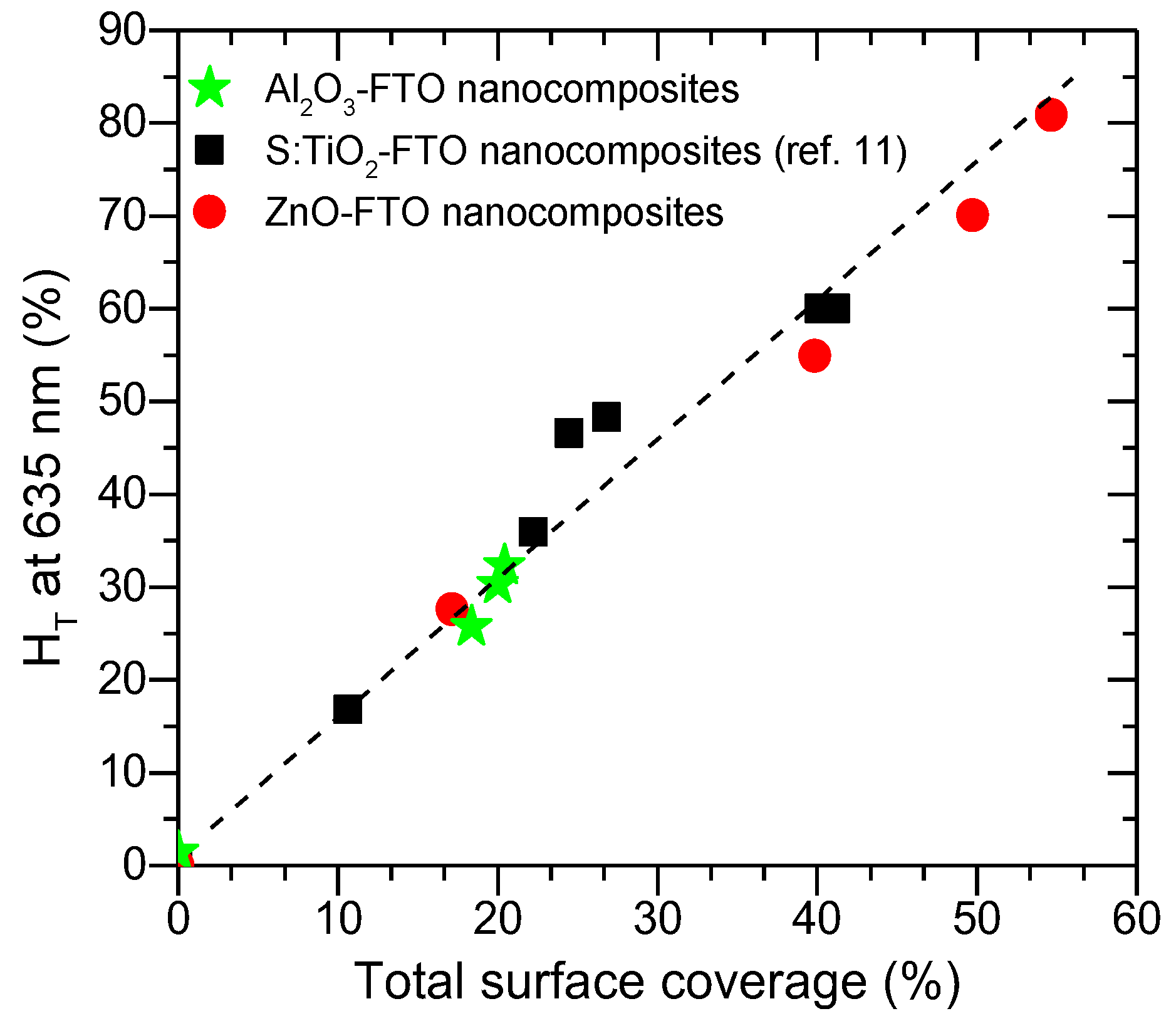
Of the three parameters examined here, one concludes that the bottom radius r
0 affects the optical scattering most while the top radius r
1 shows least influence and height h affects the optical scattering by affecting the appearance of optical fringes. As the bottom radius r
0 is essentially related to the projected area of the nanoparticle agglomerates, one would expect a direct correlation between the total surface coverage and the optical scattering for such type of nanocomposites. As is seen in
Figure 11, when drawing H
T at 635 nm against the total surface coverage for Al
2O
3-FTO, S:TiO
2-FTO and ZnO-FTO nanocomposites, a fairly straight line is observed, which is consistent with the simulated results. When varying the nanoparticle suspension concentration, the total surface coverage of Al
2O
3-FTO nanocomposites remains relatively constant, and consequently their H
T(λ) remains almost invariant. Further work is ongoing to develop a more complex model to simulate the H
T of final nanocomposites, which would require more sophisticated physical considerations such as the interaction among nanoparticle agglomerates, and/or the free carrier absorption within the FTO film in the near-infrared range, etc.