Performance Assessment of Ordered Porous Electrospun Honeycomb Fibers for the Removal of Atmospheric Polar Volatile Organic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of OPM
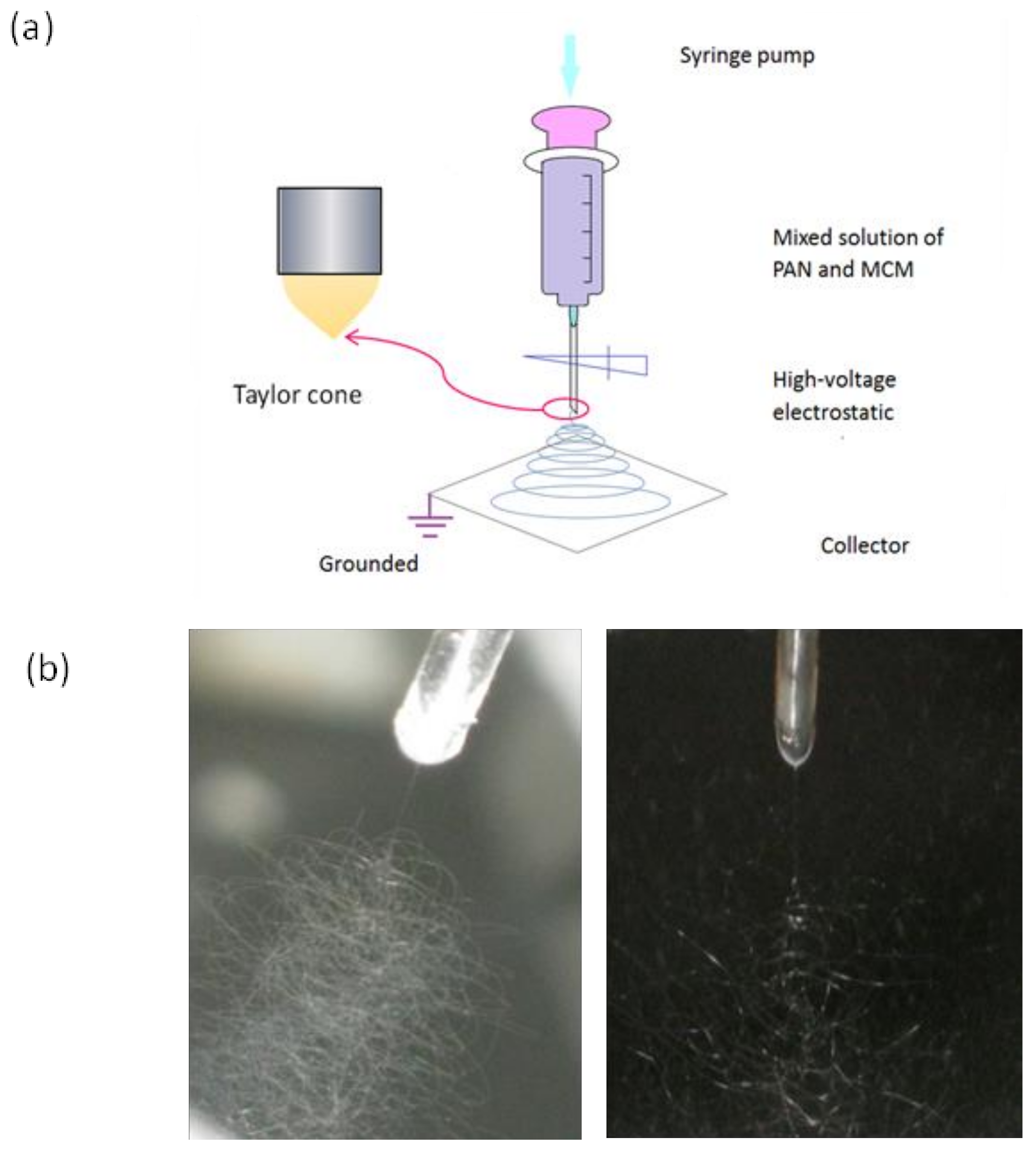
2.3. Synthesis of Electrospun Honeycomb Fibers Composites
2.4. Morphology
2.5. Physical Forms of the Components and Their Interactions
2.6. Hydrophobic Property
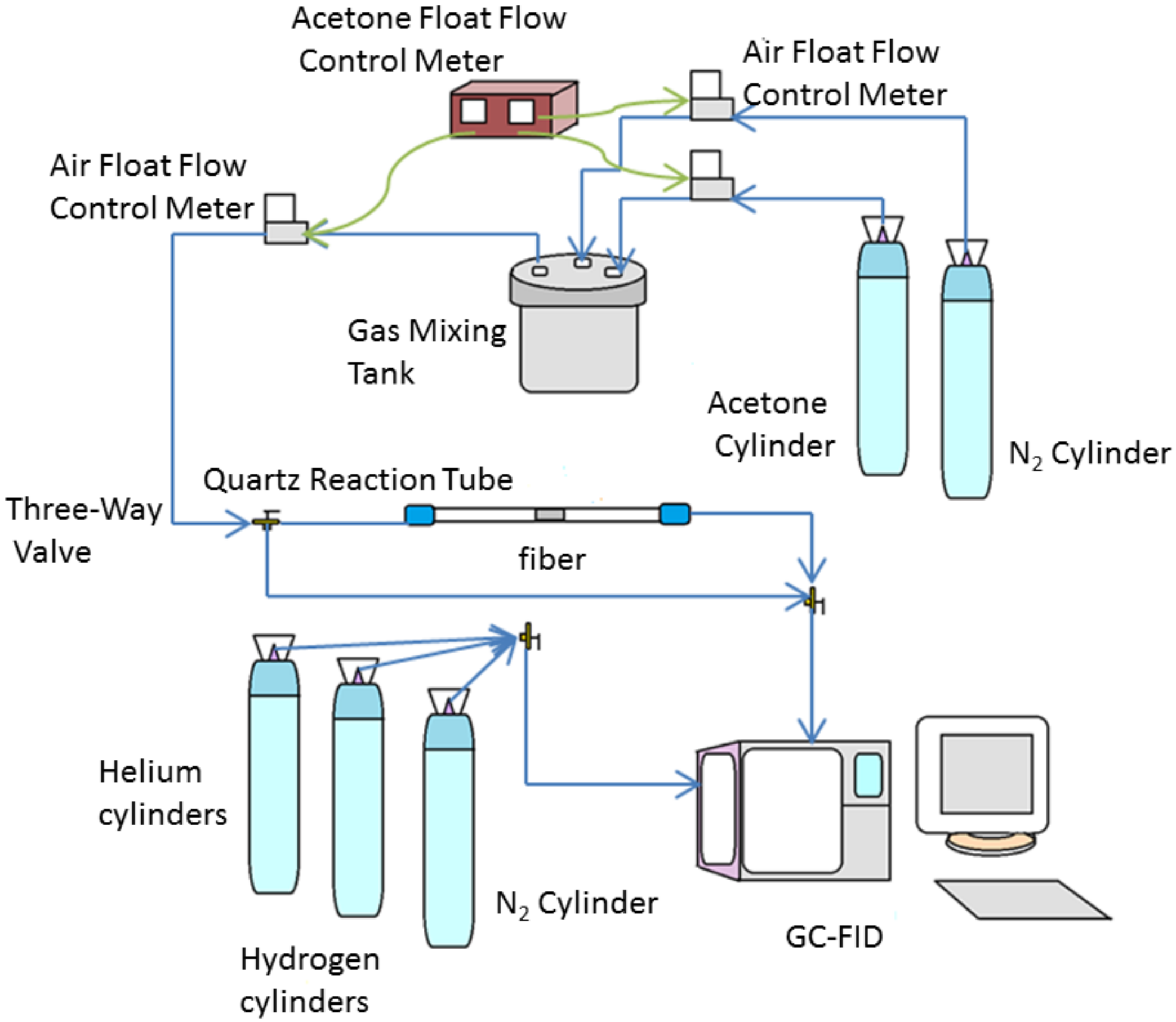
2.7. VOC Adsorption Performance Assessment
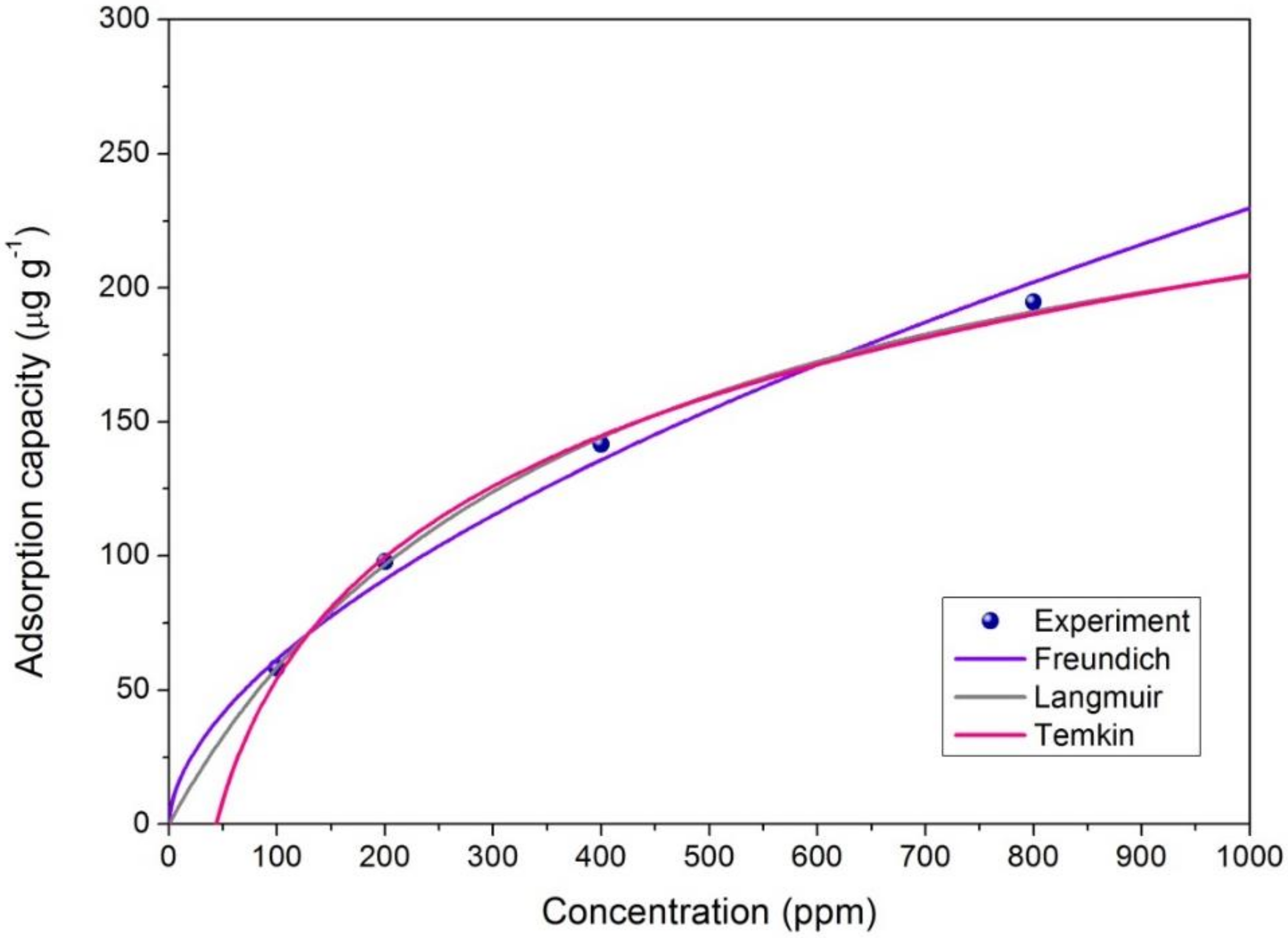
2.8. Adsorption Isotherms
2.8.1. Langmuir Isotherm
2.8.2. Freundlich Isotherm
2.8.3. Temkin Isotherm
3. Results and Discussion
3.1. Electrospinning
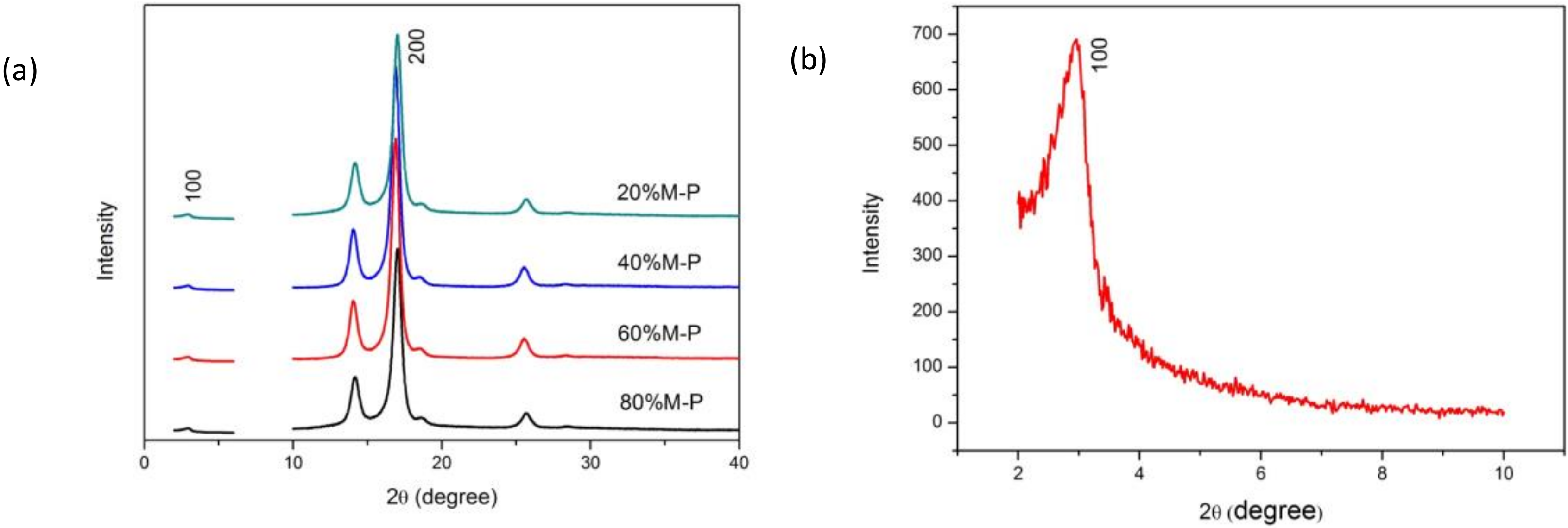
3.2. Characterization
3.2.1. Surface Area and Pore Size Distribution
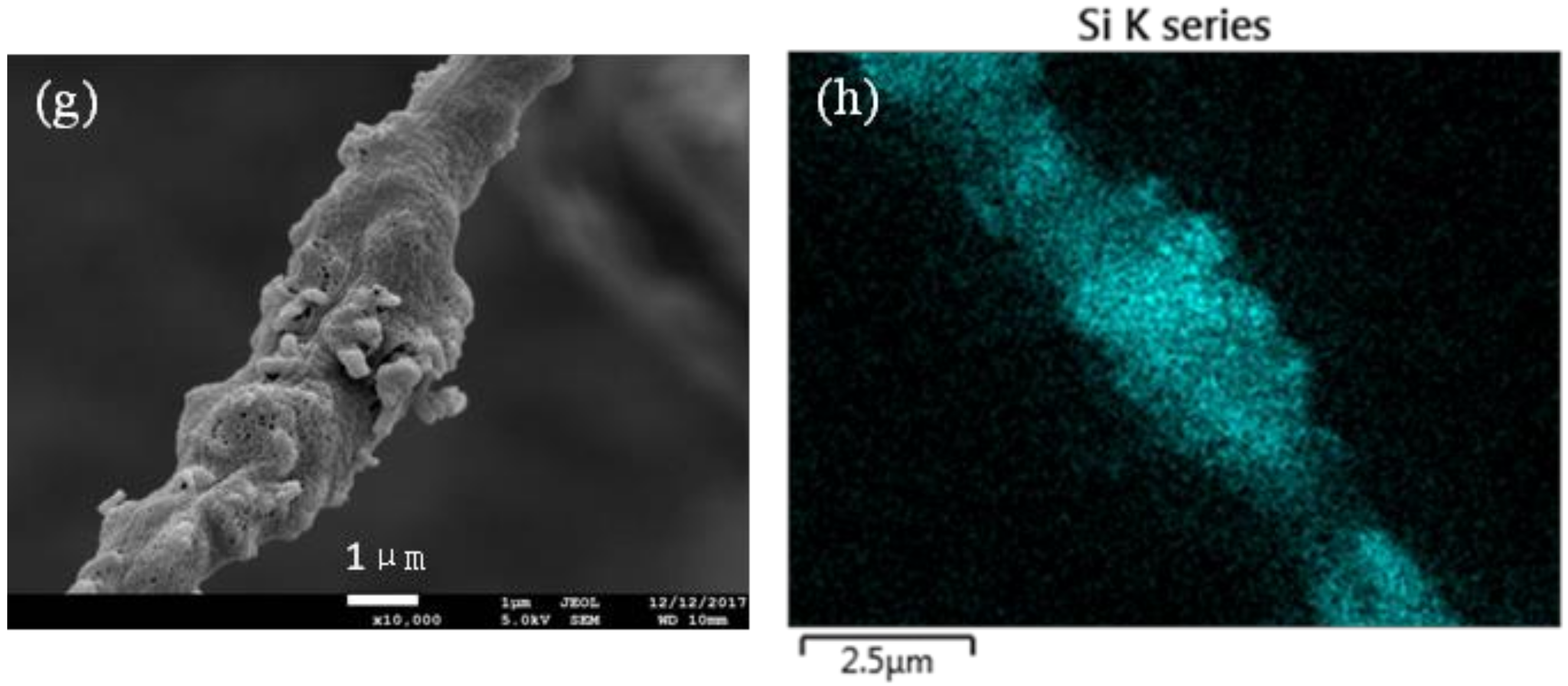
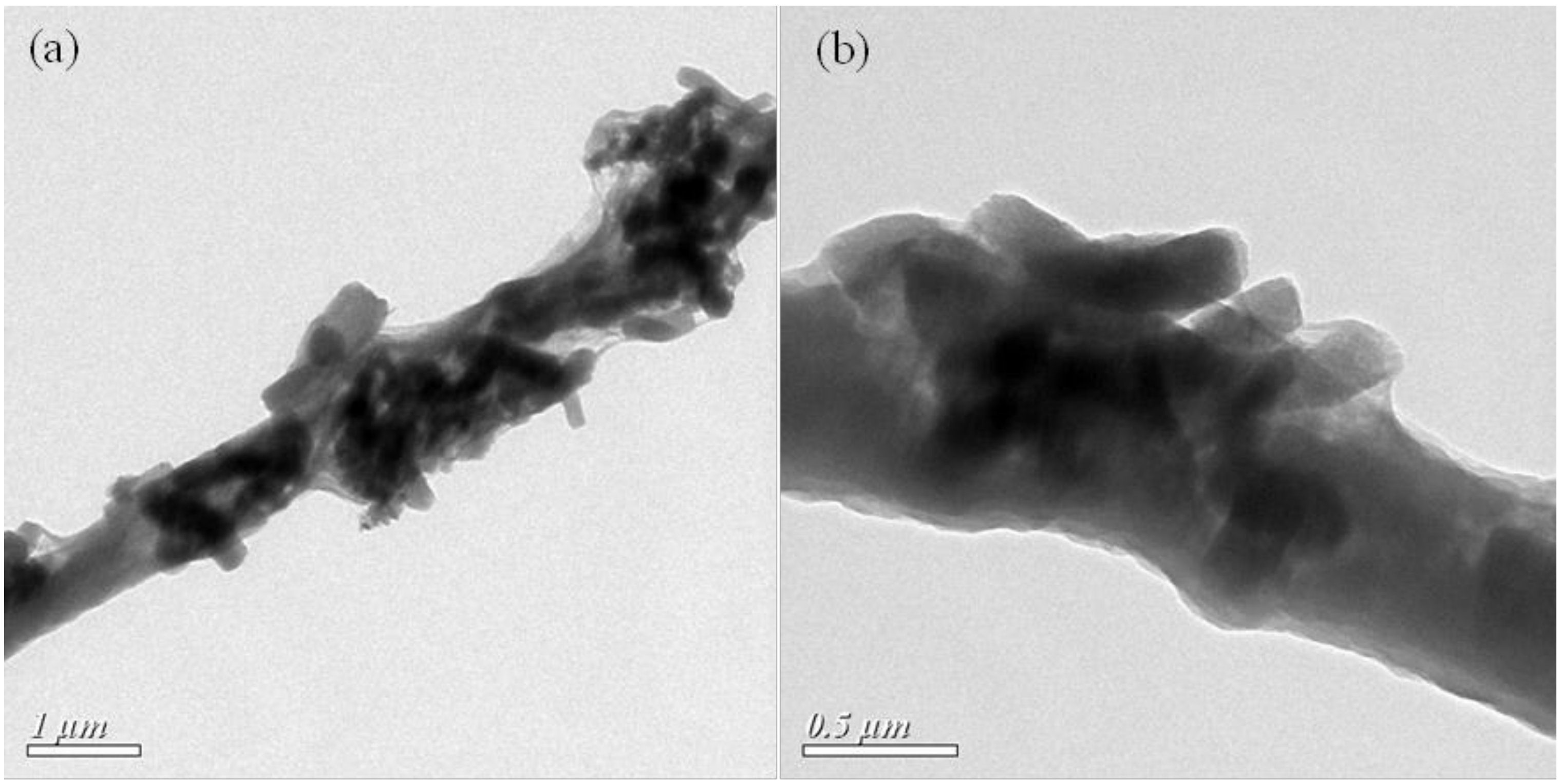
3.2.2. Morphology
3.2.3. Physical Forms
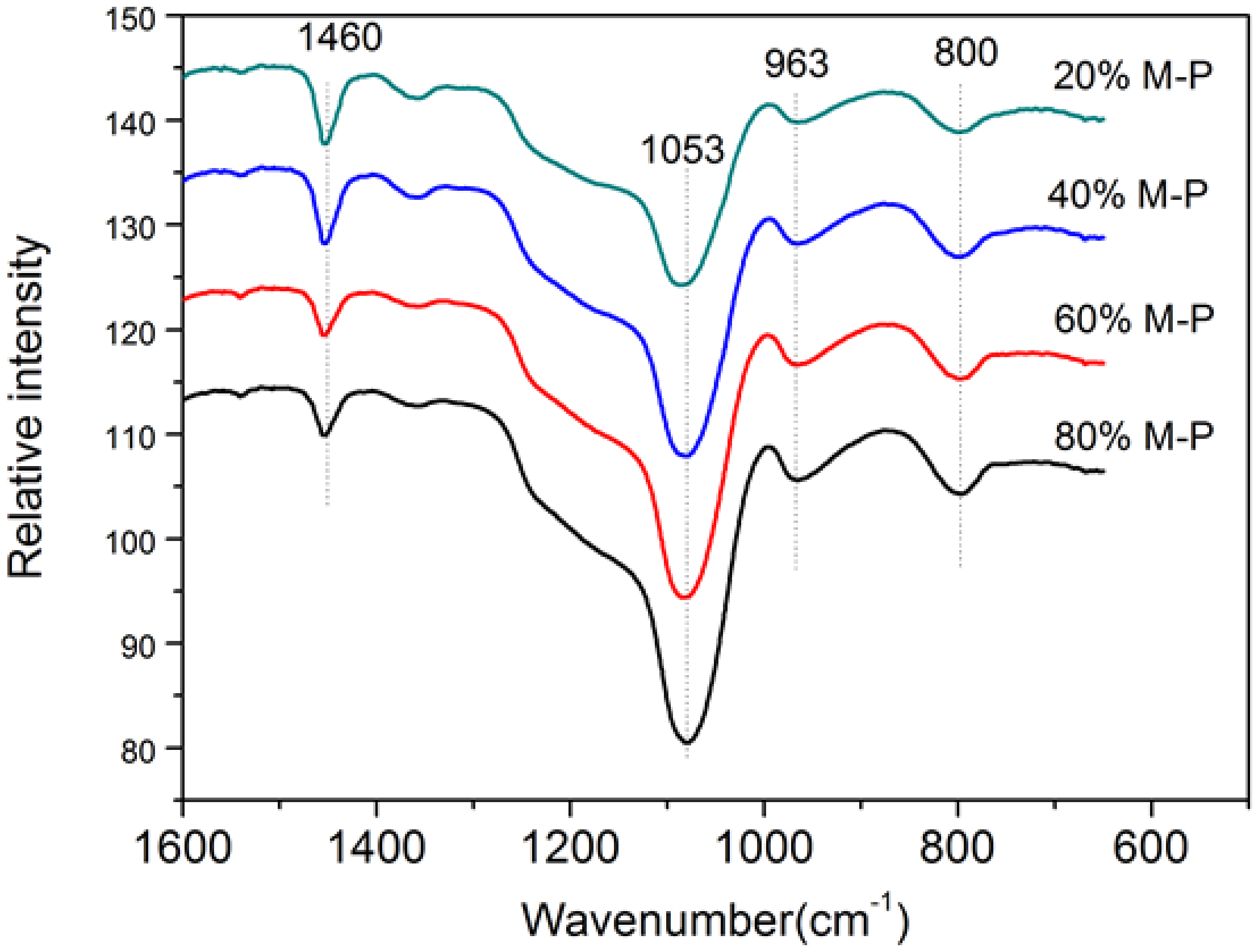
3.2.4. Functional Groups
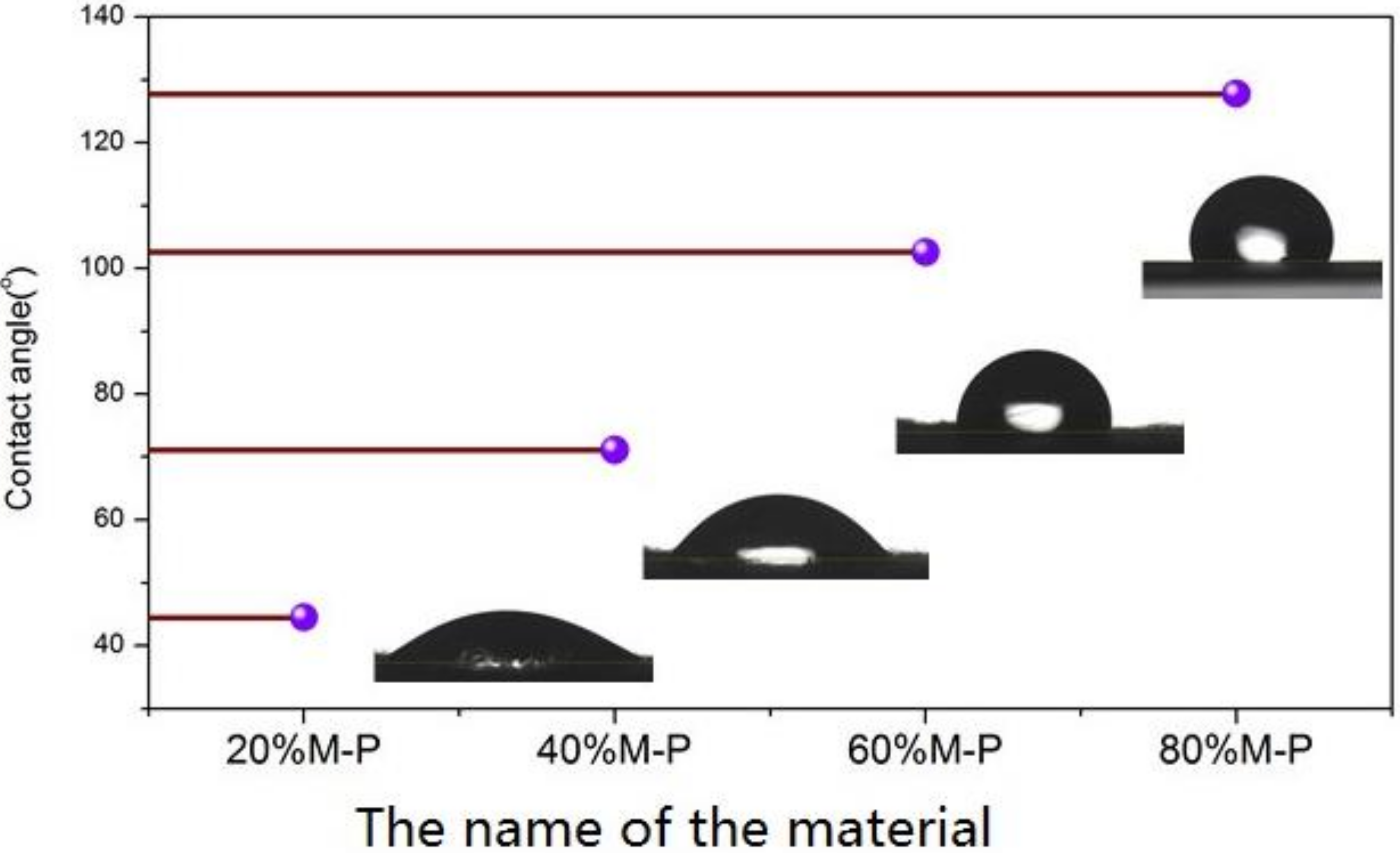
3.2.5. Hydrophobic Property
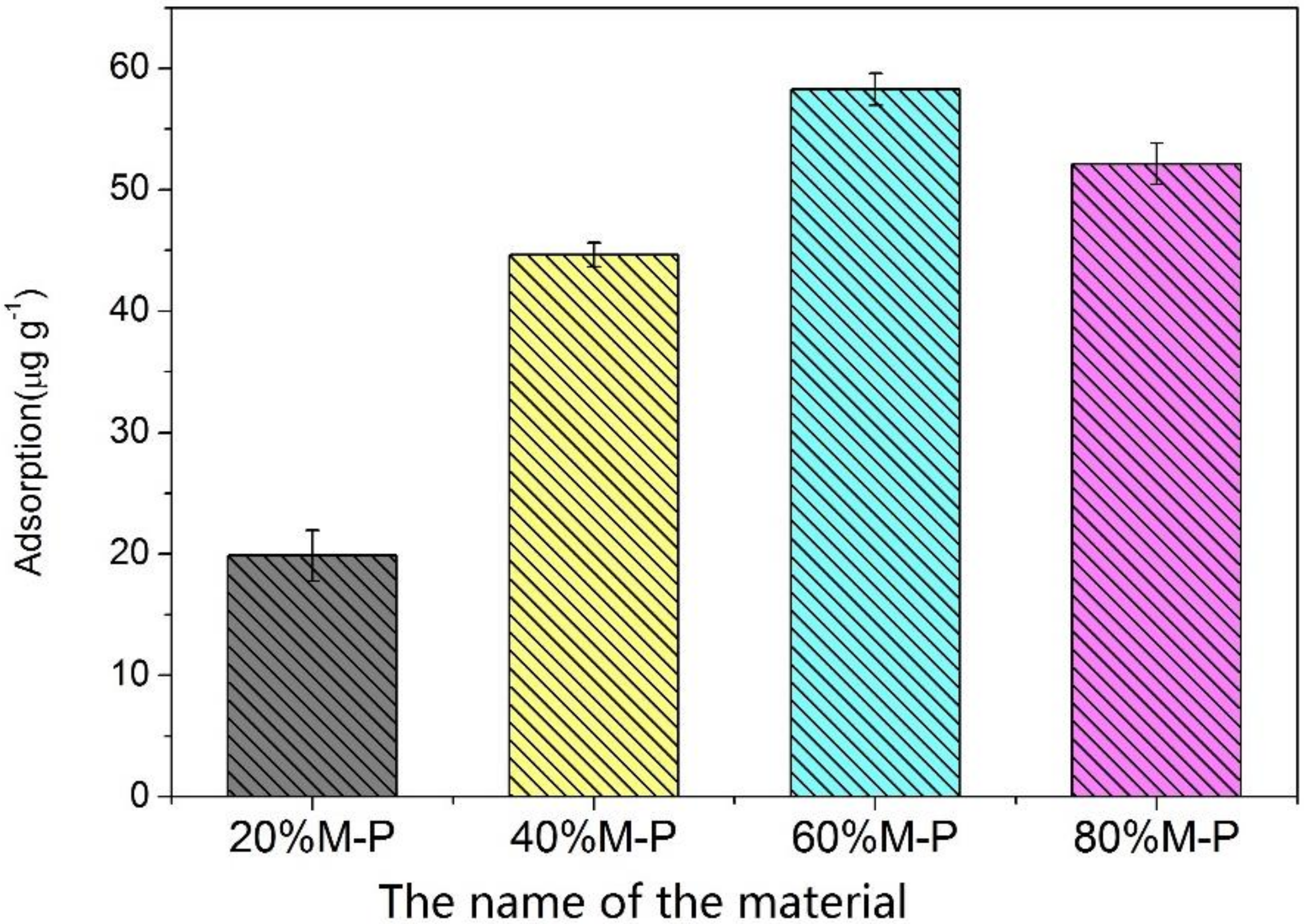
3.3. Adsorption Performance of Honeycomb Fibers
3.4. Effect of Adsorption Concentration
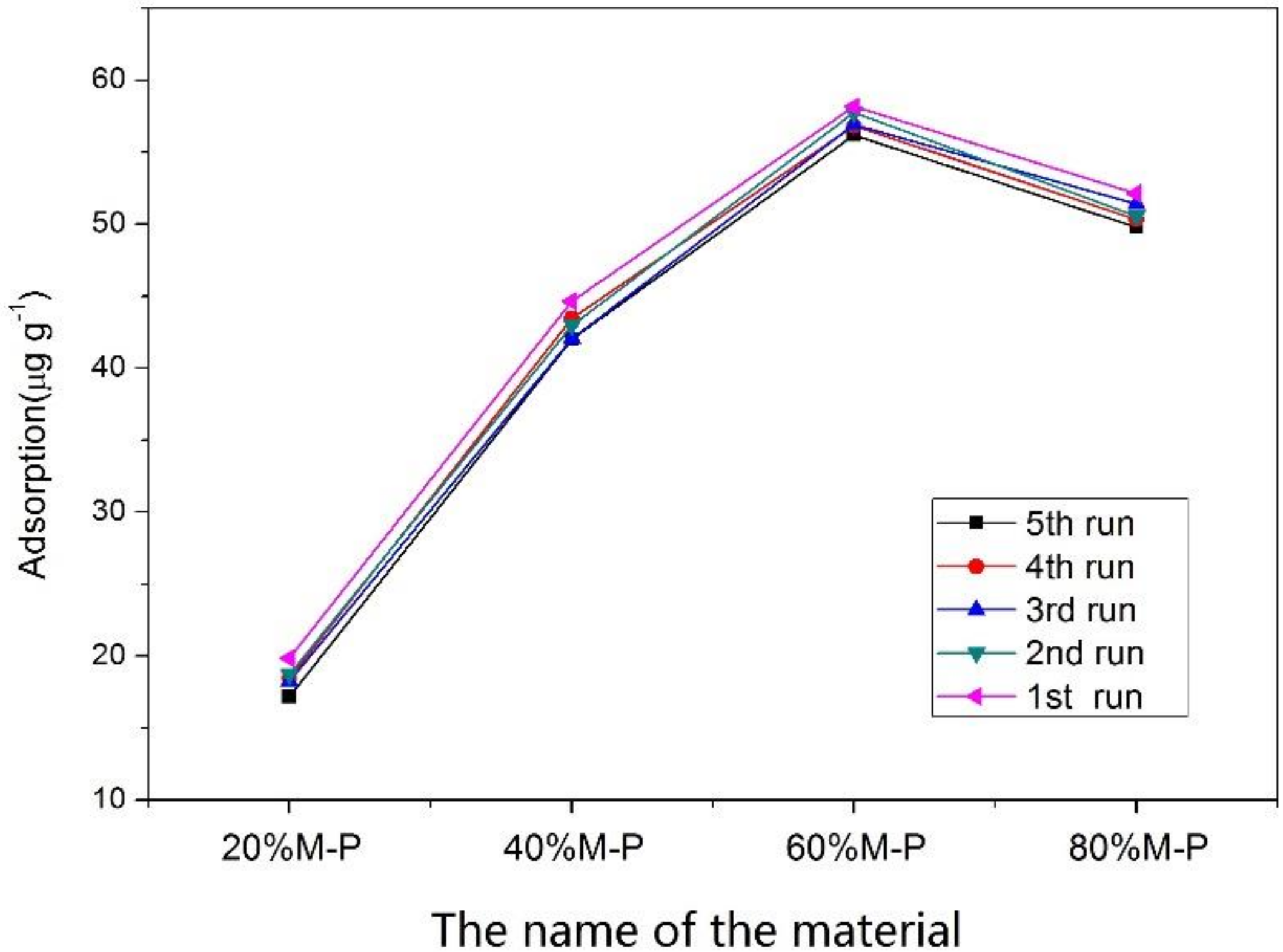
3.5. Reusability
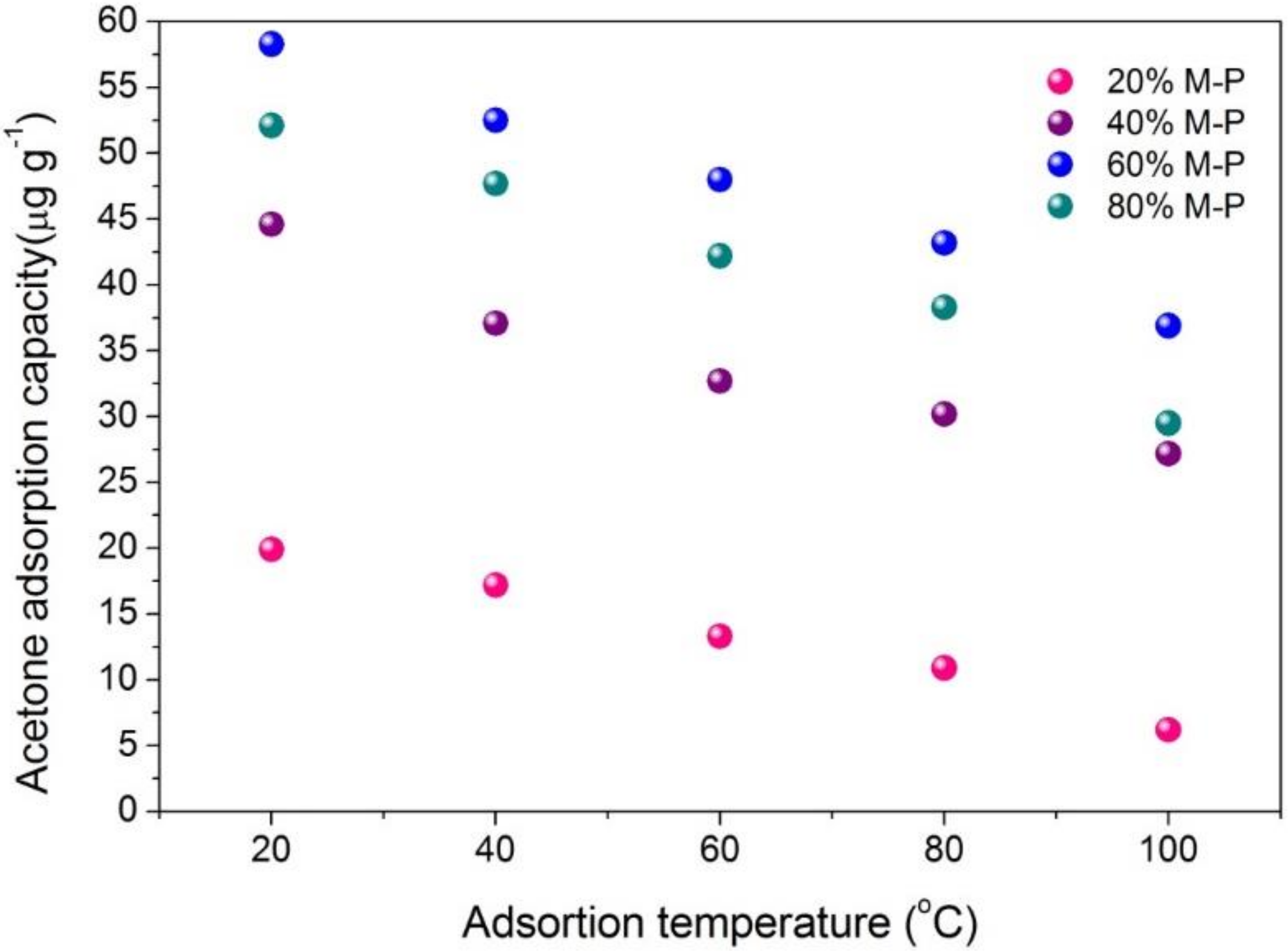
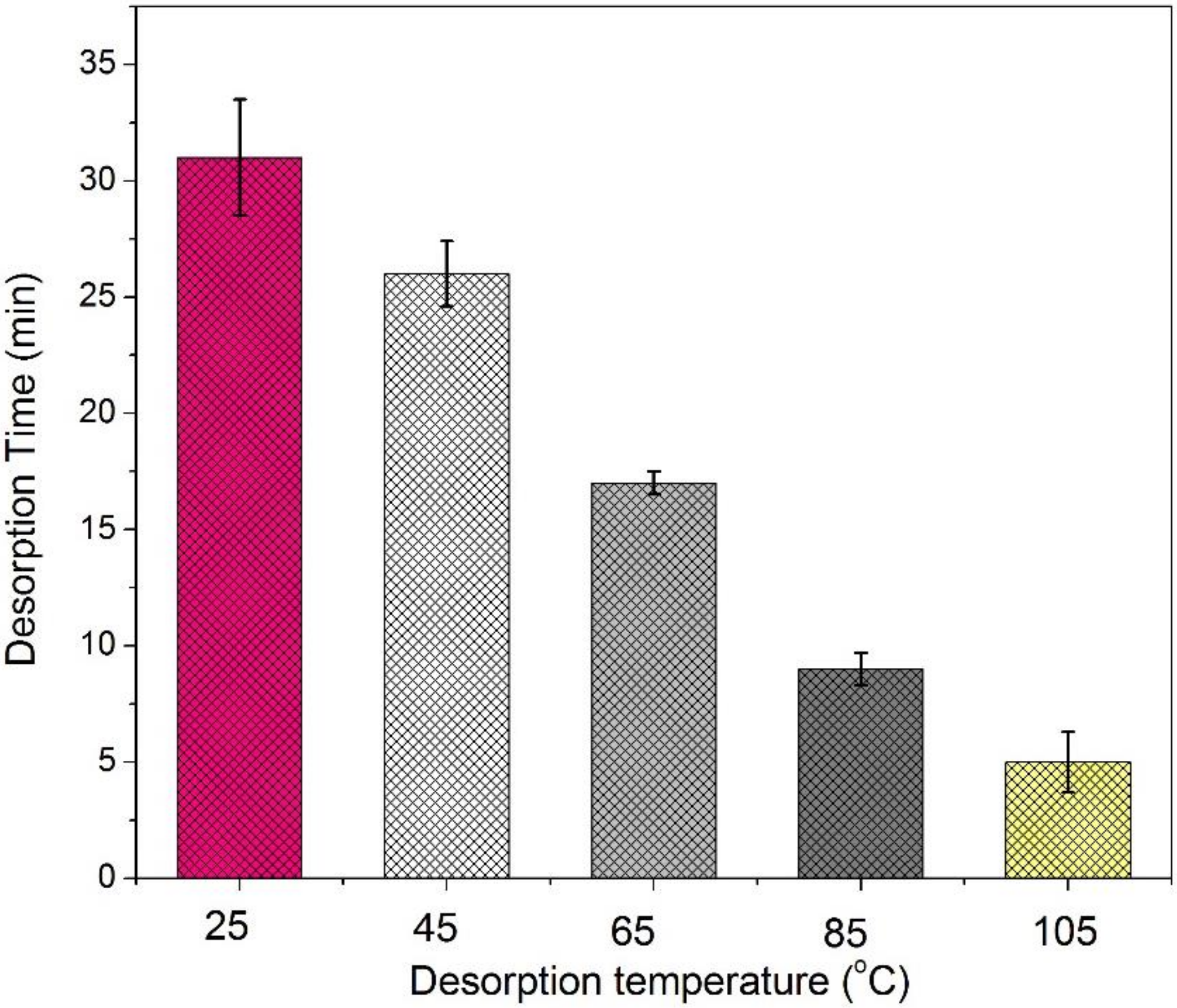
3.6. Temperature Effect on Adsorption Performance
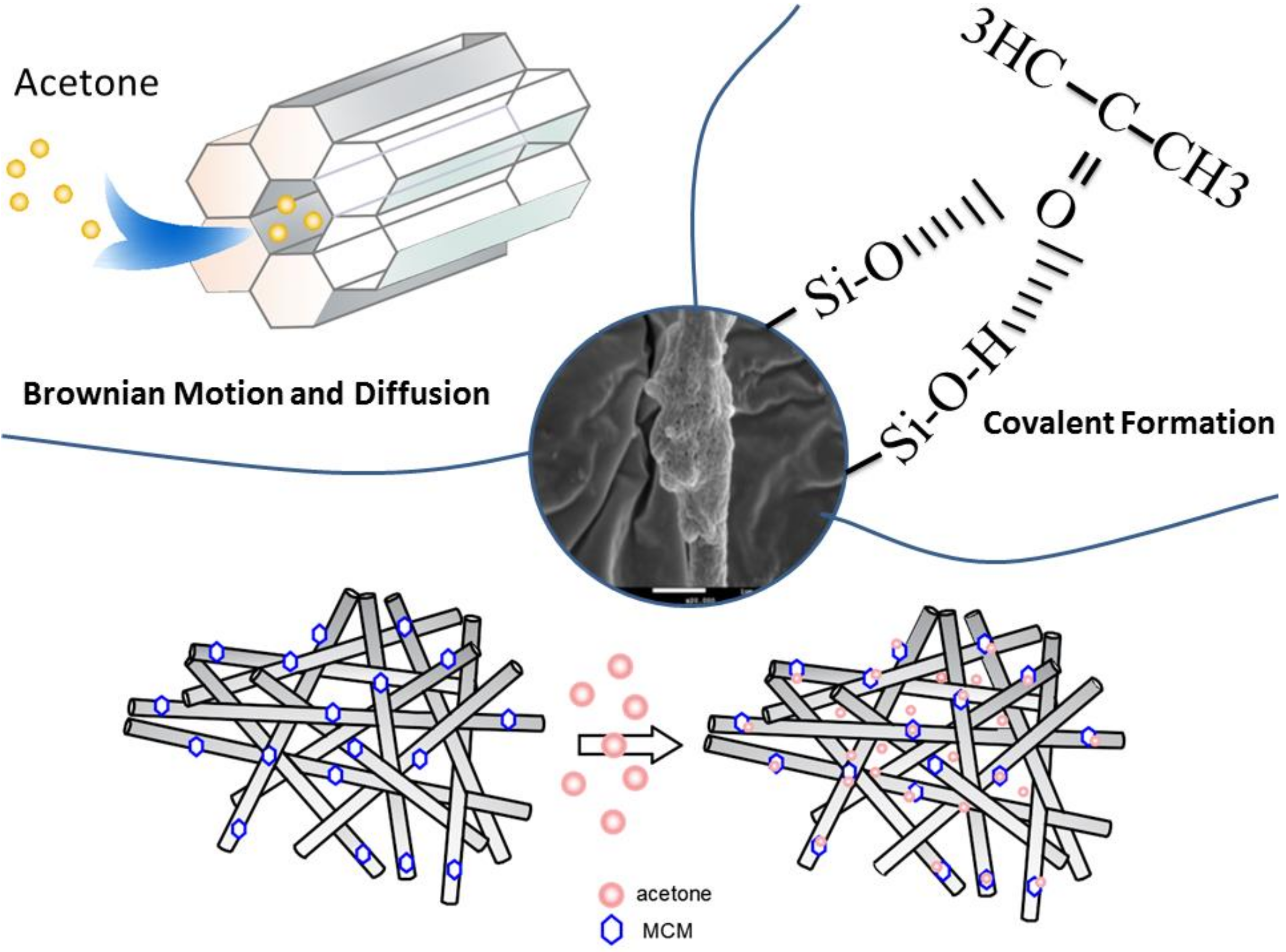
3.7. Development of Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liang, X.Y.; Chi, J.; Yang, Z. The influence of the functional group on activated carbon for acetone adsorption property by molecular simulation study. Microporous Mesoporous Mater. 2018, 262, 77–88. [Google Scholar] [CrossRef]
- Khanchi, A.; Hebbern, C.A.; Zhu, J.; Cakmak, S. Exposure to volatile organic compounds and associated health risks in windsor, Canada. Atmos. Environ. 2015, 120, 152–159. [Google Scholar] [CrossRef]
- Vellingiri, K.; Kumar, P.; Deep, A.; Kim, K.H. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem. Eng. J. 2017, 307, 1116–1126. [Google Scholar] [CrossRef]
- Li, S.; Tian, S.; Feng, Y.; Lei, J.; Wang, P. A comparative investigation on absorption performances of three expanded graphite-based complex materials for toluene. J. Hazard. Mater. 2010, 183, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Wu, Y.T.; Yu, Y.H.; Nguyen, V.H.; Lu, K.T.; Wu, J.C.; Chang, L.M.; Kuo, C.W. Enhanced xylene removal by photocatalytic oxidation using fiber-illuminated honeycomb reactor at ppb level. J. Hazard. Mater. 2013, 262, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Li, C.; Heber, A.J.; Ni, J.; Huang, H. Biofiltration of a mixture of ethylene, ammonia, n-butanol, and acetone gases. Bioresour. Technol. 2013, 127, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.E.; Bonelli, P.R.; Cukierman, A.L.; Ribeiro Carrott, M.M.; Carrott, P.J. Adsorption of volatile organic compounds onto activated carbon cloths derived from a novel regenerated cellulosic precursor. J. Hazard. Mater. 2010, 177, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, M.; Shariaty, P.; Atkinson, J.D.; Hashisho, Z.; Phillips, J.H.; Anderson, J.E.; Nichols, M. Using microwave heating to improve the desorption efficiency of high molecular weight VOC from beaded activated carbon. Environ. Sci. Technol. 2015, 49, 4536–4542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, L.; Hang, X.; Chen, Z.Y. Preparation of zeolitic imidazolate framework-8/graphene oxide composites with enhanced VOCs adsorption capacity. Microporous Mesoporous Mater. 2016, 225, 488–493. [Google Scholar] [CrossRef]
- Pokharel, P.; Dai, S.L. High performance polyurethane nanocomposite films prepared from a masterbatch of graphene oxide in polyether polyol. Chem. Eng. J. 2014, 253, 356–365. [Google Scholar] [CrossRef]
- Al-Attabi, R.; Dumée, L.F.; Schütz, J.A.; Morsi, Y. Pore engineering towards highly efficient electrospun nanofibrous membranes for aerosol particle removal. Sci. Total Environ. 2018, 625, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Li, J.J.; Zhang, M.; Williams, G.R. High-quality janus nanofibers prepared using three-fluid electrospinning. Chem. Commun. 2017, 53, 4542–4545. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, J.J.; Yu, D.G.; Williams, G.R.; Yang, J.H.; Wang, X. Influence of the drug distribution in electrospun gliadin fibers on drug-release behavior. Eur. J. Pharm. Sci. 2017, 106, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, D.G.; Zhang, L.L.; Liu, X.K.; Deng, Y.C.; Zhao, M. Electrospun hypromellose-based hydrophilic composites for rapid dissolution of poorly water-soluble drug. Carbohydr. Polym. 2017, 174, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Han, J.K.; Pant, H.R.; Choi, N.J.; Kim, C.S. Composite electrospun fly ash/polyurethane fibers for absorption of volatile organic compounds from air. Chem. Eng. J. 2013, 230, 244–250. [Google Scholar]
- Scholten, E.; Bromberg, L.; Rutledge, G.C.; Hatton, T.A. Electrospun polyurethane fibers for absorption of volatile organic compounds from air. ACS Appl. Mater. Interfaces 2011, 3, 3902–3909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Du, Y.; Feng, Q.; Huang, F.; Lu, K.; Liu, J.; Wei, Q. Nanostructures and surface nanomechanical properties of polyacrylonitrile/graphene oxide composite nanofibers by electrospinning. J. Appl. Polym. Sci. 2013, 128, 1152–1157. [Google Scholar] [CrossRef]
- Al-Attabi, R.; Dumée, L.F.; Kong, L.; Schütz, J.A.; Morsi, Y. High efficiency poly(acrylonitrile) electrospun nanofiber membranes for airborne nanomaterials filtration. Adv. Eng. Mater. 2017, 20, 1700572. [Google Scholar] [CrossRef]
- Oh, K.J.; Park, D.W.; Kim, S.S.; Park, S.W. Breakthrough data analysis of adsorption of volatile organic compounds on granular activated carbon. Korean J. Chem. Eng. 2010, 27, 632–638. [Google Scholar] [CrossRef]
- Delage, F.; Pascaline Pré, A.; Cloirec, P.L. Mass transfer and warming during adsorption of high concentrations of VOCs on an activated carbon bed: Experimental and theoretical analysis. Environ. Sci. Technol. 2000, 34, 309–316. [Google Scholar] [CrossRef]
- Li, L.Q.; Song, J.F.; Yao, X.L.; Huang, G.J.; Liu, Z. Adsorption of volatile organic compounds on three activated carbon samples: Effect of pore structure. J. Cent. South Univ. 2012, 19, 3530–3539. [Google Scholar] [CrossRef]
- Hata, H.; Saeki, S.; Kimura, T.; Sugahara, Y.; Kuroda, K. Adsorption of taxol into ordered mesoporous silicas with various pore diameters. Chem. Mater. 1999, 11, 1110–1119. [Google Scholar] [CrossRef]
- Viveiros, R.; Lopes, M.I.; Heggie, W.; Casimiro, T. Green approach on the development of lock-and-key polymers for API purification. Chem. Eng. J. 2017, 308, 229–239. [Google Scholar] [CrossRef]
- Ansell, R.J. Characterization of the binding properties of molecularly imprinted polymers. In Molecularly Imprinted Polymers in Biotechnology; Springer: Cham, Switzerland, 2015; Volume 150, pp. 51–93. [Google Scholar]
- Ellwanger, A.; Berggren, C.; Bayoudh, S.; Crecenzi, C.; Karlsson, L.; Owens, P.K.; Ensing, K.; Cormack, P.; Herrington, D.; Sellergren, S.B. Evaluation of methods aimed at complete removal of template from molecularly imprinted polymers. Analyst 2001, 126, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, X.K.; Chen, X.H.; Yu, D.G.; Yang, Y.Y.; Liu, P. Electrospun hydrophilic janus nanocomposites for the rapid onset of therapeutic action of helicid. ACS Appl. Mater. Interfaces 2018, 10, 2859–2867. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Liu, Z.P.; Yu, D.G.; Wang, K.; Liu, P.; Chen, X.H. Colon-specific pulsatile drug release provided by electrospun shellac nanocoating on hydrophilic amorphous composites. Int. J. Nanomed. 2018, 13, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.K.; Shao, W.Y.; Luo, M.Y.; Bian, J.Y.; Yu, D.G. Electrospun blank nanocoating for improved sustained release profiles from medicated gliadin nanofibers. Nanomaterials 2018, 8, 184. [Google Scholar]
- Bore, M.T.; Rathod, S.B.; Ward, T.L.; Datye, A.K. Hexagonal mesostructure in powders produced by evaporation-induced self-assembly of aerosols from aqueous tetraethoxysilane solutions. Langmuir 2008, 19, 256–264. [Google Scholar] [CrossRef]
- Janus, R.; Kuśtrowski, P.; Dudek, B.; Piwowarska, Z.; Kochanowski, A.; Michalik, M.; Cool, P. Removal of methyl–ethyl ketone vapour on polyacrylonitrile-derived carbon/mesoporous silica nanocomposite adsorbents. Microporous Mesoporous Mater. 2011, 145, 65–73. [Google Scholar] [CrossRef]
- Furtado, A.M.B.; Wang, Y.; Glover, T.G.; Levan, M.D. MCM-41 impregnated with active metal sites: Synthesis, characterization, and ammonia adsorption. Microporous Mesoporous Mater. 2011, 142, 730–739. [Google Scholar] [CrossRef]
- Chauhan, D.; Afreen, S.; Mishra, S.; Sankararamakrishnan, N. Synthesis, characterization and application of zinc augmented aminated PAN nanofibers towards decontamination of chemical and biological contaminants. J. Ind. Eng. Chem. 2017, 55, 50–64. [Google Scholar] [CrossRef]
- Kosuge, K.; Kubo, S.; Kikukawa, N.; Takemori, M. Effect of pore structure in mesoporous silicas on VOC dynamic adsorption/desorption performance. Langmuir 2007, 23, 3095–3102. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.; Bai, H.; Karthik, M. Ordered mesoporous silica particles and Si-MCM-41 for the adsorption of acetone: A comparative study. Sep. Purif. Technol. 2009, 64, 265–272. [Google Scholar] [CrossRef]

| Sample | SBET a | Pore Volume | Pore Size | Fiber Diameter (nm) | |
|---|---|---|---|---|---|
| (m2g−1) | (m2g) | (nm) | Range | Mean | |
| 20% M-P | 62 | 0.02 | 2.12 | 52–90 | 65 |
| 40% M-P | 113 | 0.03 | 2.16 | 68–184 | 103 |
| 60% M-P | 121 | 0.05 | 2.19 | 71–218 | 119 |
| 80% M-P | 219 | 0.03 | 3.66 | 113–306 | 199 |
| Adsorption | Langmuir Isotherm | Freundlich Isotherm | Temkin Isotherm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Kd | qm | R2 | Kf | n | R2 | BT | AT | R2 | |
| 60% M-P | 0.0036 | 277.78 | 0.9988 | 4.39 | 1.75 | 0.9880 | 65.36 | 0.023 | 0.9925 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Tao, H.; Yu, D.; Chang, C. Performance Assessment of Ordered Porous Electrospun Honeycomb Fibers for the Removal of Atmospheric Polar Volatile Organic Compounds. Nanomaterials 2018, 8, 350. https://doi.org/10.3390/nano8050350
Wang Y, Tao H, Yu D, Chang C. Performance Assessment of Ordered Porous Electrospun Honeycomb Fibers for the Removal of Atmospheric Polar Volatile Organic Compounds. Nanomaterials. 2018; 8(5):350. https://doi.org/10.3390/nano8050350
Chicago/Turabian StyleWang, Yixin, Hong Tao, Dengguang Yu, and Changtang Chang. 2018. "Performance Assessment of Ordered Porous Electrospun Honeycomb Fibers for the Removal of Atmospheric Polar Volatile Organic Compounds" Nanomaterials 8, no. 5: 350. https://doi.org/10.3390/nano8050350






