A Facile Method for the Preparation of Colored Bi4Ti3O12−x Nanosheets with Enhanced Visible-Light Photocatalytic Hydrogen Evolution Activity
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of the Bi4Ti3O12−x Nanosheet Photocatalyst
2.2. Characterization
2.3. Photocatalytic Activity
3. Results and Discussion
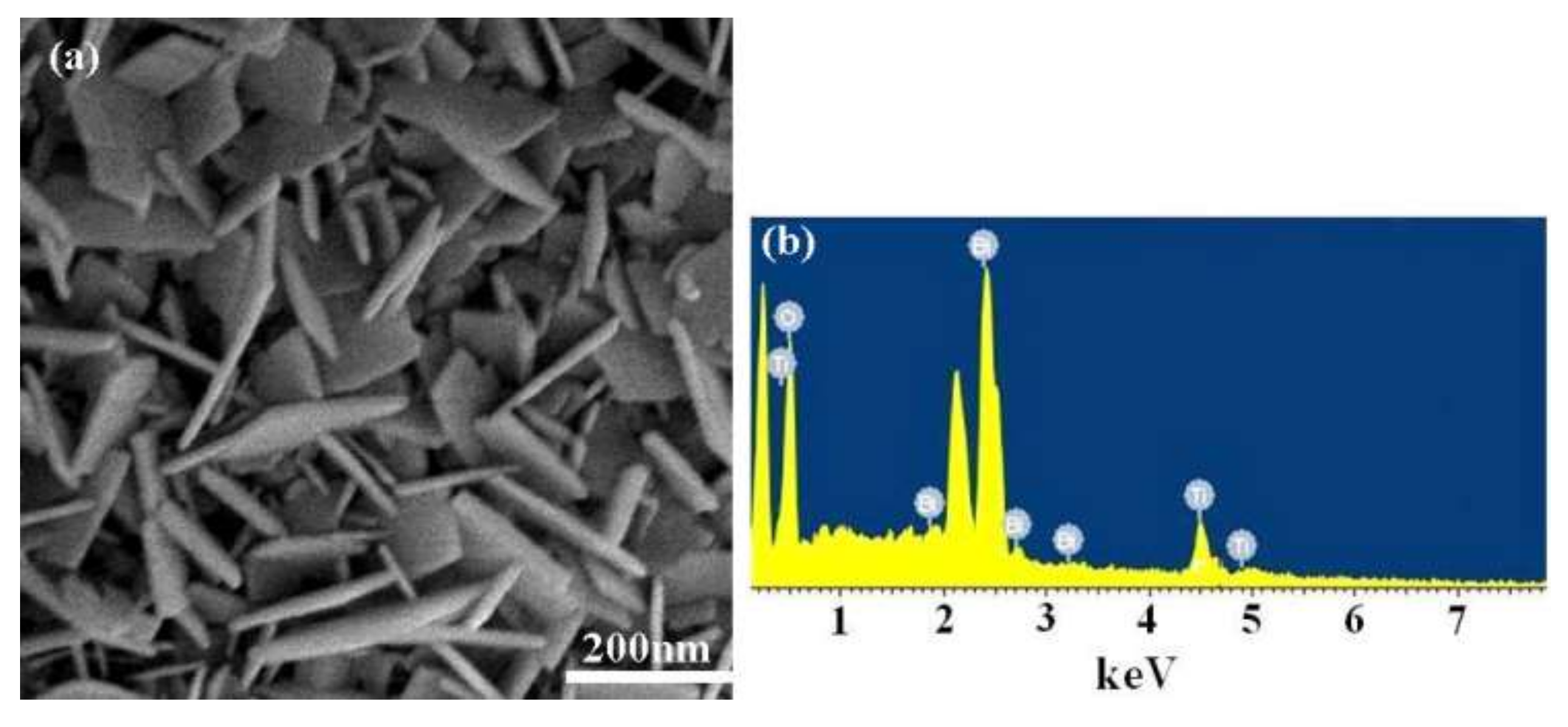
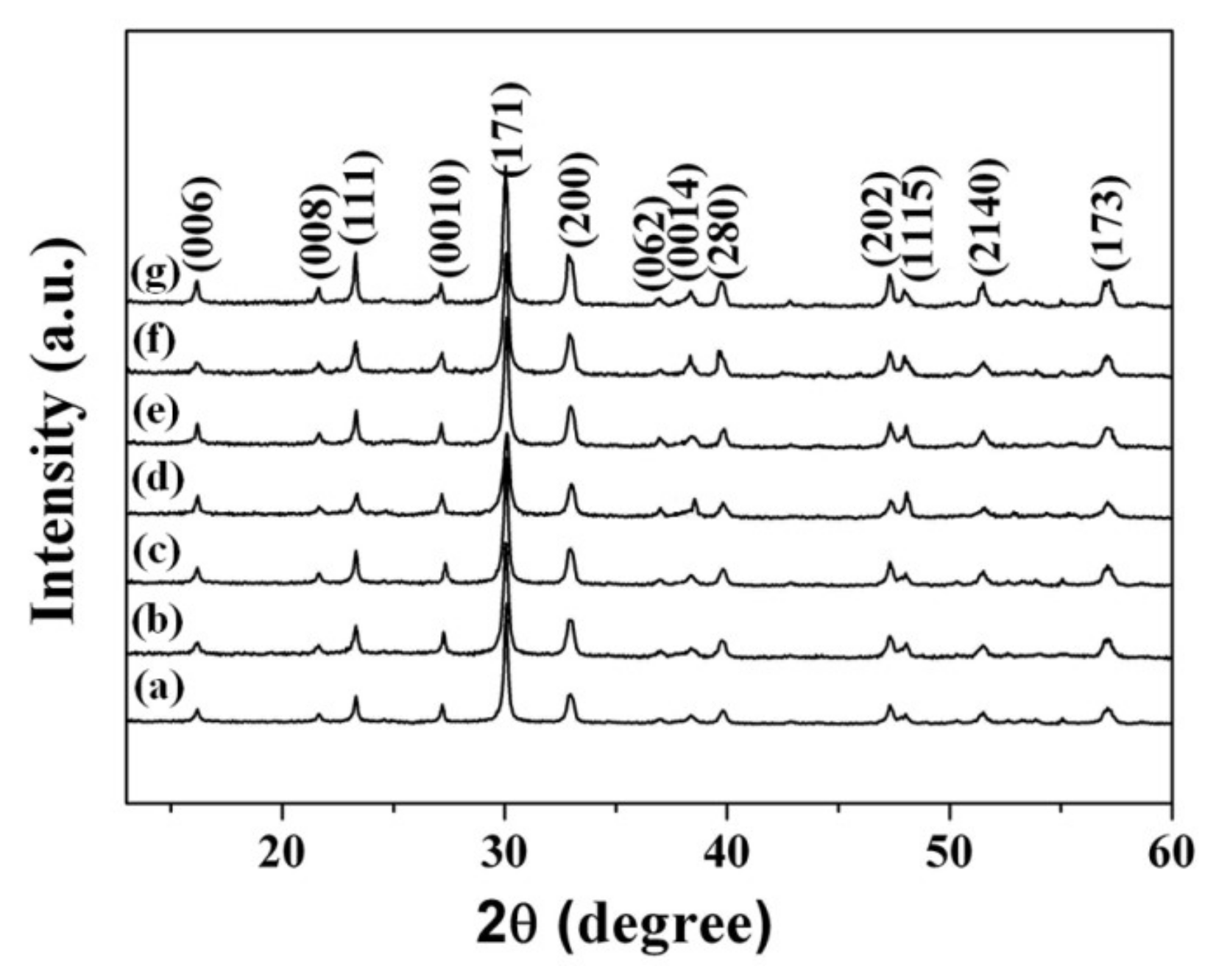
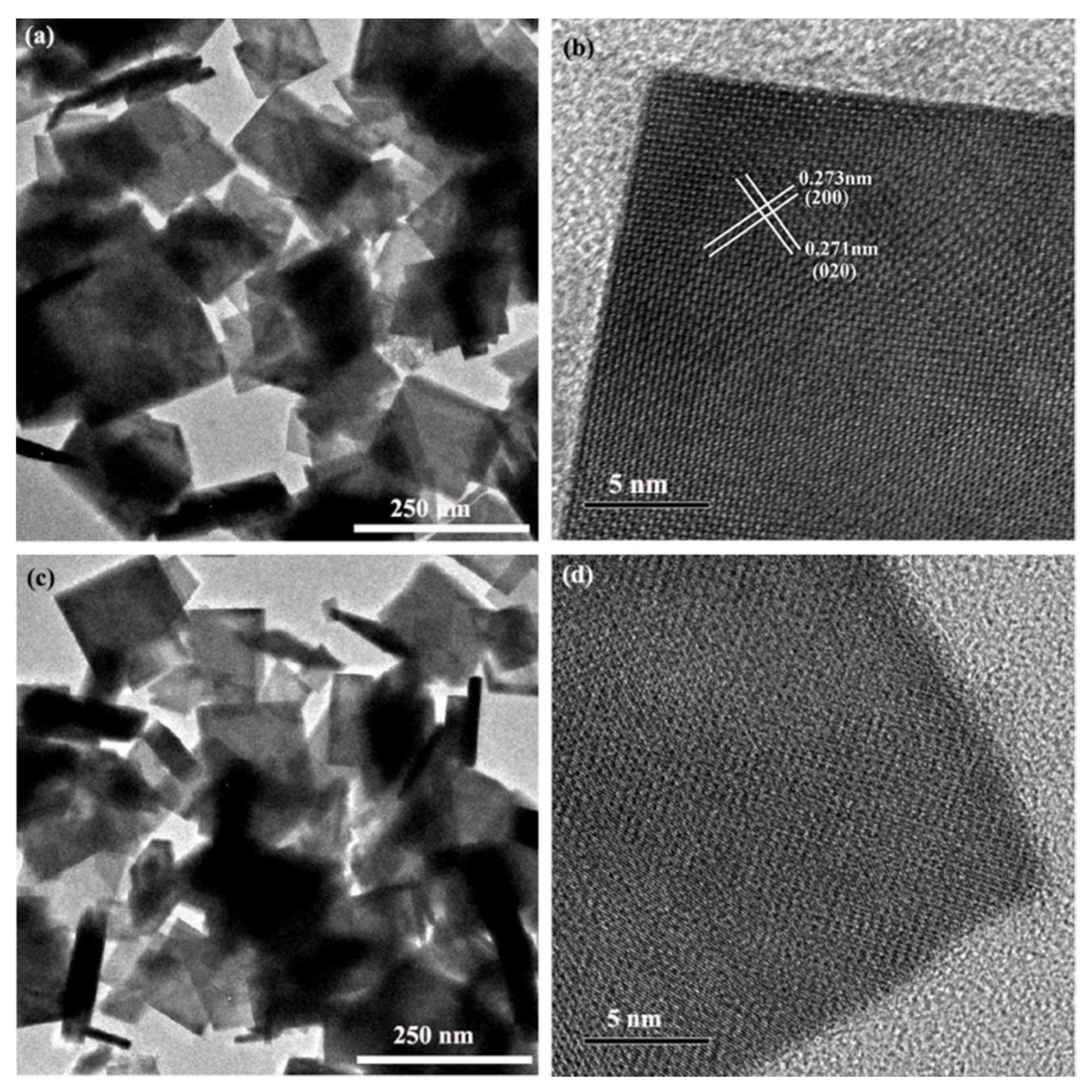
3.1. Morphology, Structure, and UV-Vis Spectra of Bi4Ti3O12 and Bi4Ti3O12−x
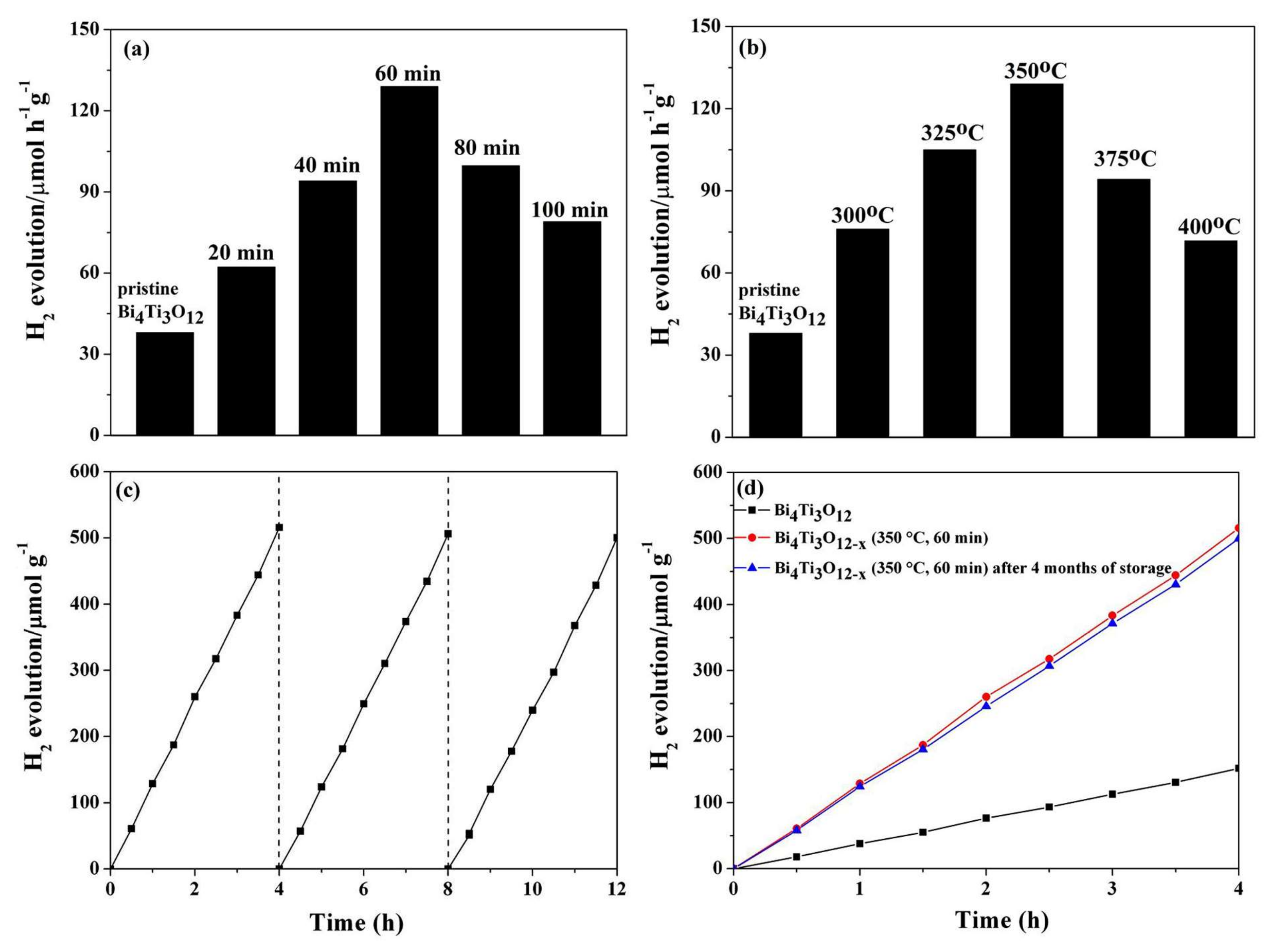
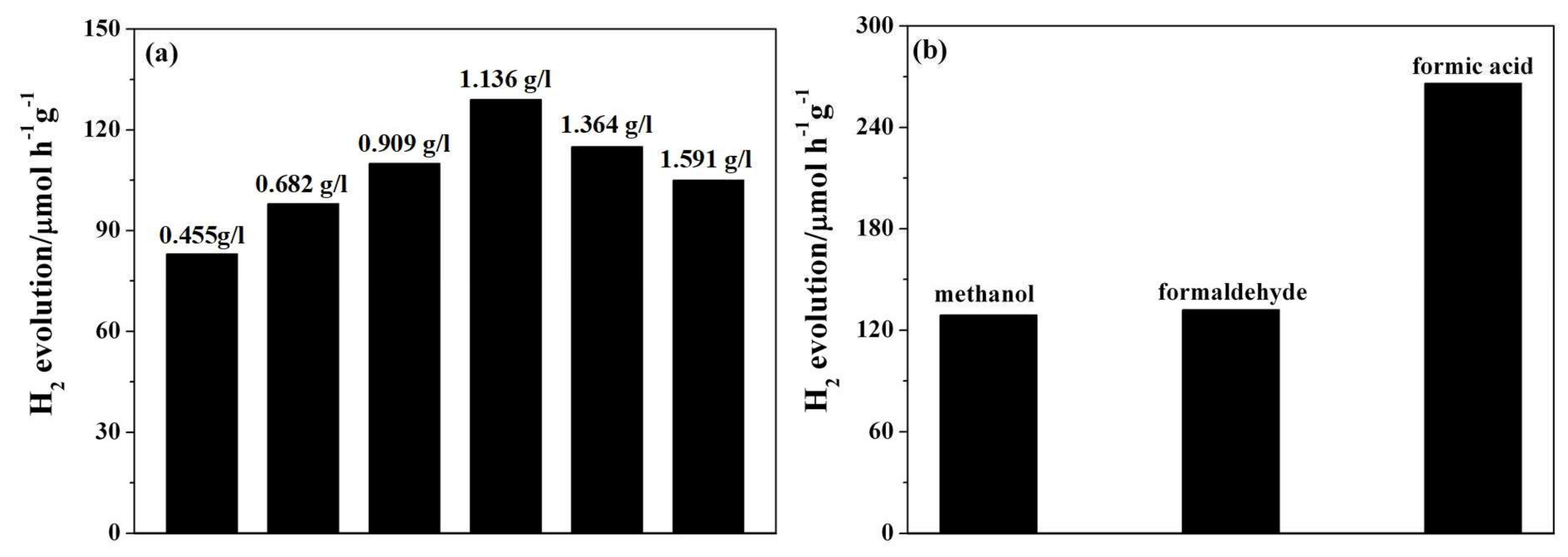
3.2. Photocatalytic Performance and Stability
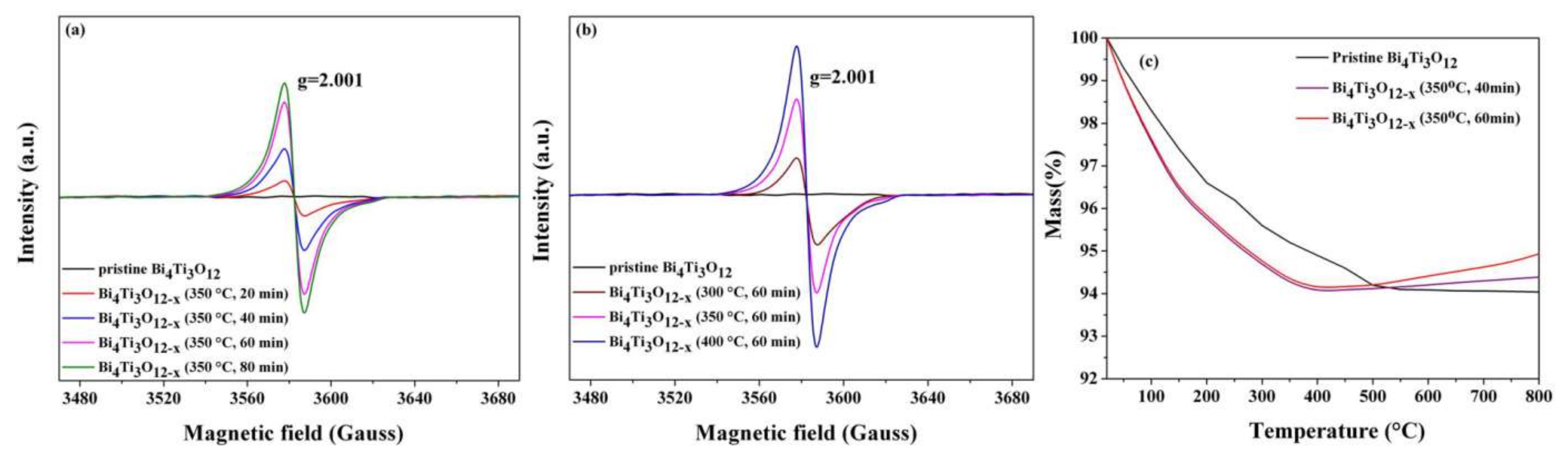
3.3. Surface Oxygen Vacancy Formation
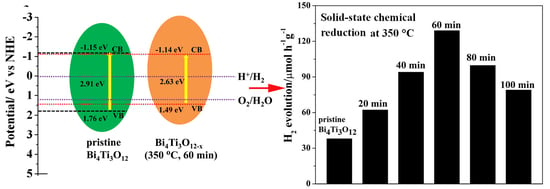
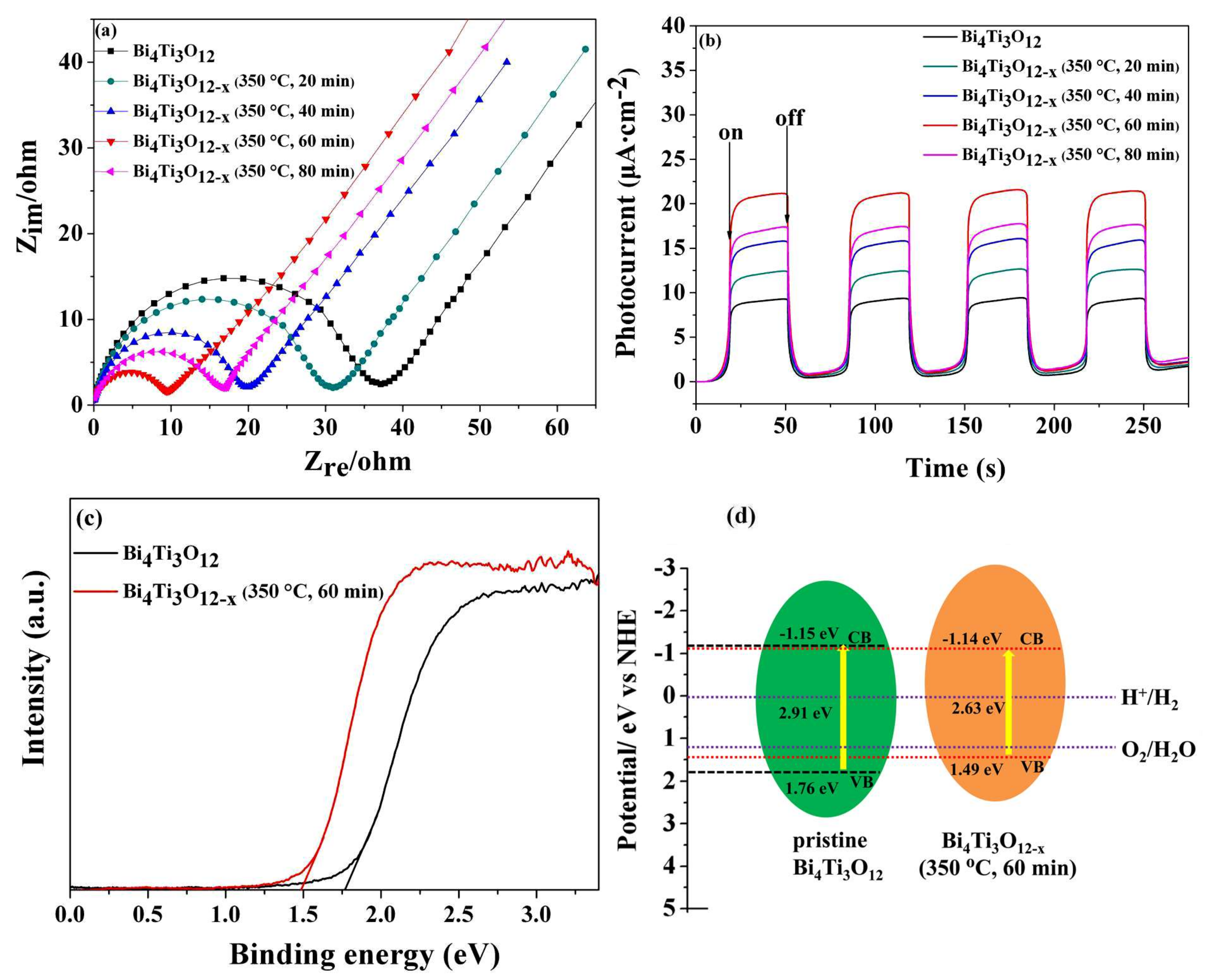
3.4. Mechanism of Enhanced Photocatalytic Activity of Bi4Ti3O12−x
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, W.; Tadé, M.O.; Shao, Z.P. Research progress of perovskite materials in photocatalysis-and photovoltaics-related energy conversion and environmental treatment. Chem. Soc. Rev. 2015, 44, 5371–5408. [Google Scholar] [CrossRef] [PubMed]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.X.; Tang, J.W. Visible-light driven heterojunction photocatalysts for water splitting–a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X. Titanium dioxide nanomaterials: Self-structural modifications. Chem. Rev. 2014, 114, 9890–9918. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, Y.; Dong, Y.; Chen, R.S.; Xiang, L.; Komarneni, S. Defect-rich ZnO nanosheets of high surface area as an efficient visible-light photocatalyst. Appl. Catal. B 2016, 192, 8–16. [Google Scholar] [CrossRef]
- Yu, J.G.; Yu, Y.F.; Zhou, P.; Xiao, W.; Cheng, B. Morphology-dependent photocatalytic H2-production activity of CdS. Appl. Catal. B 2014, 156, 184–191. [Google Scholar] [CrossRef]
- Kisch, H. Semiconductor photocatalysis-mechanistic and synthetic aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.F.; Luo, W.; Huang, Y.H.; Yu, J.C.; Hu, X.L. Synthesis of porous Bi4Ti3O12 nanofibers by electrospinning and their enhanced visible-light-driven photocatalytic properties. Nanoscale 2013, 5, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Jiang, H.; Jin, W.L.; Shi, C.K. Enhanced photocatalytic performance over Bi4Ti3O12 nanosheets with controllable size and exposed {001} facets for Rhodamine B degradation. Appl. Catal. B 2016, 180, 698–706. [Google Scholar] [CrossRef]
- Park, B.H.; Kang, B.S.; Bu, S.D.; Noh, T.W.; Lee, J.; Jo, W. Lanthanum-substituted bismuth titanate for use in non-volatile memories. Nature 1999, 401, 682–684. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Li, L.; Zhang, X. One-dimensional visible-light-driven bifunctional photocatalysts based on Bi4Ti3O12 nanofiber frameworks and Bi2XO6 (X = Mo, W) nanosheets. Appl. Catal. B 2014, 160–161, 757–766. [Google Scholar] [CrossRef]
- Kudo, A.; Hijii, S. H2 or O2 Evolution from aqueous solutions on layered oxide photocatalysts consisting of Bi3+ with 6s2 configuration and d0 transition metal ions. Chem. Lett. 1999, 28, 1103–1104. [Google Scholar] [CrossRef]
- Yao, W.; Xu, X.; Wang, H.; Zhou, J.T.; Yang, X.; Zhang, Y.; Shang, S.; Huang, B. Photocatalytic property of perovskite bismuth titanate. Appl. Catal. B 2004, 52, 109–116. [Google Scholar] [CrossRef]
- Zhao, W.; Jia, Z.; Lei, E.; Wang, L.; Li, Z.; Dai, Y. Photocatalytic degradation efficacy of Bi4Ti3O12 micro-scale platelets over methylene blue under visible light. J. Phys. Chem. Solids 2013, 74, 1604–1607. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, M.; Liu, S.; Wang, L.; Xiu, Z.; Zhou, Y.; Qiu, Z.; Zhang, A.; Ma, Q. Preparation and photocatalytic property of perovskite Bi4Ti3O12 films. Mater. Chem. Phys. 2009, 114, 716–721. [Google Scholar] [CrossRef]
- Hou, D.F.; Hu, X.; Hu, P.; Zhang, W.; Zhang, M.; Huang, Y.H. Bi4Ti3O12 nanofibers-BiOI nanosheets p-n junction: Facile synthesis and enhanced visible-light photocatalytic activity. Nanoscale 2013, 5, 9764–9772. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Tan, H.; Zhu, W.B.; Sun, Z.; Ma, Y.; Wang, E. Electrospun Cr-doped Bi4Ti3O12/Bi2Ti2O7 heterostructure fibers with enhanced visible-light photocatalytic properties. J. Mater. Chem. A 2015, 3, 6586–6591. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Li, L.; Gao, H.; Zhang, X. BiOCl nanosheet/Bi4Ti3O12 nanofiber heterostructures with enhanced photocatalytic activity. Catal. Commun. 2015, 58, 122–126. [Google Scholar] [CrossRef]
- Zheng, C.X.; Yang, H.; Cui, Z.M.; Zhang, H.M.; Wang, X.X. A novel Bi4Ti3O12/Ag3PO4 heterojunction photocatalyst with enhanced photocatalytic performance. Nanoscale Res. Lett. 2017, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, J.H.; Gao, Z.Q.; Zhu, X.; Liu, Y.; Wei, Z.B.; Zhao, W.; Sun, C. A simple and effective method for fabricating novel p-n heterojunction photocatalyst g-C3N4/Bi4Ti3O12 and its photocatalytic performances. Appl. Catal. B 2016, 192, 57–71. [Google Scholar] [CrossRef]
- Kim, H.G.; Hwang, D.; Lee, J. An undoped, single-phase oxide photocatalyst working under visible light. J. Am. Chem. Soc. 2004, 126, 8912–8913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Dai, R.Y.; Hu, S.R. Study of the role of oxygen vacancies as active sites in reduced graphene oxide-modified TiO2. Phys. Chem. Chem. Phys. 2017, 19, 7307–7315. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Wang, L.; Wu, T.; Zhang, Z.Y.; Borchardt, D.; Feng, P.Y. Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. J. Am. Chem. Soc. 2010, 132, 11856–11857. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Wang, Z.Y.; Huang, B.B.; Ma, Y.D.; Liu, Y.Y.; Qin, X.Y.; Zhang, X.Y.; Dai, Y. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.H.; Yao, W.Q.; Ma, X.G.; Pan, C.S.; Zong, R.L.; Zhu, Y.F. Surface oxygen vacancy induced visible activity and enhanced UV activity of ZnO1−x photocatalyst. Catal. Sci. Technol. 2013, 3, 3136–3146. [Google Scholar] [CrossRef]
- Ling, Y.C.; Wang, G.M.; Reddy, J.; Wang, C.C.; Zhang, J.Z.; Li, Y. The influence of oxygen content on the thermal activation of hematite nanowires. Angew. Chem. Int. Ed. 2012, 51, 4074–4079. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.L.; Yates, J.T. Surface science studies of the photoactivation of TiO2-new photochemical processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium- reduced black titania. Energy Environ. Sci. 2013, 6, 3007–3014. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. H-Doped black titania with very high solar absorption and excellent photocatalysis enhanced by localized surface plasmon resonance. Adv. Funct. Mater. 2013, 23, 5444–5450. [Google Scholar] [CrossRef]
- Cushing, S.K.; Meng, F.; Zhang, J.Y.; Ding, B.F.; Chen, C.K.; Chen, C.J.; Liu, R.S.; Bristow, A.D.; Bright, J.; Zheng, P.; et al. Effects of defects on photocatalytic activity of hydrogen-treated titanium oxide nanobelts. ACS Catal. 2017, 7, 1742–1748. [Google Scholar] [CrossRef]
- Lv, Y.H.; Yao, W.Q.; Zong, R.L.; Zhu, Y.F. Fabrication of wide-range-visible photocatalyst Bi2WO6−x nanoplates via surface oxygen vacancies. Sci. Rep. 2016, 6, 19347. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Krol, R.V.D. Selective photoreduction of nitric oxide to nitrogen by nanostructured TiO2 photocatalysts: Role of oxygen vacancies and iron dopant. J. Am. Chem. Soc. 2012, 134, 9369–9375. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Dai, Y.; Huang, B. Density functional characterization of the electronic structure and optical properties of N-doped, La-doped, and N/La-codoped SrTiO3. J. Phys. Chem. C 2009, 113, 5658–5663. [Google Scholar] [CrossRef]
- Mou, P.; Pal, U.; Jiménez, J.M.G.Y.; Pérezrodríguez, F. Effects of crystallization and dopant concentration on the emission behavior of TiO2: Eu nanophosphors. Nanoscale Res. Lett. 2012, 7, 1. [Google Scholar]
- Zhang, H.J.; Chen, G.; Li, X. Synthesis and visible light photocatalysis water splitting property of chromium-doped Bi4Ti3O12. Solid State Ionics 2009, 180, 1599–1603. [Google Scholar] [CrossRef]
- Hou, J.G.; Cao, R.; Wang, Z.; Jiao, S.Q.; Zhu, H.M. Chromium-doped bismuth titanate nanosheets as enhanced visible-light photocatalysts with a high percentage of reactive {110} facets. J. Mater. Chem. 2011, 21, 7296–7301. [Google Scholar] [CrossRef]
- Chen, Z.W.; Jiang, X.Y.; Zhu, C.B.; Shi, C.K. Chromium-modified Bi4Ti3O12 photocatalyst: Application forhydrogen evolution and pollutant degradation. Appl. Catal. B 2016, 199, 241–251. [Google Scholar] [CrossRef]
- Yang, P.J.; Ou, H.H.; Fang, Y.X.; Wang, X.C. A facile steam reforming strategy to delaminate layered carbon nitride semiconductors for photoredox catalysis. Angew. Chem. Int. Ed. 2017, 56, 3992–3996. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Shen, S.H.; Guo, L.J.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Allured, B.; DelaCruz, S.; Darling, T.; Huda, M.N.; Subramanian, V. Enhancing the visible light absorbance of Bi2Ti2O7 through Fe-substitution and its effects on photocatalytic hydrogen evolution. Appl. Catal. B 2014, 144, 261–268. [Google Scholar] [CrossRef]
- Choi, H.J.; Kang, M. Hydrogen production from methanol/water decomposition in a liquid photosystem using the anatase structure of Cu loaded TiO2. Int. J. Hydrogen Energy 2007, 32, 3841–3848. [Google Scholar] [CrossRef]
- Yang, X.; Salzmann, C.; Shi, H.; Malcolm, L.; Green, H.; Xiao, T. The role of photoinduced defects in TiO2 and its effects on hydrogen evolution from aqueous methanol solution. J. Phys. Chem. C 2008, 112, 10784–10789. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.; Pujari, S.; Muley, G.; Pagare, B.; Gambhire, A. Visible-light-activated nanocomposite photocatalyst of Cr2O3/SnO2. J. Nanostruct. Chem. 2013, 3, 46. [Google Scholar] [CrossRef]
- Chen, J.; Ollis, D.F.; Rulkens, W.H.; Bruning, H. Photocatalyzed oxidation of alcohols and organochlorides in the presence of native TiO2 and metallized TiO2 suspensions. Part (II): Photocatalytic mechanisms. Water Res. 1999, 33, 669–676. [Google Scholar] [CrossRef]
- Gupta, S.; De Leon, L.; Subramanian, V. Mn-modified Bi2Ti2O7 photocatalysts: Bandgap engineered multifunctional photocatalysts for hydrogen generation. Phys. Chem. Chem. Phys. 2014, 16, 12719–12727. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.H.; Pan, C.S.; Ma, X.G.; Zong, R.L.; Bai, X.J.; Zhu, Y.F. Production of visible activity and UV performance enhancement of ZnO photocatalyst via vacuum deoxidation. Appl. Catal. B 2013, 138–139, 26–32. [Google Scholar] [CrossRef]
- Lv, Y.H.; Liu, Y.F.; Zhu, Y.Y.; Zhu, Y.F. Surface oxygen vacancy induced photocatalytic performance enhancement of a BiPO4 nanorod. J. Mater. Chem. A 2014, 2, 1174–1182. [Google Scholar] [CrossRef]
- Ischenko, V.; Polarz, S.; Grote, D.; Stavarache, V.; Fink, K. Zinc oxide nanoparticles with defects. Adv. Funct. Mater. 2005, 15, 1945–1954. [Google Scholar] [CrossRef]
- Lv, Y.H.; Zhu, Y.Y.; Zhu, Y.F. Enhanced photocatalytic performance for the BiPO4–x nanorod induced by surface oxygen vacancy. J. Phys. Chem. C 2013, 117, 18520–18528. [Google Scholar] [CrossRef]
- Zhang, L.W.; Wang, L.; Zhu, Y.F. Synthesis and performance of BaAl2O4 with a wide spectral range of optical absorption. Adv. Funct. Mater. 2007, 173, 781–790. [Google Scholar] [CrossRef]
- Zhuang, W.; Li, L.; Zhu, J.; An, R.; Lu, L.; Lu, X.; Wu, X.; Ying, H. Facile synthesis of mesoporous MoS2-TiO2 nanofibers for ultrastable lithium ion battery anodes. Chemelectrochem 2015, 2, 374–381. [Google Scholar] [CrossRef]
- Li, L.; Shi, K.; Tu, R.; Qian, Q.; Li, D.; Yang, Z.; Lu, X. Black TiO2(B)/anatase bicrystalline TiO2-x nanofibers with enhanced photocatalytic performance. Chin. J. Catal. 2015, 36, 1943–1948. [Google Scholar] [CrossRef]
- Li, J.L.; Zhang, M.; Guan, Z.J.; Li, Q.Y.; He, C.Q.; Yang, J.J. Synergistic effect of surface and bulk single-electron-trapped oxygen vacancy of TiO2 in the photocatalytic reduction of CO2. Appl. Catal. B 2017, 206, 300–307. [Google Scholar] [CrossRef]
- Haider, Z.; Kang, Y.S. Facile preparation of hierarchical TiO2 nano structures: Growth mechanism and enhanced photocatalytic H2 production from water splitting using methanol as a sacrificial reagent. ACS Appl. Mater. Interfaces 2014, 6, 10342–10352. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Inc.: Chanhassen, MN, USA, 1992. [Google Scholar]
- Chu, M.W.; Ganne, M.; Caldes, M.T.; Brohan, L. X-ray photoelectron spectroscopy and high resolution electron microscopy studies of Aurivillius compounds: Bi4−xLaxTi3O12(x = 0, 0.5, 0.75, 1.0, 1.5, and 2.0). J. Appl. Phys. 2002, 91, 3178–3187. [Google Scholar] [CrossRef]
- Leelavathi, A.; Madras, G.; Ravishankar, N. Origin of enhanced photocatalytic activity and photoconduction in high aspect ratio ZnO nanorods. Phys. Chem. Chem. Phys. 2013, 15, 10795–10802. [Google Scholar] [CrossRef] [PubMed]
- Han, X.G.; He, H.Z.; Kuang, Q.; Zhou, X.; Zhang, X.H.; Xu, T.; Xie, Z.X.; Zheng, L.S. Controlling morphologies and tuning the related properties of nano/microstructured ZnO crystallites. J. Phys. Chem. C 2009, 113, 584–589. [Google Scholar] [CrossRef]
- Jovalekic, C.; Pavlovic, M.; Osmokrovic, P.; Atanasoska, L. X-ray photoelectron spectroscopy study of Bi4Ti3O12 ferroelectric ceramics. Appl. Phys. Lett. 1998, 72, 1051–1053. [Google Scholar] [CrossRef]
- He, H.; Yin, J.; Li, Y.; Zhang, Y.; Qiu, H.; Xu, J.; Xu, T.; Wang, C. Size controllable synthesis of single-crystal ferroelectric Bi4Ti3O12 nanosheet dominated with {001} facets toward enhanced visible-light-driven photocatalytic activities. Appl. Catal. B 2014, 156–157, 35–43. [Google Scholar] [CrossRef]
- Kong, M.; Li, Y.; Chen, X.; Tian, T.; Fang, P.; Zheng, F.; Zhao, X. Tuning the relative concentration ratio of bulk defects to surface defects in TiO2 nanocrystals leads to high photocatalytic efficiency. J. Am. Chem. Soc. 2011, 133, 16414–16417. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Chattopadhyay, S.; Jana, D.; Banerjee, A.; Manik, S.; Pradhan, S.K.; Sutradhar, M.; Sarkar, A. Annealing effect on nano-ZnO powder studied from positron lifetime and optical absorption spectroscopy. J. Appl. Phys. 2006, 100, 114328. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, K.; Wang, L.; Wang, B.; Li, Y. Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods. J. Am. Chem. Soc. 2009, 131, 3140–3141. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Xiao, C.; Zhang, J.; Fan, S.; An, R.; Cheng, Q.; Xie, J.; Zhou, M.; Ye, B.; Xie, Y. Vacancy associates promoting solar-driven photocatalytic activity of ultrathin bismuth oxychloride nanosheets. J. Am. Chem. Soc. 2013, 135, 10411–10417. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Becker, W.G.; Truong, M.M.; Ai, C.C.; Hamel, N.N. Interfacial factors that affect the photoefficiency of semiconductor-sensitized oxidations in nonaqueous media. ACS J. Phys. Chem. 1989, 93, 4882–4886. [Google Scholar] [CrossRef]
- Pei, R.; Cheng, Z.; Wang, E.; Yang, X. Amplification of antigen-antibody interactions based on biotin labeled protein-streptavidin network complex using impedance spectroscopy. Biosens. Bioelectron. 2001, 16, 355–361. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; He, J. Controlled fabrication and photocatalytic properties of a three-dimensional ZnO nanowire/reduced graphene oxide/CdS heterostructure on carbon cloth. Nanoscale 2013, 5, 1129–11297. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zong, R.; Li, C.; Liu, D.; Liu, Y.; Zhu, Y.F. Enhancement of visible photocatalytic activity via Ag@C3N4 core–shell plasmonic composite. Appl. Catal. B 2014, 147, 82–91. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Jeon, J.P.; Kang, J.; Han, B.; Yu, J.S. Oxygen-deficient zirconia (ZrO2−x): A new material for solar light absorption. Sci. Rep. 2016, 6, 27218. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Zhao, Y.J.; Luo, J.Y.; Xia, Y.Y. Oxygen vacancy in LiTiPO5 and LiTi2(PO4)3: A first-principles study. Phys. Lett. A 2011, 375, 934–938. [Google Scholar] [CrossRef]
- Dette, C.; Perez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]



| Samples | Band Gap (eV) |
|---|---|
| Bi4Ti3O12 | 2.91 |
| Bi4Ti3O12−x (350 °C, 20 min) | 2.83 |
| Bi4Ti3O12−x (350 °C, 40 min) | 2.74 |
| Bi4Ti3O12−x (350 °C, 60 min) | 2.63 |
| Bi4Ti3O12−x (350 °C, 80 min) | 2.57 |
| Bi4Ti3O12−x (350 °C, 100 min) | 2.48 |
| Bi4Ti3O12−x (300 °C, 60 min) | 2.77 |
| Bi4Ti3O12−x (400 °C, 60 min) | 2.39 |
| Sample | Light Source | Reactant Solution | H2 Evolution Rate/μmol·g−1·h−1 | Reference |
|---|---|---|---|---|
| Bi4Ti3O12−x (350 °C, 60 min) | 300 W Xe Lamp (λ > 400 nm) | 200 mL water + 20 mL methanol | 129 | This work |
| Bi4Ti3O12 | 350 W high pressure Xe lamp (λ > 400 nm) | 400 mL water + 20 mL methanol | 36 | [37] |
| Bi4Ti2.6Cr0.4O12 | 350 W high pressure Xe lamp (λ > 400 nm) | 400 mL water + 20 mL methanol | 58.1 | [37] |
| Bi4Ti3O12 | 300 W Xe Lamp (λ > 400 nm) | 400 mL water + 20 mL methanol | 42 | [38] |
| Bi4Ti2.6Cr0.4O12 | 300 W Xe Lamp (λ > 400 nm) | 400 mL water + 20 mL methanol | 98 | [38] |
| Bi4Ti2.6Cr0.4O12 | 300 W Xe Lamp (λ > 420 nm) | 400 mL water + 30 mL methanol | 117 | [39] |
| Sample | τ1 (ps) | τ2 (ps) | τ3 (ns) | I1 (%) | I2 (%) | I3 (%) | I1/I2 |
|---|---|---|---|---|---|---|---|
| Bi4Ti3O12 | 193 | 376 | 2.33 | 50.24 | 47.78 | 1.98 | 1.05 |
| Bi4Ti3O12−x (350 °C, 20 min) | 196 | 387 | 2.47 | 46.26 | 51.97 | 1.77 | 0.89 |
| Bi4Ti3O12−x (350 °C, 40 min) | 199 | 389 | 2.49 | 38.72 | 59.64 | 1.64 | 0.65 |
| Bi4Ti3O12−x (350 °C, 60 min) | 205 | 393 | 2.77 | 23.87 | 74.24 | 1.89 | 0.32 |
| Bi4Ti3O12−x (350 °C, 80 min) | 209 | 396 | 2.92 | 36.48 | 61.57 | 1.95 | 0.59 |
| Bi4Ti3O12−x (350 °C, 100 min) | 214 | 402 | 3.05 | 41.73 | 56.42 | 1.85 | 0.74 |
| Bi4Ti3O12−x (400 °C, 60 min) | 216 | 405 | 3.11 | 44.86 | 53.33 | 1.81 | 0.84 |
| Samples | BET Specific Surface Area (m2/g) |
|---|---|
| Bi4Ti3O12 | 6.45 |
| Bi4Ti3O12−x (350 °C, 20 min) | 6.39 |
| Bi4Ti3O12−x (350 °C, 40 min) | 6.35 |
| Bi4Ti3O12−x (350 °C, 60 min) | 6.32 |
| Bi4Ti3O12−x (350 °C, 80 min) | 6.46 |
| Bi4Ti3O12−x (350 °C, 100 min) | 6.48 |
| Bi4Ti3O12−x (300 °C, 60 min) | 6.38 |
| Bi4Ti3O12−x (400 °C, 60 min) | 6.51 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, Z.; Lu, Z. A Facile Method for the Preparation of Colored Bi4Ti3O12−x Nanosheets with Enhanced Visible-Light Photocatalytic Hydrogen Evolution Activity. Nanomaterials 2018, 8, 261. https://doi.org/10.3390/nano8040261
Zhang Y, Chen Z, Lu Z. A Facile Method for the Preparation of Colored Bi4Ti3O12−x Nanosheets with Enhanced Visible-Light Photocatalytic Hydrogen Evolution Activity. Nanomaterials. 2018; 8(4):261. https://doi.org/10.3390/nano8040261
Chicago/Turabian StyleZhang, Yizeng, Zhiwu Chen, and Zhenya Lu. 2018. "A Facile Method for the Preparation of Colored Bi4Ti3O12−x Nanosheets with Enhanced Visible-Light Photocatalytic Hydrogen Evolution Activity" Nanomaterials 8, no. 4: 261. https://doi.org/10.3390/nano8040261
APA StyleZhang, Y., Chen, Z., & Lu, Z. (2018). A Facile Method for the Preparation of Colored Bi4Ti3O12−x Nanosheets with Enhanced Visible-Light Photocatalytic Hydrogen Evolution Activity. Nanomaterials, 8(4), 261. https://doi.org/10.3390/nano8040261