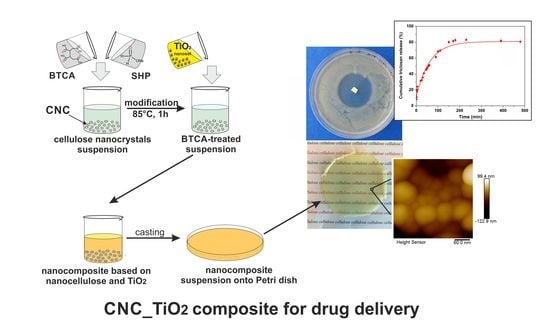
Hybrid Drug Delivery Patches Based on Spherical Cellulose Nanocrystals and Colloid Titania—Synthesis and Antibacterial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
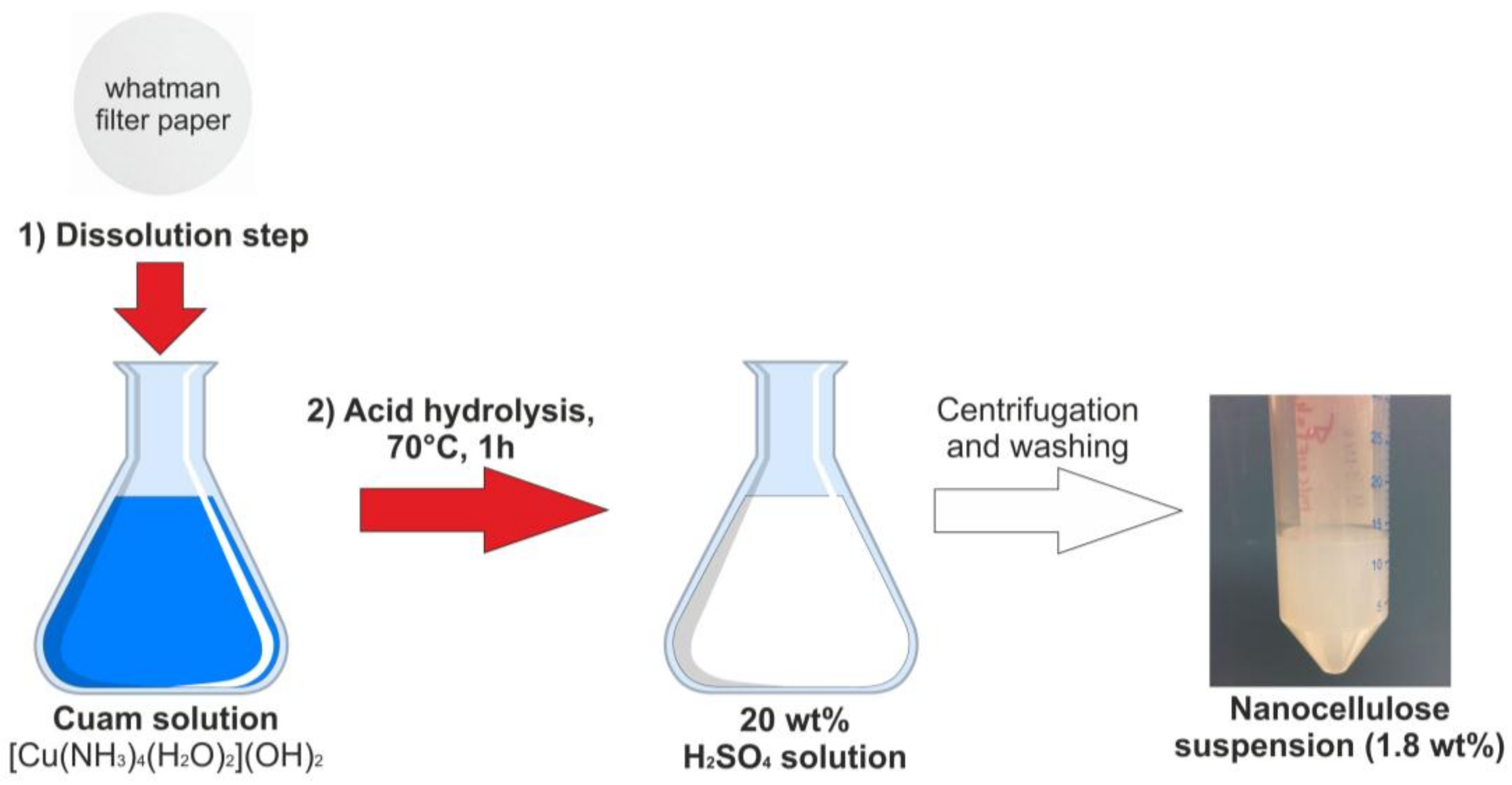
2.2. Synthesis of Spherical Cellulose Nanocrystals
2.3. Bionanocomposite Films Preparation
2.4. Characterization
2.5. In Vitro Drug Release
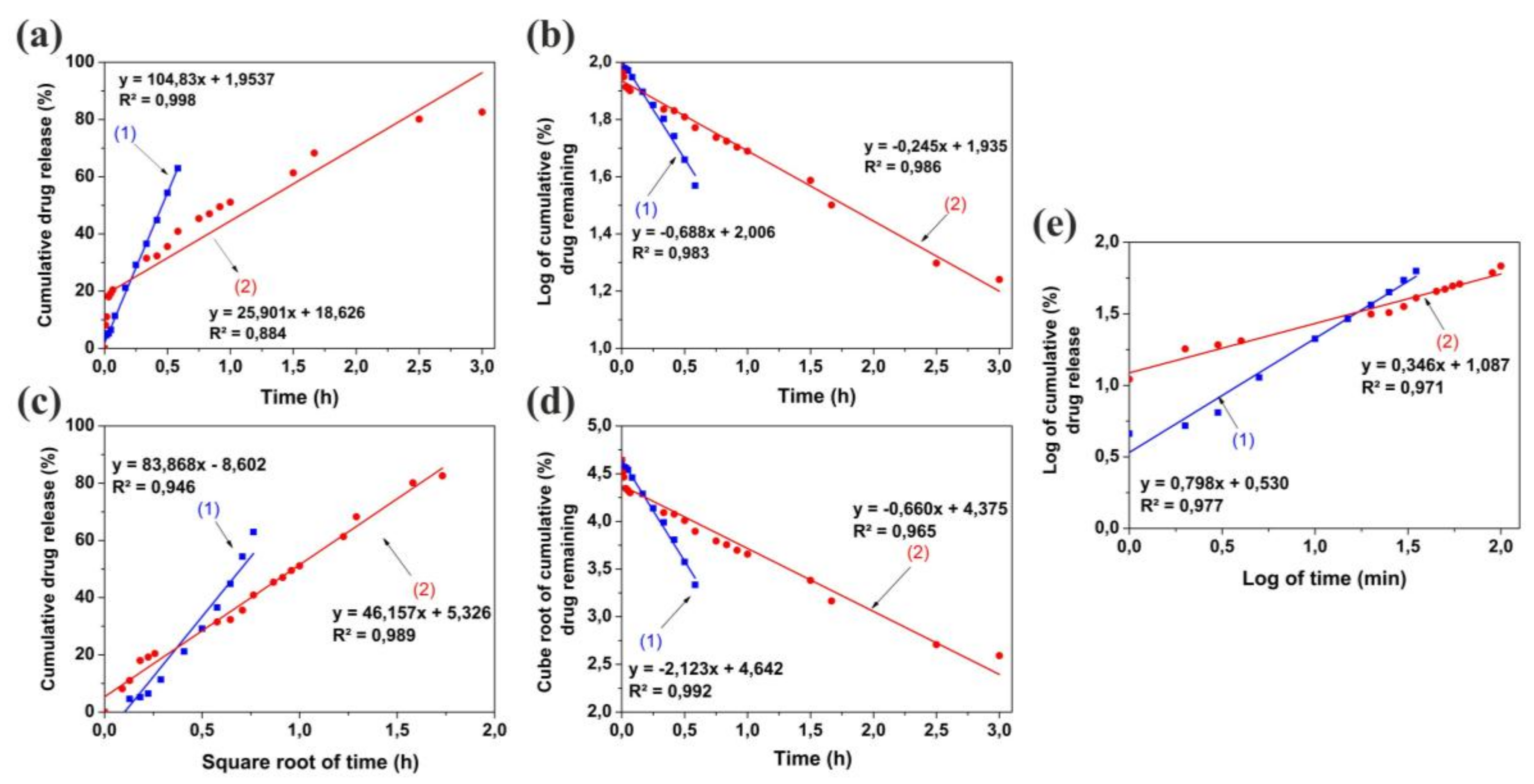
2.6. Mathematical Modelling of Release Kinetics
2.7. In Vitro Antibacterial Studies
2.8. Molecular Model Compounds
3. Results and Discussion
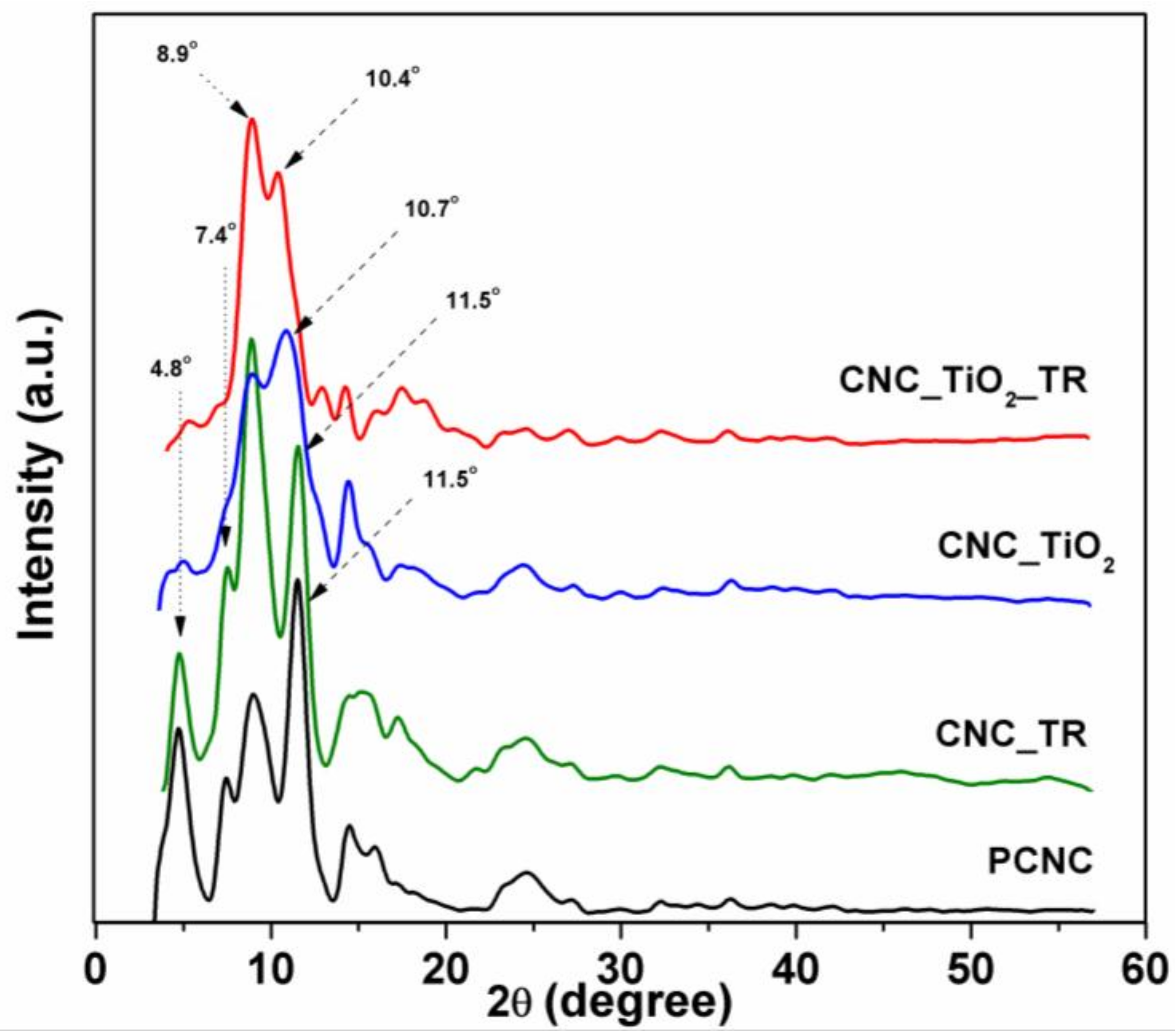
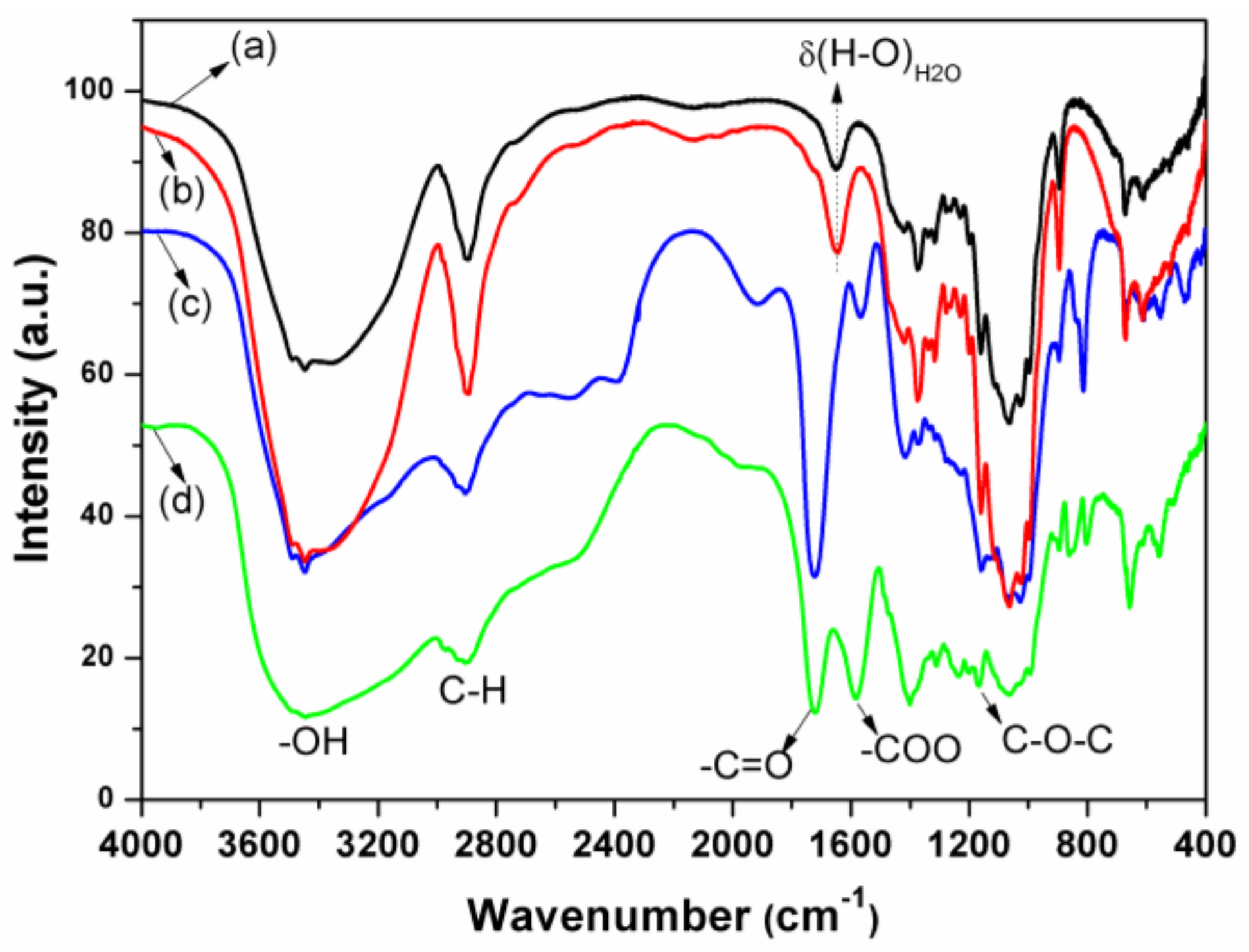
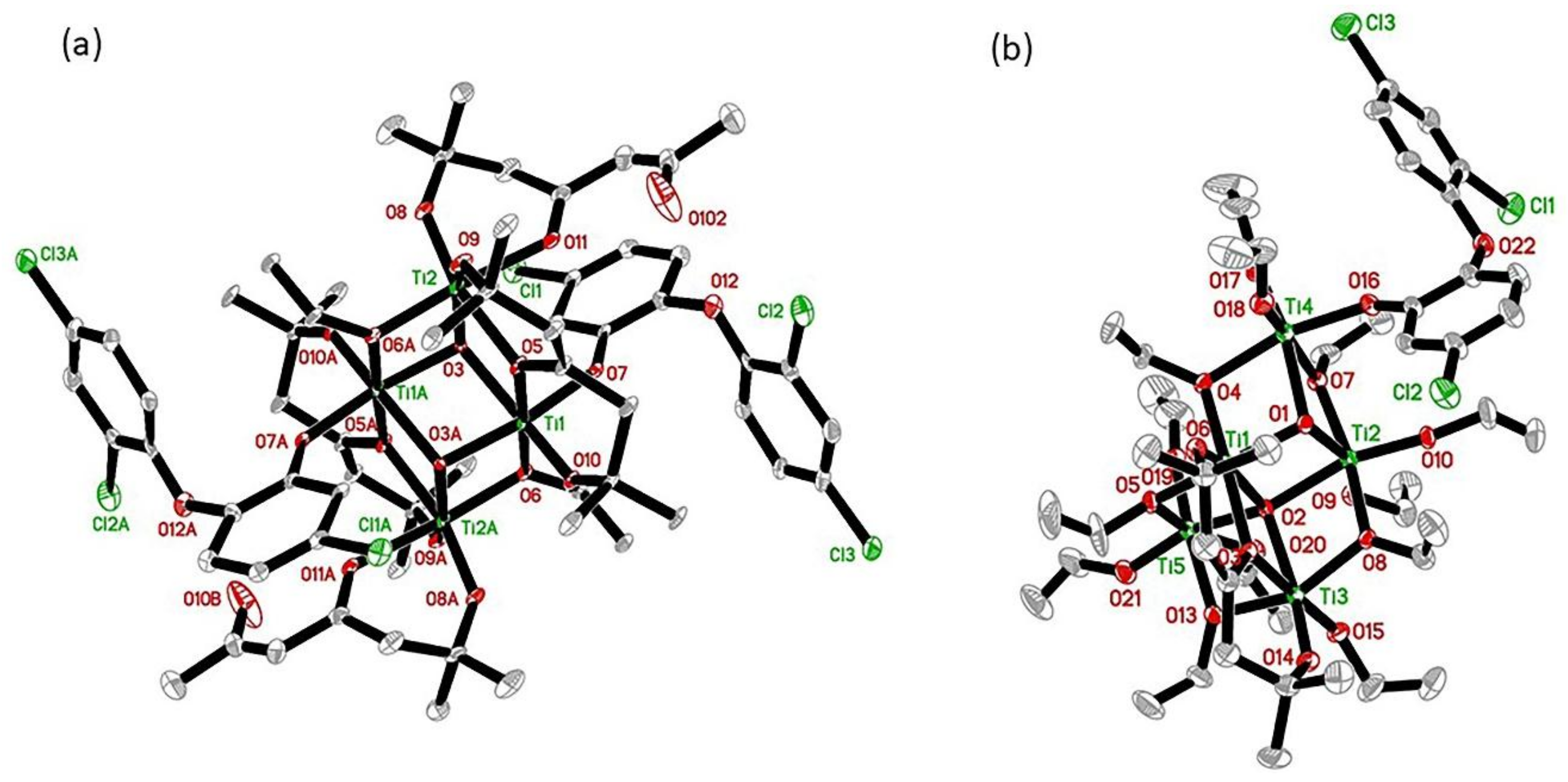
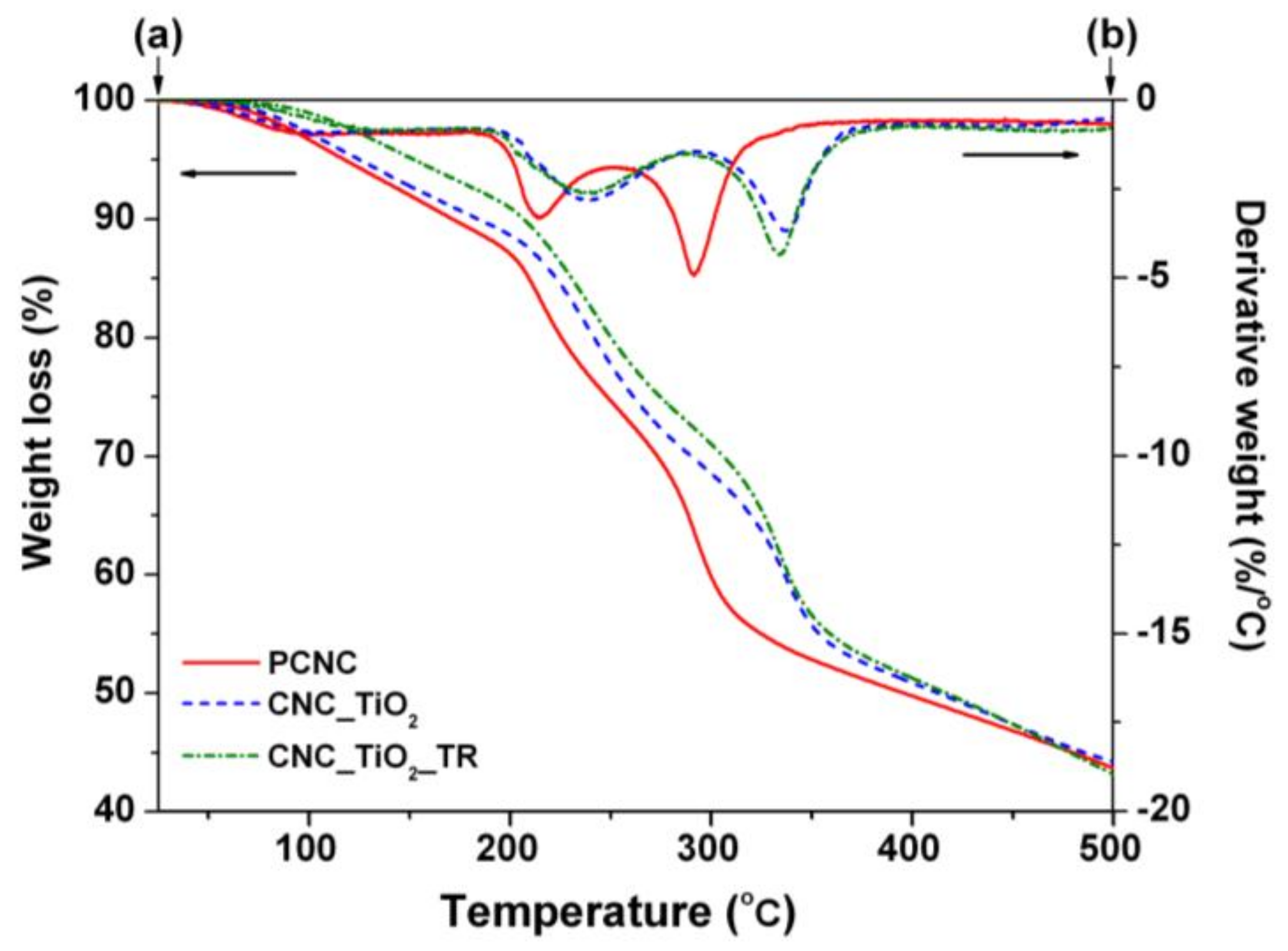
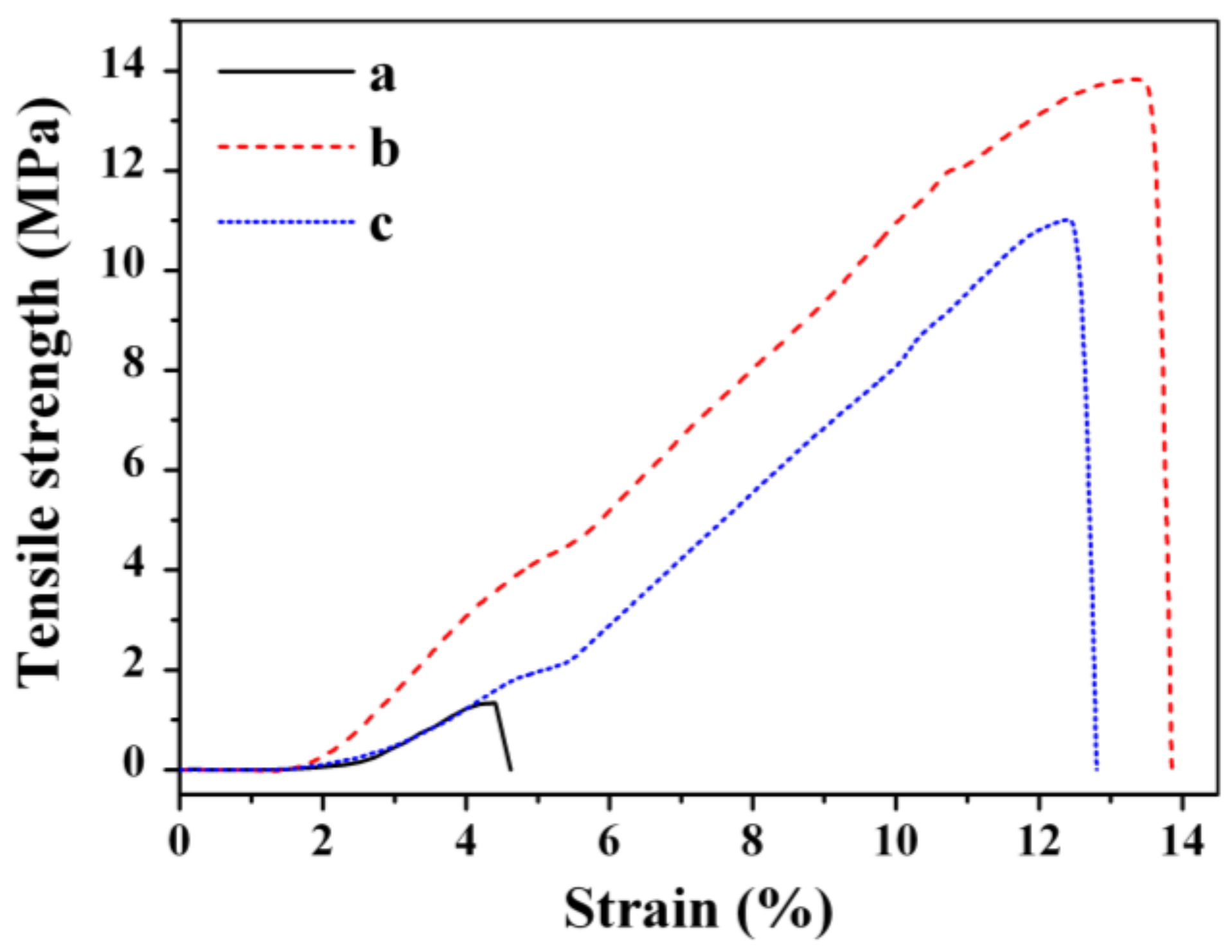
3.1. Preparation and Characterization of Nanocellulose Based-Nanocomposites
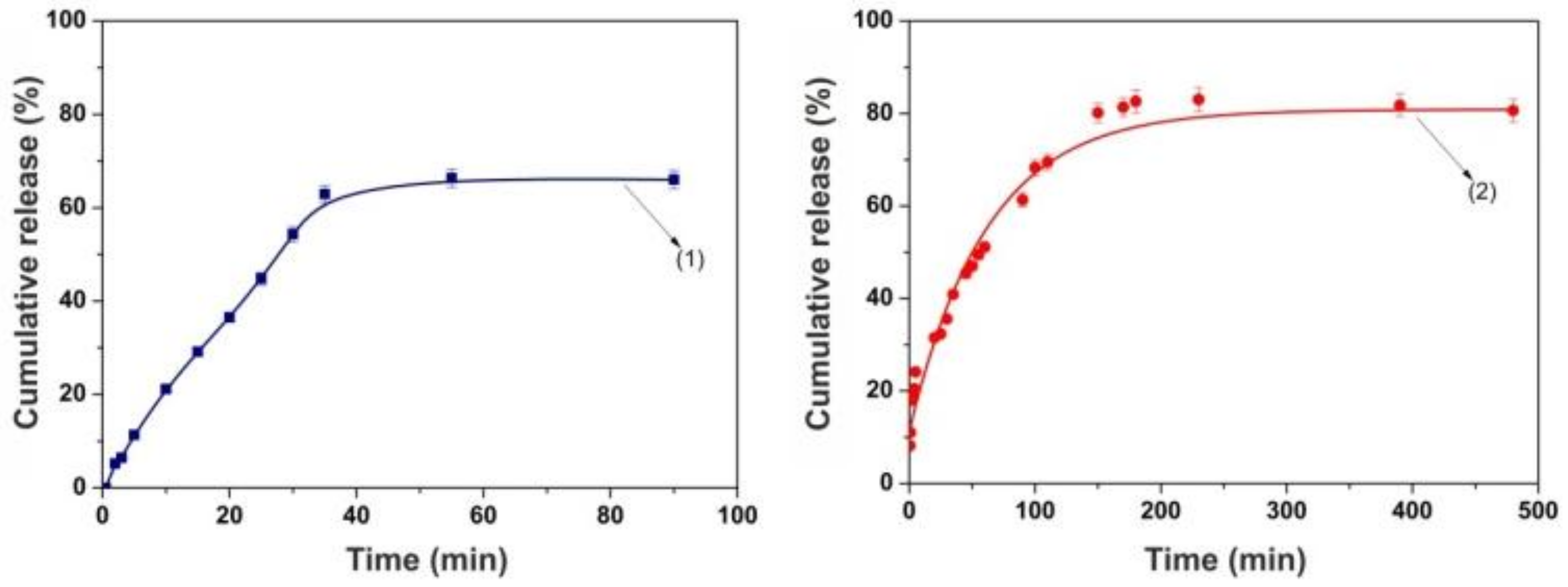
3.2. In Vitro Drug Release Studies and Kinetics
3.3. Antimicrobial Activity of the Obtained Nanocomposites
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cohen, M.L. Changing patterns of infectious disease. Nature 2000, 406, 762–767. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Allen, H.K.; Donato, J.; Wang, H.H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010, 8, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Cantas, L.; Shah, S.Q.A.; Cavaco, L.M.; Manaia, C.M.; Walsh, F.; Popowska, M.; Garelick, H.; Bürgmann, H.; Sørum, H. A brief multi-disciplinary review on antimicrobial resistance in medicine and its linkage to the global environmental microbiota. Front. Microbiol. 2013, 4, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.W.; Luong, J.H.T. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Juang, T.Y.; Chen, Y.C.; Tsai, C.C. Nanoscale organic/inorganic hybrids based on self-organized dendritic macromolecules on montmorillonites. Appl. Clay Sci. 2010, 48, 103–110. [Google Scholar] [CrossRef]
- Depan, D.; Venkata Surya, P.K.C.; Girase, B.; Misra, R.D.K. Organic/inorganic hybrid network structure nanocomposite scaffolds based on grafted chitosan for tissue engineering. Acta Biomater. 2011, 7, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Parola, S.; Julián-López, B.; Carlos, L.D.; Sanchez, C. Optical Properties of Hybrid Organic-Inorganic Materials and their Applications. Adv. Funct. Mater. 2016, 26, 6506–6544. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.; French, A.; Concha, M.; DeLucca, A.; Wu, Q. Nanocellulose-Based Biosensors: Design, Preparation, and Activity of Peptide-Linked Cotton Cellulose Nanocrystals Having Fluorimetric and Colorimetric Elastase Detection Sensitivity. Engineering 2013, 5, 20–28. [Google Scholar] [CrossRef]
- Hood, M.; Mari, M.; Muñoz-Espí, R. Synthetic Strategies in the Preparation of Polymer/Inorganic Hybrid Nanoparticles. Materials 2014, 7, 4057–4087. [Google Scholar] [CrossRef] [PubMed]
- Wicklein, B.; Salazar-Alvarez, G. Functional hybrids based on biogenic nanofibrils and inorganic nanomaterials. J. Mater. Chem. A 2013, 1, 5469. [Google Scholar] [CrossRef]
- Letchford, J.K.; Jackson, K.; Wasserman, B.; Ye, L.; Hamad, W.; Burt, H. The use of nanocrystalline cellulose for the binding and controlled release of drugs. Int. J. Nanomed. 2011, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.M.A.; Gomes, M.E.; Reis, R.L. The Potential of Cellulose Nanocrystals in Tissue Engineering Strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Guise, C.; Fangueiro, R. Biomedical Applications of Nanocellulose; Springer: Dordrecht, The Netherlands, 2016; pp. 155–169. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, J.-Y.; Li, H.-Q.; Zhao, A.Z.-J.; Wang, Y.; Zhang, K.-Q.; Sun, H.-T.; Lai, Y.-K. Recent advances on smart TiO2 nanotube platforms for sustainable drug delivery applications. Int. J. Nanomed. 2017, 12, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Aw, M.S.; Addai-Mensah, J.; Losic, D. A multi-drug delivery system with sequential release using titania nanotube arrays. Chem. Commun. 2012, 48, 3348–3350. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, A.; Engelbrecht, T.; Neubert, R.H.H.; Goebel, A.S.B. New nanosized technologies for dermal and transdermal drug delivery. A review. J. Biomed. Nanotechnol. 2010, 6, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Basavaraj, K.H.; Johnsy, G.; Navya, M.A.; Rashmi, R.; Siddaramaiah. Biopolymers as transdermal drug delivery systems in dermatology therapy. Crit. Rev. Ther. Drug Carrier Syst. 2010, 27, 155–185. [Google Scholar] [PubMed]
- Tanwar, H.; Sachdeva, R. Transdermal drug delivery system: A review. Int. J. Pharm. Sci. Res. 2016, 7, 2274–2290. [Google Scholar] [CrossRef]
- Kolakovic, R.; Peltonen, L.; Laukkanen, A.; Hirvonen, J.; Laaksonen, T. Nanofibrillar cellulose films for controlled drug delivery. Eur. J. Pharm. Biopharm. 2012, 82, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Moritz, S.; Wiegand, C.; Wesarg, F.; Hessler, N.; Miller, F.; Kralisch, D.; Hipler, U.-C.; Fischer, D. Active wound dressings based on bacterial nanocellulose as drug delivery system for octenidine. Int. J. Pharm. 2014, 471, 45–55. [Google Scholar] [CrossRef]
- Huang, L.; Chen, X.; Nguyen, T.X.; Tang, H.; Zhang, L.; Yang, G. Nano-cellulose 3D-networks as controlled-release drug carriers. J. Mater. Chem. B 2013, 1, 2976. [Google Scholar] [CrossRef]
- da Silva, E.P.; Guilherme, M.R.; Garcia, F.P.; Nakamura, C.V.; Cardozo-Filho, L.; Alonso, C.G.; Rubira, A.F.; Kunita, M.H. Drug release profile and reduction in the in vitro burst release from pectin/HEMA hydrogel nanocomposites crosslinked with titania. RSC Adv. 2016, 6, 19060–19068. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Hiekkataipale, P.; Malm, J.; Karppinen, M.; Ikkala, O.; Ras, R.H.A. Inorganic Hollow Nanotube Aerogels by Atomic Layer Deposition onto Native Nanocellulose Templates. ACS Nano 2011, 5, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Galkina, O.L.; Ivanov, V.K.; Agafonov, A.V; Seisenbaeva, G.A.; Kessler, V.G. Cellulose nanofiber-titania nanocomposites as potential drug delivery systems for dermal applications. J. Mater. Chem. B 2015, 3, 1688–1698. [Google Scholar] [CrossRef]
- Galkina, O.L.; Önneby, K.; Huang, P.; Ivanov, V.K.; Agafonov, A.V.; Seisenbaeva, G.A.; Kessler, V.G. Antibacterial and photochemical properties of cellulose nanofiber–titania nanocomposites loaded with two different types of antibiotic medicines. J. Mater. Chem. B 2015, 3, 7125–7134. [Google Scholar] [CrossRef]
- Food and Drug Administration. Safety and Effectiveness of Consumer Antiseptics: Topical Antimicrobial Drug Products for Over-the-Counter Human Use. Fed. Regist. 2016, 81, 61106–61130. [Google Scholar]
- Lim, H.; Hoag, S.W. Plasticizer effects on physical-mechanical properties of solvent cast Soluplus® films. AAPS PharmSciTech 2013, 14, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.J.; Camps, R.; Díaz, A.; Franco, L.; Rodríguez-Galán, A.; Puiggalí, J. Electrospinning of polylactide and polycaprolactone mixtures for preparation of materials with tunable drug release properties. J. Polym. Res. 2011, 18, 1903–1917. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Ahuja, N.; Katare, O.P.; Singh, B. Studies on dissolution enhancement and mathematical modeling of drug release of a poorly water-soluble drug using water-soluble carriers. Eur. J. Pharm. Biopharm. 2007, 65, 26–38. [Google Scholar] [CrossRef]
- Gomez Escalada, M.; Russell, A.D.; Maillard, J.-Y.; Ochs, D. Triclosan-bacteria interactions: Single or multiple target sites? Lett. Appl. Microbiol. 2005, 41, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S.; Villalobos, M.; Cranston, E.D. Benchmarking Cellulose Nanocrystals: From the Laboratory to Industrial Production. Langmuir 2017, 33, 1583–1598. [Google Scholar] [CrossRef] [PubMed]
- Satyamurthy, P.; Vigneshwaran, N. A novel process for synthesis of spherical nanocellulose by controlled hydrolysis of microcrystalline cellulose using anaerobic microbial consortium. Enzyme Microb. Technol. 2013, 52, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Elder, T.J.; Pu, Y.; Ragauskas, A.J. Facile synthesis of spherical cellulose nanoparticles. Carbohydr. Polym. 2007, 69, 607–611. [Google Scholar] [CrossRef]
- Xiong, R.; Zhang, X.; Tian, D.; Zhou, Z.; Lu, C. Comparing microcrystalline with spherical nanocrystalline cellulose from waste cotton fabrics. Cellulose 2012, 19, 1189–1198. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Kessler, V.G.; Seisenbaeva, G. a.; Unell, M.; Håkansson, S. Chemically triggered biodelivery using metal-organic sol-gel synthesis. Angew. Chem. Int. Ed. 2008, 47, 8506–8509. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; John Wiley & Sons/Wiley: Hoboken, NJ, USA, 2017; pp. 1–49. [Google Scholar]
- Poletto, M.; Ornaghi Júnior, H.L.; Zattera, A.J. Native cellulose: Structure, characterization and thermal properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Asri, S.E.; Ismail, A.F. Physicochemical properties of “green” nanocrystalline cellulose isolated from recycled newspaper. RSC Adv. 2015, 5, 29842–29849. [Google Scholar] [CrossRef]
- Yang, C.Q.; Xu, Y.; Wang, D. FT-IR Spectroscopy Study of the Polycarboxylic Acids Used for Paper Wet Strength Improvement. Ind. Eng. Chem. Res. 1996, 5885, 4037–4042. [Google Scholar] [CrossRef]
- Svensson, F.G.; Seisenbaeva, G.A.; Kessler, V.G. Mixed-Ligand Titanium “Oxo Clusters”: Structural Insights into the Formation and Binding of Organic Molecules and Transformation into Oxide Nanostructures on Hydrolysis and Thermolysis. Eur. J. Inorg. Chem. 2017, 2017, 4117–4122. [Google Scholar] [CrossRef]
- George, J.; Ramana, K.V.; Sabapathy, S.N.; Jagannath, J.H.; Bawa, A.S. Characterization of chemically treated bacterial (Acetobacter xylinum) biopolymer: Some thermo-mechanical properties. Int. J. Biol. Macromol. 2005, 37, 189–194. [Google Scholar] [CrossRef]
- Patil, N.V.; Netravali, A.N. Nonedible Starch Based “Green” Thermoset Resin Obtained via Esterification Using a Green Catalyst. ACS Sustain. Chem. Eng. 2016, 4, 1756–1764. [Google Scholar] [CrossRef]
- Wang, N.; Ding, E.; Cheng, R. Thermal degradation behaviors of spherical cellulose nanocrystals with sulfate groups. Polymer 2007, 48, 3486–3493. [Google Scholar] [CrossRef]
- Schütz, C.; Sort, J.; Bacsik, Z.; Oliynyk, V.; Pellicer, E.; Fall, A.; Wågberg, L.; Berglund, L.; Bergström, L.; Salazar-Alvarez, G. Hard and Transparent Films Formed by Nanocellulose–TiO2 Nanoparticle Hybrids. PLoS ONE 2012, 7, e45828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kockisch, S.; Rees, G.D.; Tsibouklis, J.; Smart, J.D. Mucoadhesive, triclosan-loaded polymer microspheres for application to the oral cavity: Preparation and controlled release characteristics. Eur. J. Pharm. Biopharm. 2005, 59, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Pappas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Denyer, S.P.; Maillard, J.-Y. Cellular impermeability and uptake of biocides and antibiotics in Gram-negative bacteria. J. Appl. Microbiol. 2002, 92, 35S–45S. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Broughton, R.M.; Akdag, A.; Worley, S.D.; Huang, T.-S. Antimicrobial Fibers Created via Polycarboxylic Acid Durable Press Finishing. Text. Res. J. 2007, 77, 604–611. [Google Scholar] [CrossRef]
- Alimohammadi, F.; Gashti, M.P.; Shamei, A. A novel method for coating of carbon nanotube on cellulose fiber using 1,2,3,4-butanetetracarboxylic acid as a cross-linking agent. Prog. Org. Coat. 2012, 74, 470–478. [Google Scholar] [CrossRef]
- Orhan, M.; Kut, D.; Gunesoglu, C. Improving the antibacterial activity of cotton fabrics finished with triclosan by the use of 1,2,3,4-butanetetracarboxylic acid and citric acid. J. Appl. Polym. Sci. 2009, 111, 1344–1352. [Google Scholar] [CrossRef]
- Yazhini Bharathi, K.; Prabu Gurumallesh, H.; Nandhini Rathna, J. Synthesis and coating of zno-btca composite on cotton for antibacterial activity—Science research library. J. Environ. Appl. Biores. 2015, 3, 150–154. [Google Scholar]
- Missoum, K.; Sadocco, P.; Causio, J.; Belgacem, M.N.; Bras, J. Antibacterial activity and biodegradability assessment of chemically grafted nanofibrillated cellulose. Mater. Sci. Eng. C 2014, 45, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Seisenbaeva, G.A.; Daniel, G.; Nedelec, J.-M.; Kessler, V.G. Solution equilibrium behind the room-temperature synthesis of nanocrystalline titanium dioxide. Nanoscale 2013, 5, 3330. [Google Scholar] [CrossRef] [PubMed]
- Seisenbaeva, G.A.; Moloney, M.P.; Tekoriute, R.; Hardy-Dessources, A.; Nedelec, J.-M.; Gun’ko, Y.K.; Kessler, V.G. Biomimetic Synthesis of Hierarchically Porous Nanostructured Metal Oxide Microparticles—Potential Scaffolds for Drug Delivery and Catalysis. Langmuir 2010, 26, 9809–9817. [Google Scholar] [CrossRef] [PubMed]

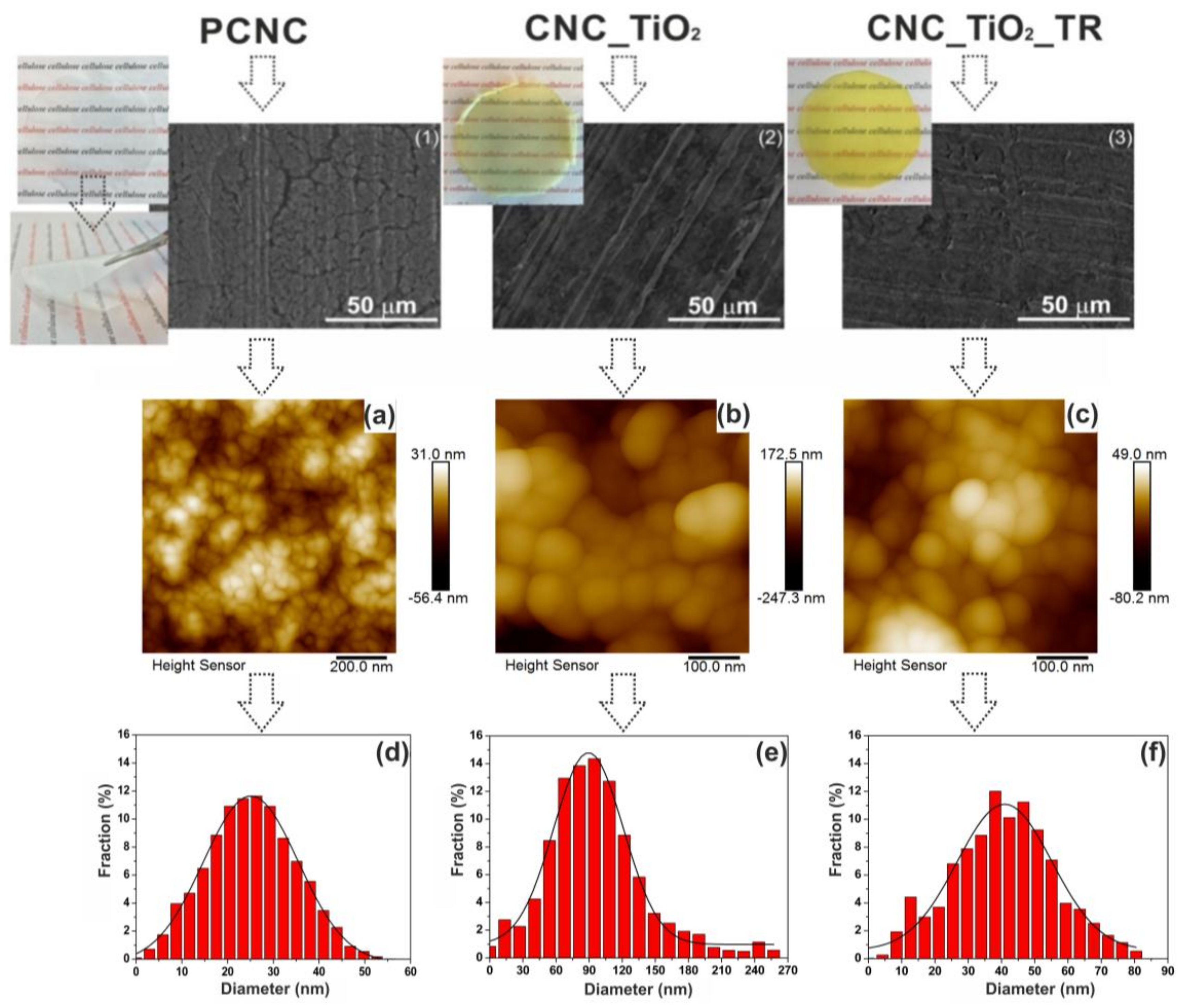
| Sample | Tensile Strength (MPa) | Strain (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| PCNC | 1.3 | 4.4 | 3.7 |
| CNC_TiO2 | 13.8 | 13.4 | 6.8 |
| CNC_TiO2_TR | 11.0 | 12.6 | 6.3 |
| Sample | Type of Bacteria | MIC (mg/mL) [36] | Diameter of Inhibition Zone (mm ± SD 1) |
|---|---|---|---|
| PCNC | E. coli CCUG24T | - | 13 ± 4 |
| CNC_TiO2 | - | 19 ± 6 | |
| CNC_TR | 0.1 | 42 ± 3 | |
| CNC_TR_TiO2 | 0.1 | 38 ± 2 | |
| PCNC | S. aureus CCUG1800T | - | 16 ± 7 |
| CNC_TiO2 | - | 26 ± 6 | |
| CNC_TR | 0.1 | 62 ± 4 | |
| CNC_TR_TiO2 | 0.1 | 56 ± 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evdokimova, O.L.; Svensson, F.G.; Agafonov, A.V.; Håkansson, S.; Seisenbaeva, G.A.; Kessler, V.G. Hybrid Drug Delivery Patches Based on Spherical Cellulose Nanocrystals and Colloid Titania—Synthesis and Antibacterial Properties. Nanomaterials 2018, 8, 228. https://doi.org/10.3390/nano8040228
Evdokimova OL, Svensson FG, Agafonov AV, Håkansson S, Seisenbaeva GA, Kessler VG. Hybrid Drug Delivery Patches Based on Spherical Cellulose Nanocrystals and Colloid Titania—Synthesis and Antibacterial Properties. Nanomaterials. 2018; 8(4):228. https://doi.org/10.3390/nano8040228
Chicago/Turabian StyleEvdokimova, Olga L., Fredric G. Svensson, Alexander V. Agafonov, Sebastian Håkansson, Gulaim A. Seisenbaeva, and Vadim G. Kessler. 2018. "Hybrid Drug Delivery Patches Based on Spherical Cellulose Nanocrystals and Colloid Titania—Synthesis and Antibacterial Properties" Nanomaterials 8, no. 4: 228. https://doi.org/10.3390/nano8040228
APA StyleEvdokimova, O. L., Svensson, F. G., Agafonov, A. V., Håkansson, S., Seisenbaeva, G. A., & Kessler, V. G. (2018). Hybrid Drug Delivery Patches Based on Spherical Cellulose Nanocrystals and Colloid Titania—Synthesis and Antibacterial Properties. Nanomaterials, 8(4), 228. https://doi.org/10.3390/nano8040228