Biocompatibility of Gd-Loaded Chitosan-Hyaluronic Acid Nanogels as Contrast Agents for Magnetic Resonance Cancer Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis and Characterization of Nanogels (NGs)
2.3. Cell Culture Conditions and Treatment
2.4. Preparation of Cell Lysate
2.5. Sulforhodamine B (SRB) Assay
2.6. LDH Assay
2.7. Intracellular ROS Measurement
2.8. Lipid Peroxidation Measurement
2.9. GSH Assay
2.10. Western Blot Assay
2.11. Comet Assay
2.12. Statistical Analysis
3. Results
3.1. Physico-Chemical Characteristics of GdDOTA and GdDOTP Based Nanogels
3.2. Effect of GdDOTA and GdDOTP Based Nanogels on Cell Viability
3.3. Effect of GdDOTA and GdDOTP Based Nanogels on Cell Membrane Integrity
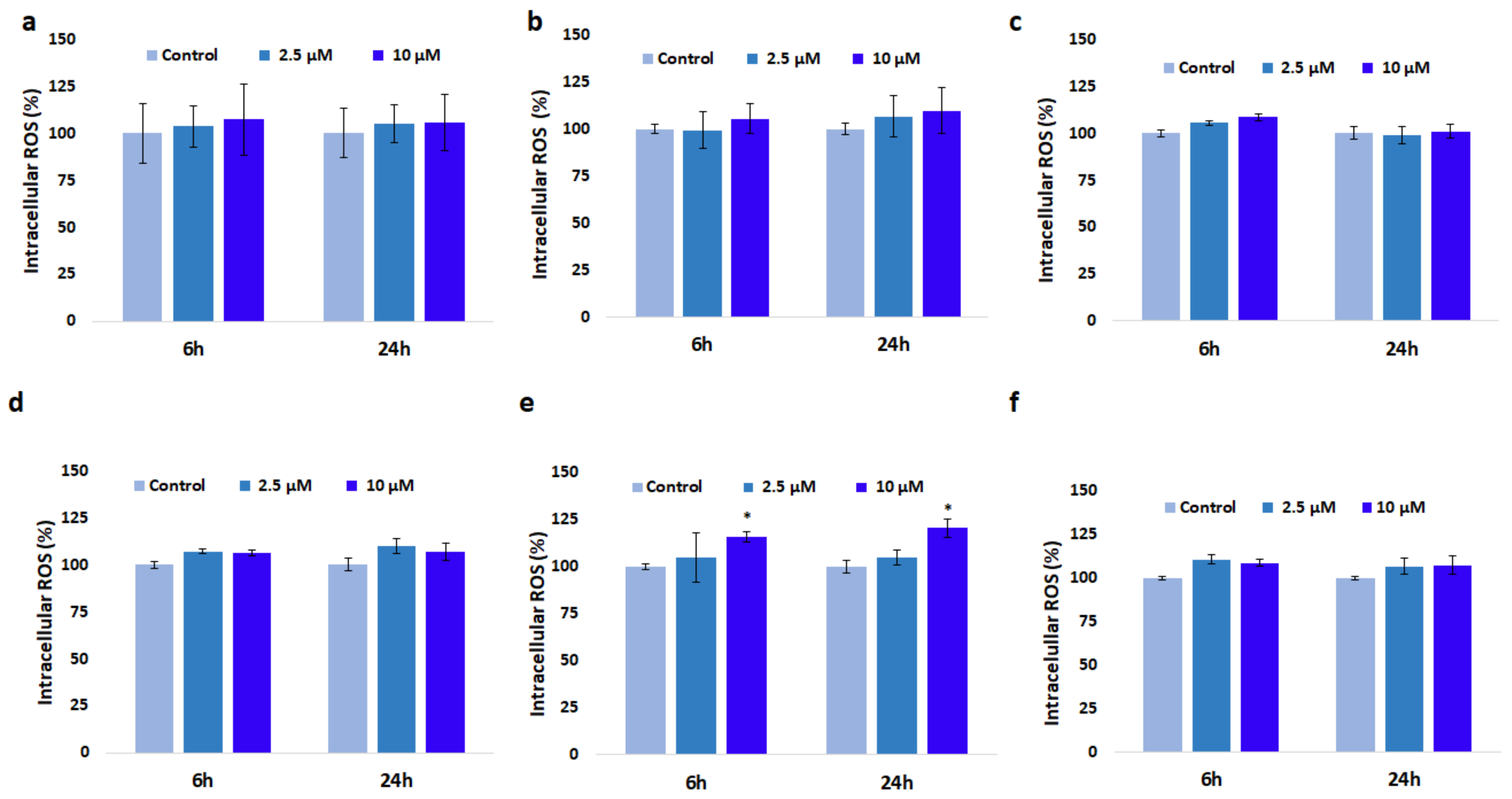
3.4. Effect of GdDOTA and GdDOTP Based Nanogels on Oxidative Stress
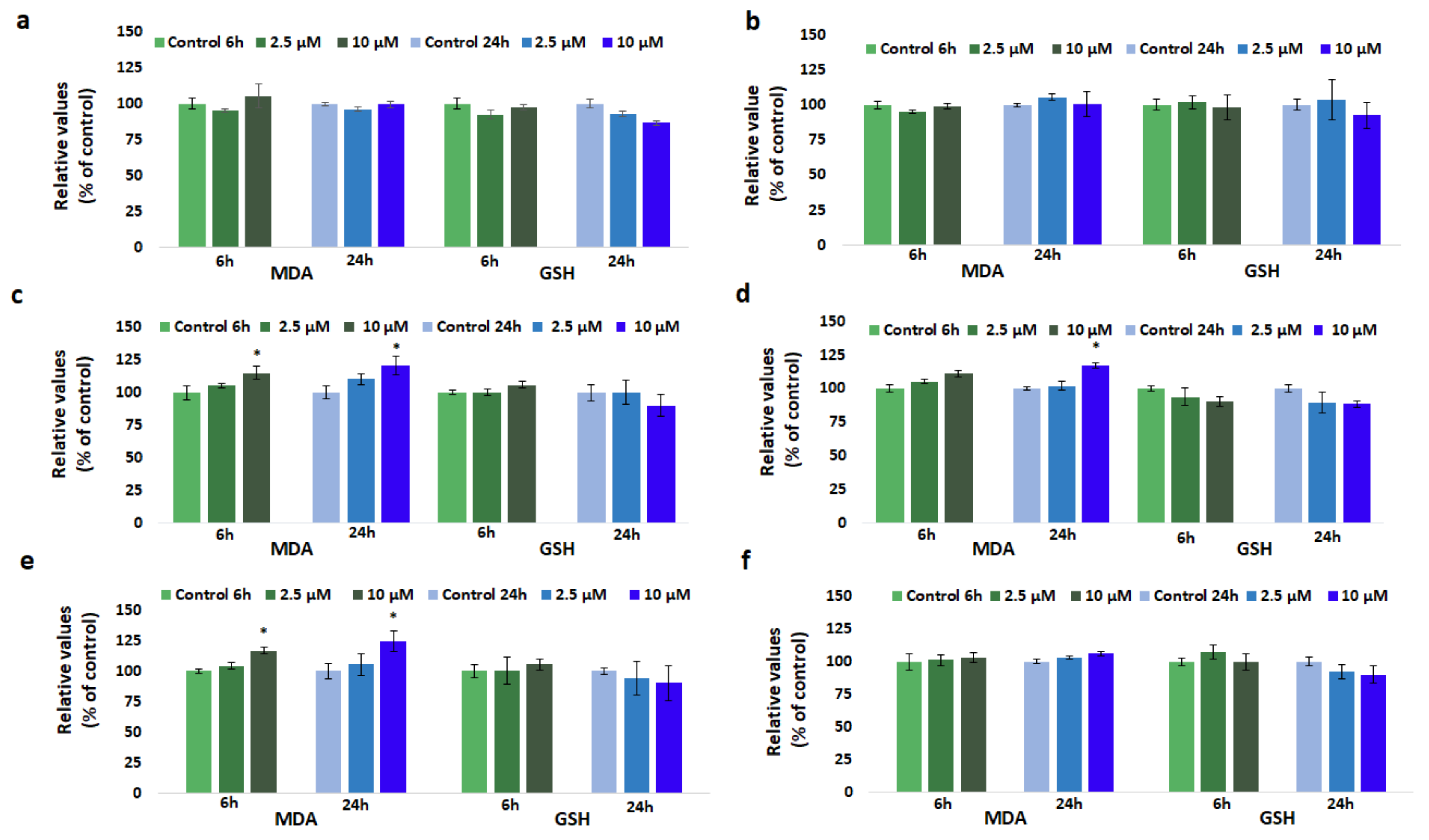
3.5. Effect of GdDOTA and GdDOTP Based Nanogels on Lipid Peroxidation
3.6. Effect of GdDOTA and GdDOTP Based Nanogels on GSH Level
3.7. Effect of GdDOTA and GdDOTP Based Nanogels on Nrf-2 Protein Expression
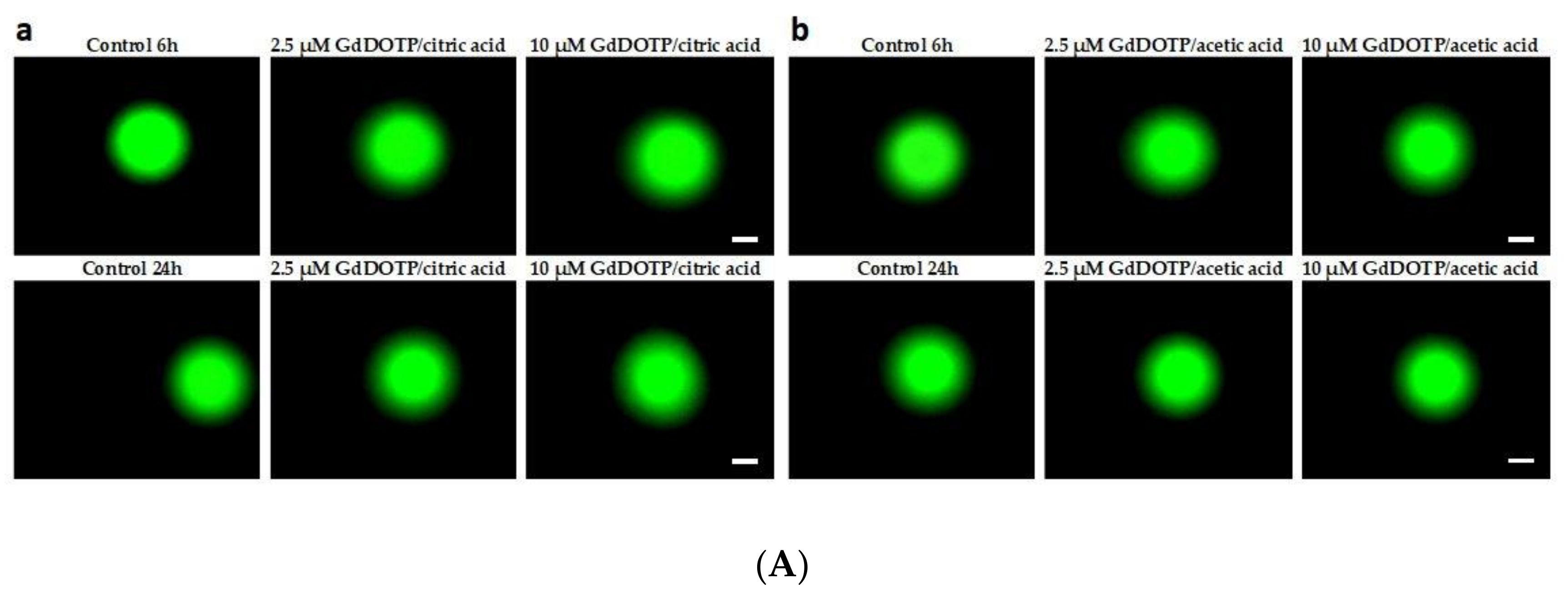
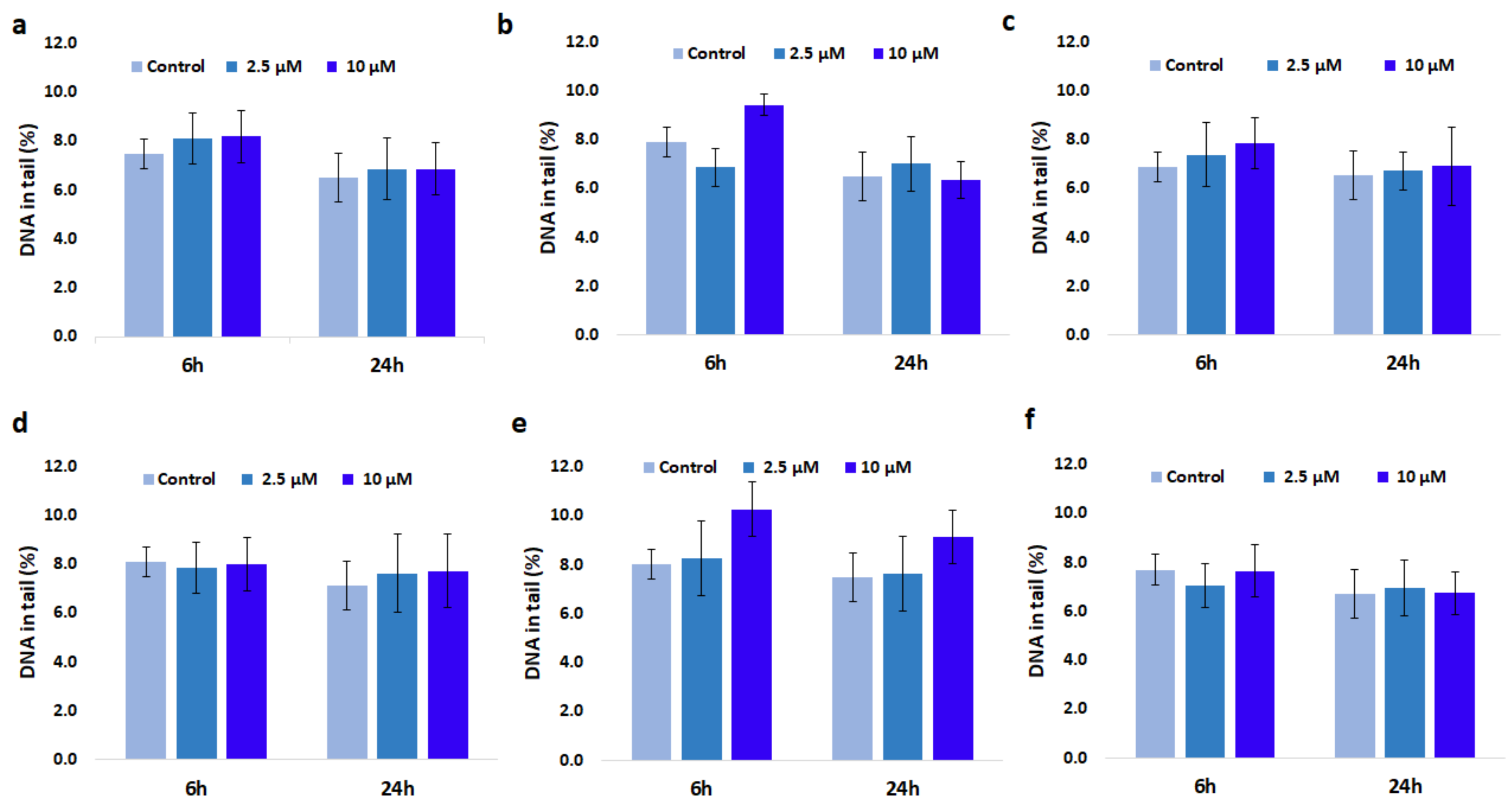
3.8. Genotoxic Effect of GdDOTA and GdDOTP Based Nanogels
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Compliance with Ethical Standards
References
- Caravan, P. Strategies for increasing the sensitivity gadolinium based MRI contrast agents. Chem. Soc. Rev. 2006, 35, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.S.; Tóth, É. Smart Contrat Agents for Magnetic Resonance Imaging. Chimia 2016, 70, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wu, A.; Chen, X. Iron Oxide Nanoparticle Based Contrast Agents for Magnetic Resonance Imaging. Mol. Pharm. 2017, 14, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Rajca, A.; Wang, Y.; Boska, M.; Paletta, J.T.; Olankitwanit, A.; Swanson, M.A.; Mitchell, D.G.; Eaton, S.S.; Eaton, G.R.; Rajca, S. Organic Radical Contrast Agents for Magnetic Resonance Imaging. J. Am. Chem. Soc. 2012, 134, 15724–15727. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lu, Z.R. Gadolinium Based Contrast Agents for MR Cancer Imaging. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2013, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Merbach, A.E.; Helm, L.; Tόth, É. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, 2nd ed.; Merbach, A.E., Helm, L., Tόth, É., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 1–152. ISBN 978-1-119-99176-2. [Google Scholar]
- Terreno, E.; Castelli, D.D.; Viale, A.; Aime, S. Challenges for molecular magnetic resonance imaging. Chem. Rev. 2010, 110, 3019–3042. [Google Scholar] [CrossRef] [PubMed]
- Idée, J.M.; Port, M.; Medina, C.; Lancelot, E.; Fayoux, E.; Ballet, S.; Corot, C. Possible involvement of gadolinium chelates in the pathophysiology of nephrogenic systemic fibrosis: A critical review. Toxicology 2008, 248, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Bertin, A.; Michou-Gallani, A.I.; Gallani, J.L.; Felder-Flesch, D. In vitro neurotoxicity of magnetic resonance imaging (MRI) contrast agents: Influence of the molecular structure and paramagnetic ion. Toxicol. In Vitro 2010, 24, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Park, W.; Hu, J.; Bae, Y.H.; Na, K. A cancer-recognizable MRI contrast agents using pH-responsive polymeric micelle. Biomaterials 2014, 35, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Botta, M.; Tei, L. Relaxivity Enhancement in Macromolecular and Nanosized GdIII-Based MRI Contrast Agents. Eur. J. Inorg. Chem. 2012, 12, 1945–1960. [Google Scholar] [CrossRef]
- Darras, V.; Nelea, M.; Winnik, F.M.; Buschmann, M.D. Chitosan modified with gadolinium diethylenetriaminepentaacetic acid for magnetic resonance imaging of DNA/chitosan nanoparticles. Carbohydr. Polym. 2010, 80, 1137–1146. [Google Scholar] [CrossRef]
- Wartenberg, N.; Fries, P.; Raccurt, O.; Guillermo, A.; Imbert, D.; Mazzanti, M.A. Gadolinium Complex Confined in Silica Nanoparticles as a Highly Efficient T1/T2 MRI Contrast Agent. Chem. Eur. J. 2013, 19, 6980–6983. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Kawano, K.; Maitani, Y.; Yokoyama, M. Polyion complex micelle MRI contrast agents from poly (ethylene glycol)-B-poly(l-lysine) block copolymers having Gd-DOTA; preparations and their control of T1-relaxivities and blood circulation characteristics. J. Control. Release 2010, 148, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Lux, J.; Chan, M.; Vander Elst, L.; Schopf, E.; Mahmoud, E.; Laurent, S.; Almutairi, A. Metal Chelating Crosslinkers Form Nanogels with High Chelation Stability. J. Mater. Chem. B Mater. Biol. Med. 2013, 1, 6359–6364. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.; Santos-Martinez, M.J.; Radomski, A.; Corrigan, O.I.; Radomski, M.W. Nanoparticles: Pharmacological and toxicological significance. Br. J. Pharmacol. 2007, 150, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Chitin and Chitosan: Sources, Production and Medical Applications. In Renewable Resources for Functional Polymers and Biomaterials: Polysaccharides, Proteins and Polyesters, 2nd ed.; RSC in Polymer Chemistry Series No. 1; Williams, P.A., Ed.; Royal Society of Chemistry: Cambridge, UK, 2011; pp. 292–318. ISBN 978-1-84973-351-9. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan—TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Karagozlu, M.Z.; Kim, S.K. Anticancer effects of chitin and chitosan derivatives. Adv. Food Nutr. Res. 2014, 72, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: A review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Kim, S.K. Antioxidant effects of chitin, chitosan, and their derivatives. Adv. Food Nutr. Res. 2014, 73, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Sonaje, K.; Chuang, E.Y.; Lin, K.J.; Yen, T.C.; Su, F.Y.; Tseng, M.T.; Sung, H.W. Opening of epithelial tight junctions and enhancement of paracellular permeation by chitosan: Microscopic, ultrastructural, and computed-tomographic observations. Mol. Pharm. 2012, 9, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhang, Y.; He, S.; Nie, F.; Teng, G.; Gu, N. Fluorescence modified chitosan coated magnetic nanoparticles for high-efficient cellular imaging. Nanoscale Res. Lett. 2009, 4, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Strijkers, G.J.; Nicolay, K. Chitosan-based systems for molecular imaging. Adv. Drug Deliv. Rev. 2010, 62, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fuentes, M.; Alonso, M.J. Chitosan-Based drug nanocarriers: Where do we stand? J. Control. Release 2012, 161, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Ragelle, H.; Vandermeulen, G.; Preéat, V. Chitosan-based siRNA delivery systems. J. Control. Release 2013, 172, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Wang, Y.; Wang, X.; Wang, Q.; Chen, M. Advances in self-assembled chitosan nanomaterials for drug delivery. Biotechnol. Adv. 2014, 32, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Ghaz-Jahanian, M.A.; Abbaspour-Aghdam, F.; Anarjan, N.; Berenjian, A.; Jafarizadeh-Malmiri, H. Application of Chitosan-Based Nanocarriers in Tumor-Targeted Drug Delivery. Mol. Biotechnol. 2015, 57, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N.; Schiller, J.; Stern, R.; Soltés, L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr. Med. Chem. 2009, 16, 1718–1745. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.A.; Kong, J.H.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Sironen, R.K.; Tammi, M.; Tammi, R.; Auvinen, P.K.; Anttila, M.; Kosma, V.M. Hyaluronan in human malignancies. Exp. Cell Res. 2011, 317, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F.; Menaa, A.; Menaa, B. Hyaluronic Acid and Derivatives for Tissue Engineering. J. Biotechnol. Biomater. 2011, S3, 1–7. [Google Scholar] [CrossRef]
- Ossipov, D.A. Nanostructured hyaluronic acid-based materials for active delivery to cancer. Expert Opin. Drug Deliv. 2010, 7, 681–703. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Ubonvan, T.; Kim, D.D. Hyaluronic Acid in Drug Delivery Systems. J. Pharm. Investig. 2010, 40, 33–43. [Google Scholar] [CrossRef]
- Jiao, Y.; Pang, X.; Zhai, G. Advances in Hyaluronic Acid-Based Drug Delivery Systems. Curr. Drug Targets 2016, 17, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Knopf-Marques, H.; Pravda, M.; Wolfova, L.; Velebny, V.; Schaaf, P.; Vrana, N.E.; Lavalle, P. Hyaluronic Acid and Its Derivatives in Coating and Delivery Systems: Applications in Tissue Engineering, Regenerative Medicine and Immunomodulation. Adv. Healthc. Mater. 2016, 5, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Thomas, R.G.; Heo, S.U.; Park, M.S.; Bae, W.K.; Heo, S.H.; Yim, N.Y.; Jeong, Y.Y. A Hyaluronic Acid-Conjugated Gadolinium Hepatocyte-Specific T1 Contrast Agent for Liver Magnetic Resonance Imaging. Mol. Imaging Biol. 2015, 17, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Payne, W.M.; Hill, T.K.; Svechkarev, D.; Holmes, M.B.; Sajja, B.R.; Mohs, A.M. Multimodal Imaging Nanoparticles Derived from Hyaluronic Acid for Integrated Preoperative and Intraoperative Cancer Imaging. Contrast Media Mol. Imaging 2017, 2017, 9616791. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Seijo, B.; Alonso, M.J. Bioadhesive hyaluronan-chitosan nanoparticles can transport genes across the ocular mucosa and transfect ocular tissue. Gene Ther. 2008, 15, 668–676. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Seijo, B.; Alonso, M.J. Novel hyaluronan-based nanocarriers for transmucosal delivery of macromolecules. Macromol. Biosci. 2008, 8, 441–450. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Seijo, B.; Alonso, M.J. Design of novel polysaccharidic nanostructures for gene delivery. Nanotechnology 2008, 19, 075105. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Seijo, B.; Alonso, M.J. Novel Hyaluronic Acid-Chitosan Nanoparticles for Ocular Gene Therapy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors. Eur. J. Pharm. Sci. 2008, 35, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Duceppe, N.; Tabrizian, M. Factors influencing the transfection efficiency of ultra-low molecular weight chitosan/hyaluronic acid nanoparticles. Biomaterials 2009, 30, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Nasti, A.; Zaki, N.M.; de Leonardis, P.; Ungphaiboon, S.; Sansongsak, P.; Rimoli, M.G.; Tirelli, N. Chitosan/TPP and chitosan/TPP hyaluronic acid nanoparticles: Systematic optimisation of the preparative process and preliminary biological evaluation. Pharm. Res. 2009, 26, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Raviña, M.; Cubillo, E.; Olmeda, D.; Novoa-Carballal, R.; Fernandez-Megia, E.; Riguera, R.; Sánchez, A.; Cano, A.; Alonso, M.J. Hyaluronic Acid/Chitosan-g-poly(ethylene glycol) Nanoparticles for Gene Therapy: An Application for pDNA and siRNA Delivery. Pharm. Res. 2010, 27, 2544–2555. [Google Scholar] [CrossRef] [PubMed]
- Gwak, S.J.; Jung, J.K.; An, S.S.; Kim, H.J.; Oh, J.S.; Pennant, W.A.; Lee, H.Y.; Kong, M.H.; Kim, K.N.; Yoon, D.H.; et al. Chitosan/TPP-Hyaluronic Acid Nanoparticles: A New Vehicle for Gene Delivery to the Spinal Cord. J. Biomater. Sci. Polym. Ed. 2012, 23, 1437–1450. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of M1R-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef] [PubMed]
- Lindborg, B.A.; Brekke, J.H.; Scott, C.M.; Chai, Y.W.; Ulrich, C.; Sandquist, L.; Kokkoli, E.; O’Brien, T.D. A chitosan-hyaluronan-based hydrogel-hydrocolloid supports in vitro culture and differentiation of human mesenchymal stem/stromal cells. Tissue Eng. Part A 2015, 21, 1952–1962. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Cheng, J.; Wu, G.; Lu, Q.; Yi, F.; Xu, J.; Zhu, J.; He, D. A Hyaluronic Acid-Chitosan-Gadolinium Nanosphere for Specific Tumor-Targeted MRI Contrast Agent. Gen. Chem. 2015, 1, 15–21. [Google Scholar] [CrossRef]
- Courant, T.; Roullin, V.G.; Cadiou, C.; Callewaert, M.; Andry, M.C.; Portefaix, C.; Hoeffel, C.; de Goltstein, M.C.; Port, M.; Laurent, S.; et al. Hydrogels incorporating GdDOTA: Towards highly efficient dual T1/T2 MRI contrast agents. Angew. Chem. Int. Ed. 2012, 51, 9119–9122. [Google Scholar] [CrossRef] [PubMed]
- Courant, T.; Roullin, V.G.; Cadiou, C.; Callewaert, M.; Andry, M.C.; Portefaix, C.; Hoeffel, C.; de Goltstein, M.C.; Port, M.; Laurent, S.; et al. Biocompatible Nanoparticles and Gadolinium Complexes for MRI applications. C. R. Chim. 2013, 16, 531–539. [Google Scholar] [CrossRef]
- Callewaert, M.; Roullin, V.G.; Cadiou, C.; Millart, E.; Van Gulik, L.; Andry, M.C.; Portefaix, C.; Hoeffel, C.; Laurent, S.; Vander Elst, L.; et al. Tuning the composition of biocompatible Gd nanohydrogels to achieve hypersensitive dual T1/T2 MRI contrast agents. J. Mater. Chem. B 2014, 2, 6397–6405. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, T.; Xiao, Y.; Yu, D.; Zhang, N. Hyaluronic Acid-Chitosan Nanoparticles to Deliver Gd-DTPA for MR Cancer Imaging. Nanomaterials 2015, 5, 1379–1396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Yu, D.; Zhang, N. Gadolinium-Loaded Chitosan Nanoparticles as Magnetic Resonance Imaging Contrast Agents for the Diagnosis of Tumor. J. Biomed. Nanotechnol. 2013, 9, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Gheran, C.V.; Voicu, S.N.; Rigaux, G.; Callewaert, M.; Chuburu, F.; Dinischiotu, A. Biological effects induced by Gadolinium nanoparticles on Lymphocyte A20 cell line. EuroBiotech J. 2017, 1, 57–64. [Google Scholar] [CrossRef]
- Rigaux, G.; Gheran, C.V.; Callewaert, M.; Cadiou, C.; Voicu, S.N.; Dinischiotu, A.; Andry, M.C.; Vander Elst, L.; Laurent, S.; Muller, R.N.; et al. Characterization of Gd loaded chitosan-TPP nanohydrogels by a multi-technique approach combining dynamic light scattering (DLS), asymetrical flow-field-flow fractionation (AF4) and atomic force microscopy (AFM) and design of positive contrast agents for molecular resonance imaging (MRI). Nanotechnology 2017, 28, 055705. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Legrand, C.; Bour, J.M.; Jacob, C.; Capiaumont, J.; Martial, A.; Marc, A.; Wudtke, M.; Kretzmer, G.; Demangel, C.; Duval, D. Lactate dehydrogenase (LDH) activity of the number of dead cells in the medium of cultured eukaryotic cells as marker. J. Biotechnol. 1992, 25, 231–243. [Google Scholar] [CrossRef]
- Del Rio, D.; Pellegrini, N.; Colombi, B.; Bianchi, M.; Serafini, M.; Torta, F.; Tegoni, M.; Musci, M.; Brighenti, F. Rapid Fluorimetric method to detect total plasma malondialdehyde with mild derivatization conditions. Clin. Chem. 2003, 49, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Wimardani, Y.S.; Suniarti, D.F.; Freisleben, H.J.; Wanandi, S.I.; Ikeda, M.A. Cytotoxic effects of chitosan against oral cancer cell lines is molecular-weight-dependent and cell-type-specific. IJOR 2012, 3, e1. [Google Scholar]
- Baharifar, H.; Amani, A. Cytotoxicity of chitosan/streptokinase nanoparticles as a function of size: An artificial neural networks study. Nanomedicine 2016, 12, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Manke, M.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. Biomed. Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox Regulation of Cell Survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Gorman, AM.; Hori, O.; Samali, A. Cellular Stress Responses: Cell Survival and Cell Death. Int. J. Biochem. Cell Biol. 2010, 2010, 2140474. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.S.; Liu, L.J.; Qu, Y.L.; OuYang, X.K.; Yang, L.Y.; Xu, Z.R. Chitosan Nanoparticles Attenuate Hydrogen Peroxide-Induced Stress Injuryin Mouse Macrophage RAW264.7 Cells. Mar. Drugs 2013, 11, 3582–3600. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; Valachová, K.; Mohyeldin, M.S.; Soltes, L. Free radical scavenger activity of chitosan and its aminated derivative. J. Appl. Pharm. Sci. 2016, 6, 195–201. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, H.; Wang, C.; Wu, Y.; Xie, J.; Jin, X.; Yang, J.; Ye, J. Genoprotective effect of hyaluronic acid against benzalkonium chloride-induced DNA damage in human corneal epithelial cells. Mol. Vis. 2011, 17, 3364–3370. [Google Scholar] [PubMed]
- Ye, J.; Wu, H.; Wu, Y.; Wang, C.; Zhang, H.; Shi, X.; Yang, J. High molecular weight hyaluronan decreases oxidative DNA damage induced by EDTA in human corneal epithelial cells. Eye 2012, 26, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
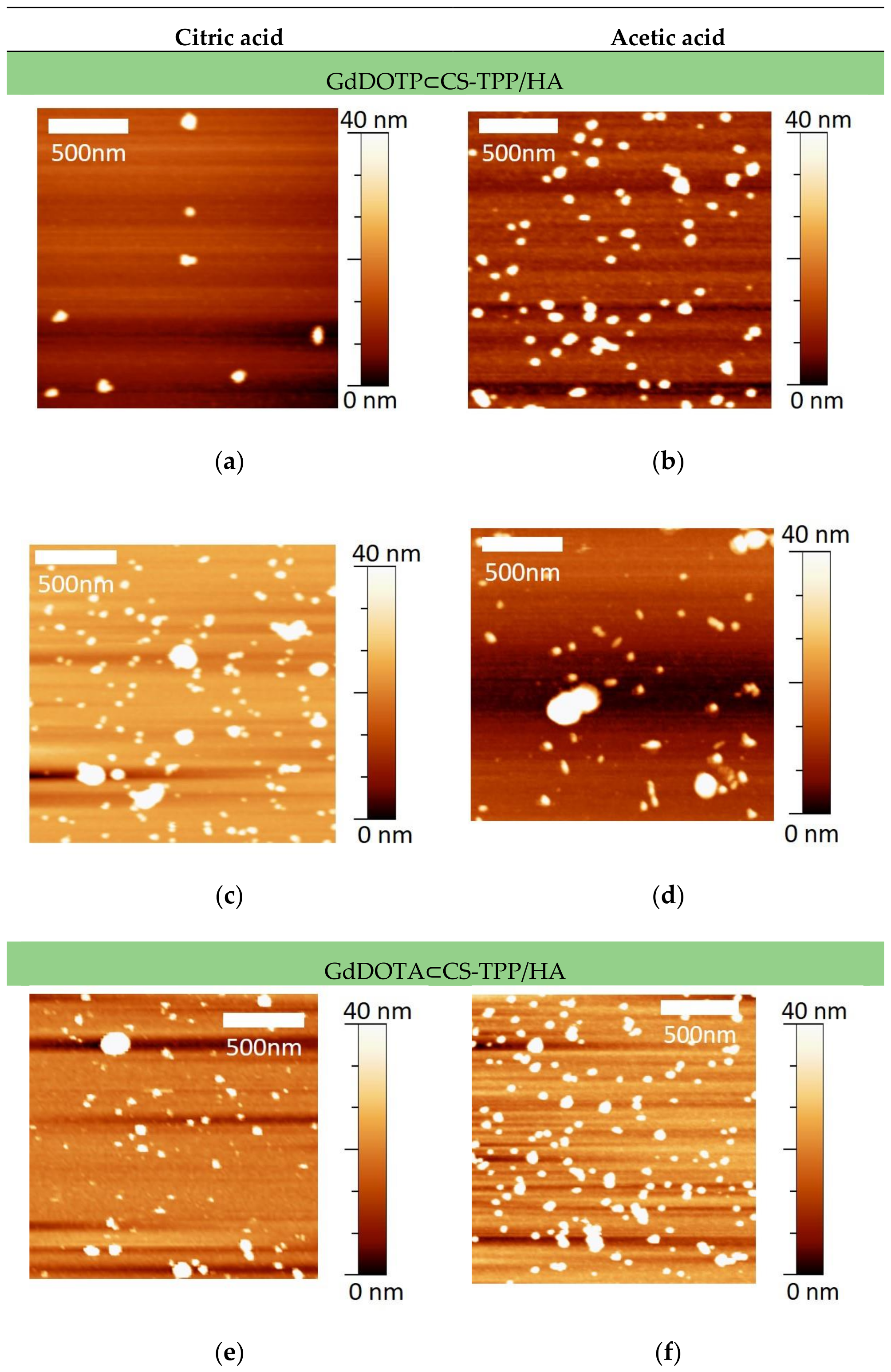


| [CS] mg/mL | 2.5 | |||
| GdCA | GdDOTP | GdDOTA | ||
| Acid | Citric | Acetic | Citric | Acetic |
| Entries | 1 | 2 | [67] | |
| DH (nm) | 242 | 197 | 217 | 393 |
| PdI | 0.2 | 0.4 | 0.2 | 0.4 |
| ζ (mV) | 31.9 | 22.7 | 30.3 | 31.9 |
| [Gd]t M | 2.39 × 10−4 | 7.82 × 10−4 | 9.87 × 10−5 | 9.07 × 10−4 |
| [CS] mg/mL | 1.5 | |||
| GdCA | GdDOTP | GdDOTA | ||
| Acid | Citric | Acetic | Citric | Acetic |
| Entries | 3 | 4 | 5 | 6 |
| DH (nm) | 199 | 234 | 186 | 185 |
| PdI | 0.2 | 0.3 | 0.2 | 0.3 |
| ζ (mV) | 22.2 | 18.9 | 20.4 | 25.3 |
| [Gd]t M | 1.11 × 10−4 | 3.30 × 10−4 | 4.90 × 10−5 | 2.64 × 10−4 |
| Diameters | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| dAFM ± sd (nm) | 69 ± 8 | 72 ± 7 | 53 ± 6 | 55 ± 6 | 51 ± 10 | 62 ± 7 |
| Time (h) | Control (Untreated Cells) | Unloaded CS-TPP/HA, [CH]polycation phase (mg·mL−1) | |||
|---|---|---|---|---|---|
| 2.5 | 1.5 | ||||
| Citric Acid | Acetic Acid | Citric Acid | Acetic Acid | ||
| 6 | 100 ± 2.05 | 96.34 ± 1.30 | 100.00 ± 3.70 | 93.00 ± 1.00 | 92.00 ± 3.31 |
| 24 | 100 ± 2.4 | 107.51 ± 6.70 | 103.55 ± 4.80 | 98.00 ± 2.70 | 103.86 ± 1.91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheran, C.V.; Rigaux, G.; Callewaert, M.; Berquand, A.; Molinari, M.; Chuburu, F.; Voicu, S.N.; Dinischiotu, A. Biocompatibility of Gd-Loaded Chitosan-Hyaluronic Acid Nanogels as Contrast Agents for Magnetic Resonance Cancer Imaging. Nanomaterials 2018, 8, 201. https://doi.org/10.3390/nano8040201
Gheran CV, Rigaux G, Callewaert M, Berquand A, Molinari M, Chuburu F, Voicu SN, Dinischiotu A. Biocompatibility of Gd-Loaded Chitosan-Hyaluronic Acid Nanogels as Contrast Agents for Magnetic Resonance Cancer Imaging. Nanomaterials. 2018; 8(4):201. https://doi.org/10.3390/nano8040201
Chicago/Turabian StyleGheran, Cecilia Virginia, Guillaume Rigaux, Maité Callewaert, Alexandre Berquand, Michael Molinari, Françoise Chuburu, Sorina Nicoleta Voicu, and Anca Dinischiotu. 2018. "Biocompatibility of Gd-Loaded Chitosan-Hyaluronic Acid Nanogels as Contrast Agents for Magnetic Resonance Cancer Imaging" Nanomaterials 8, no. 4: 201. https://doi.org/10.3390/nano8040201





