Effect of ZrO2 Nanomaterials on Wettability and Interfacial Characteristics of Al-19Cu-11Si-2Sn Filler Metal for Low Temperature Al to Cu Dissimilar Brazing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ZrO2 Reinforced Filler Metal
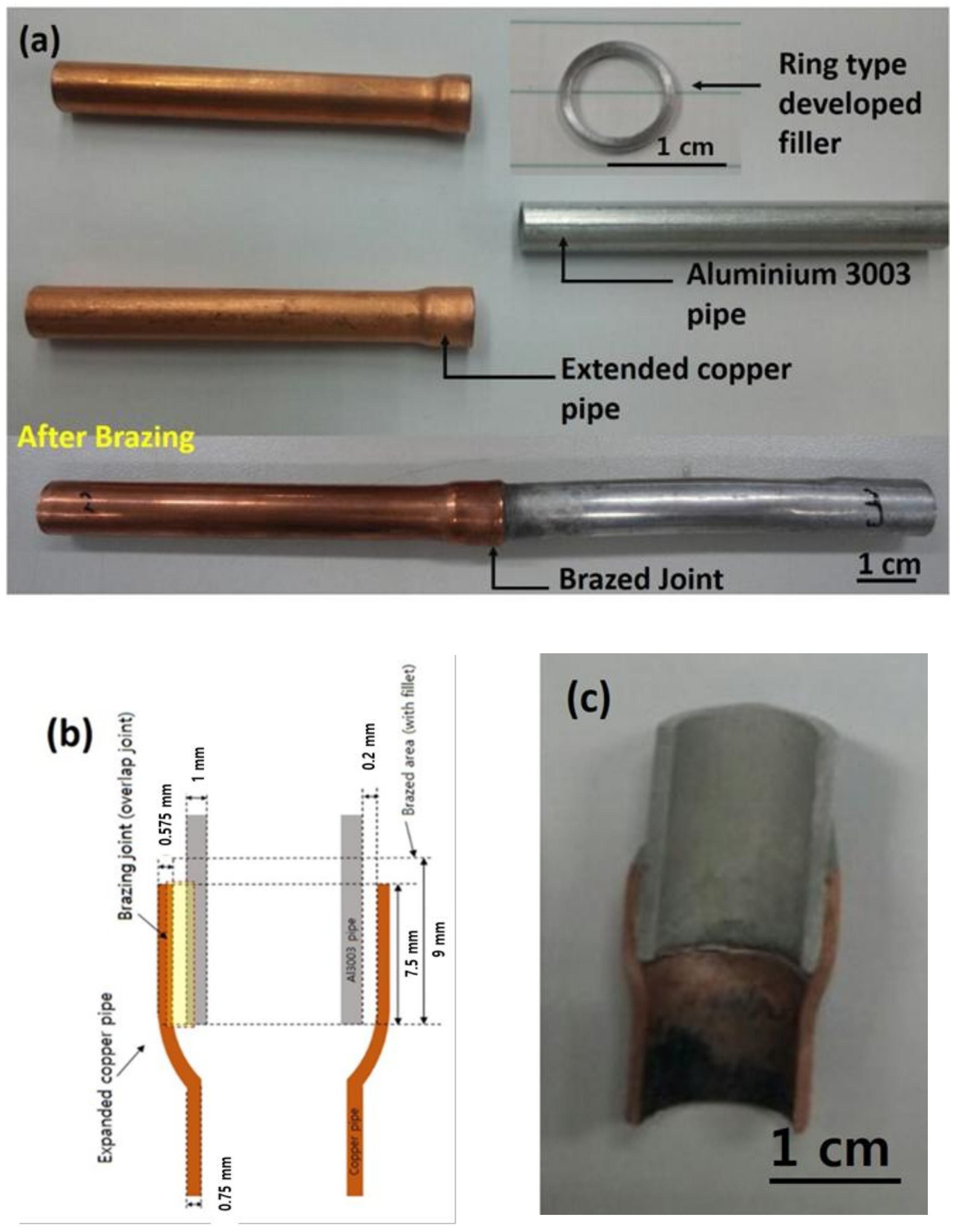
2.2. Dissimilar Al/Cu Brazing
2.3. Characterization of ZrO2-reinforced Filler Metal
2.3.1. Microstructure
2.3.2. Brazeability
2.3.3. Mechanical Properties
3. Results and Discussion
3.1. Analysis of the Filler
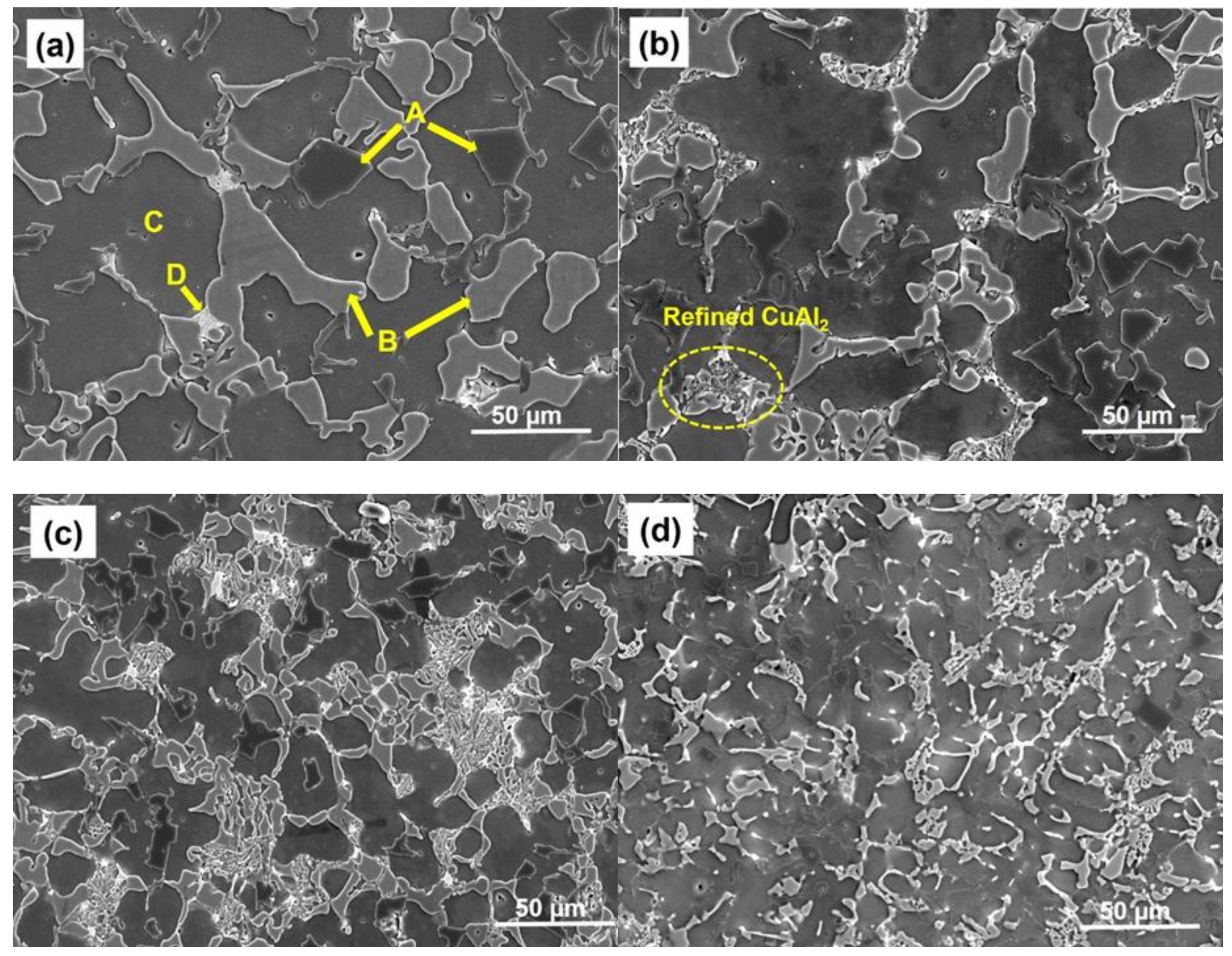
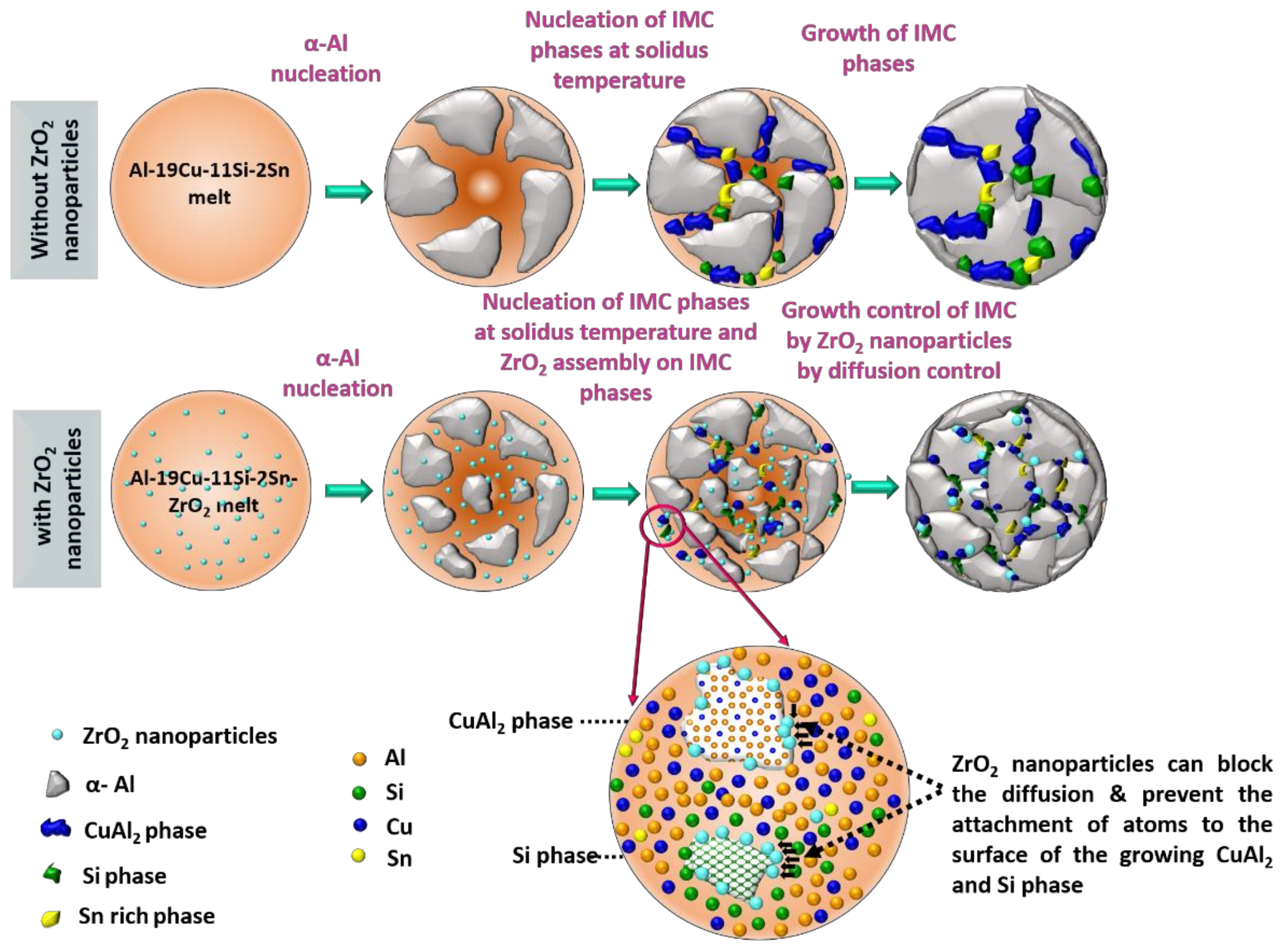
3.1.1. Microstructure of ZrO2-Reinforced Al-19Cu-11Si-2Sn Filler Metals
- (1)
- Local change in the temperature of solidification front influencing the growth of α-Al phase.
- (2)
- The ZrO2 nanomaterials reduces the diffusion of atoms to the surface of the growing Si and CuAl2 phase.
- (3)
- Engulfed ZrO2 nanomaterials onto CuAl2 or Si phase increases the free energy of their growth by distortion and thus reducing the driving force for growth.
- (4)
- Pinning of the solidification front by ZrO2 nanomaterials is explained by the Gibbs-Thomson effect.
3.1.2. Spreading Analysis of ZrO2 Reinforced Al-19Cu-11Si-2Sn Filler
3.1.3. Mechanical Properties of ZrO2 Reinforced Al-19Cu-11Si-2Sn Filler
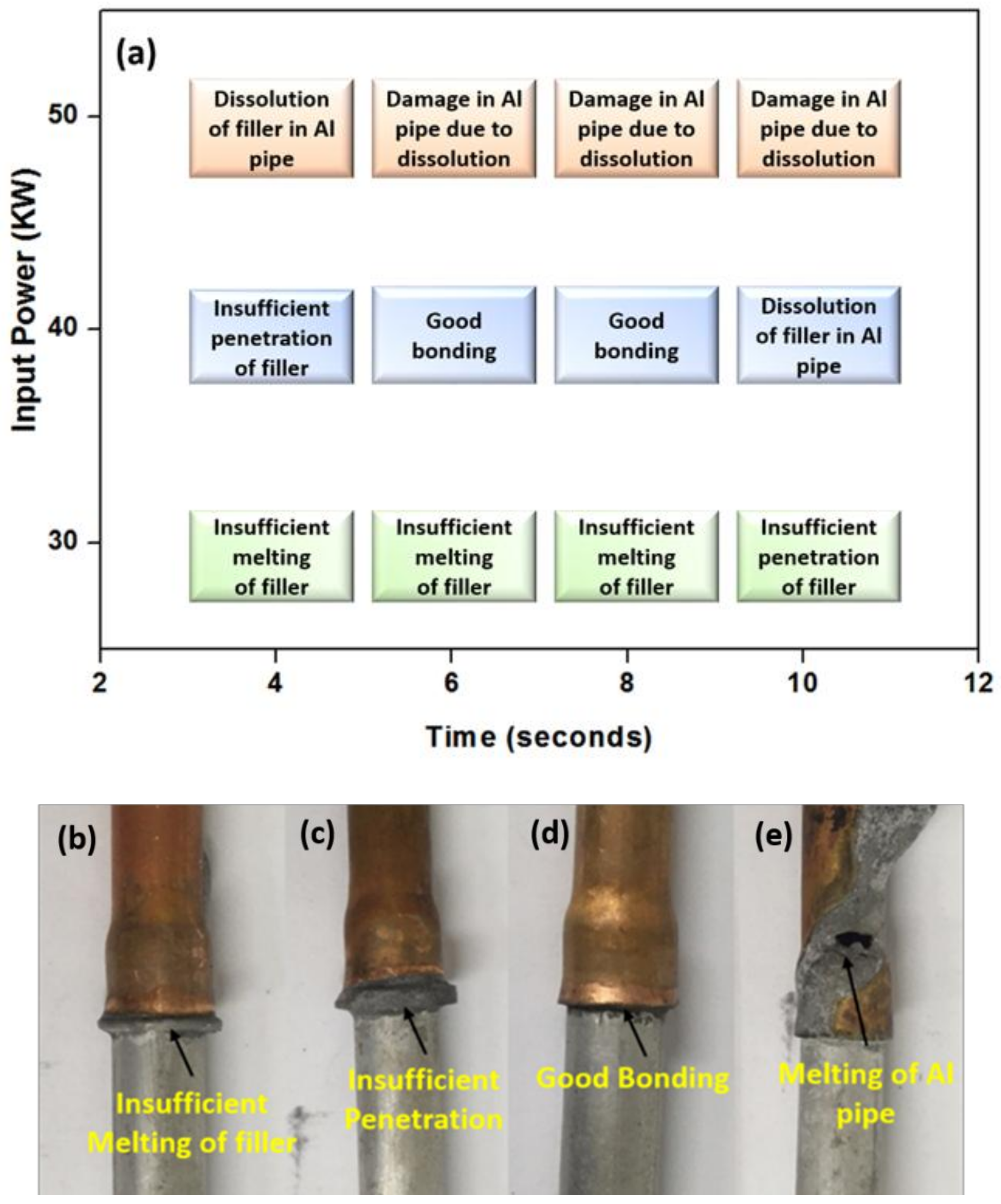
3.2. Aluminum to Copper Dissimilar Brazing
3.3. Analysis of the Al/Cu Brazed Joint
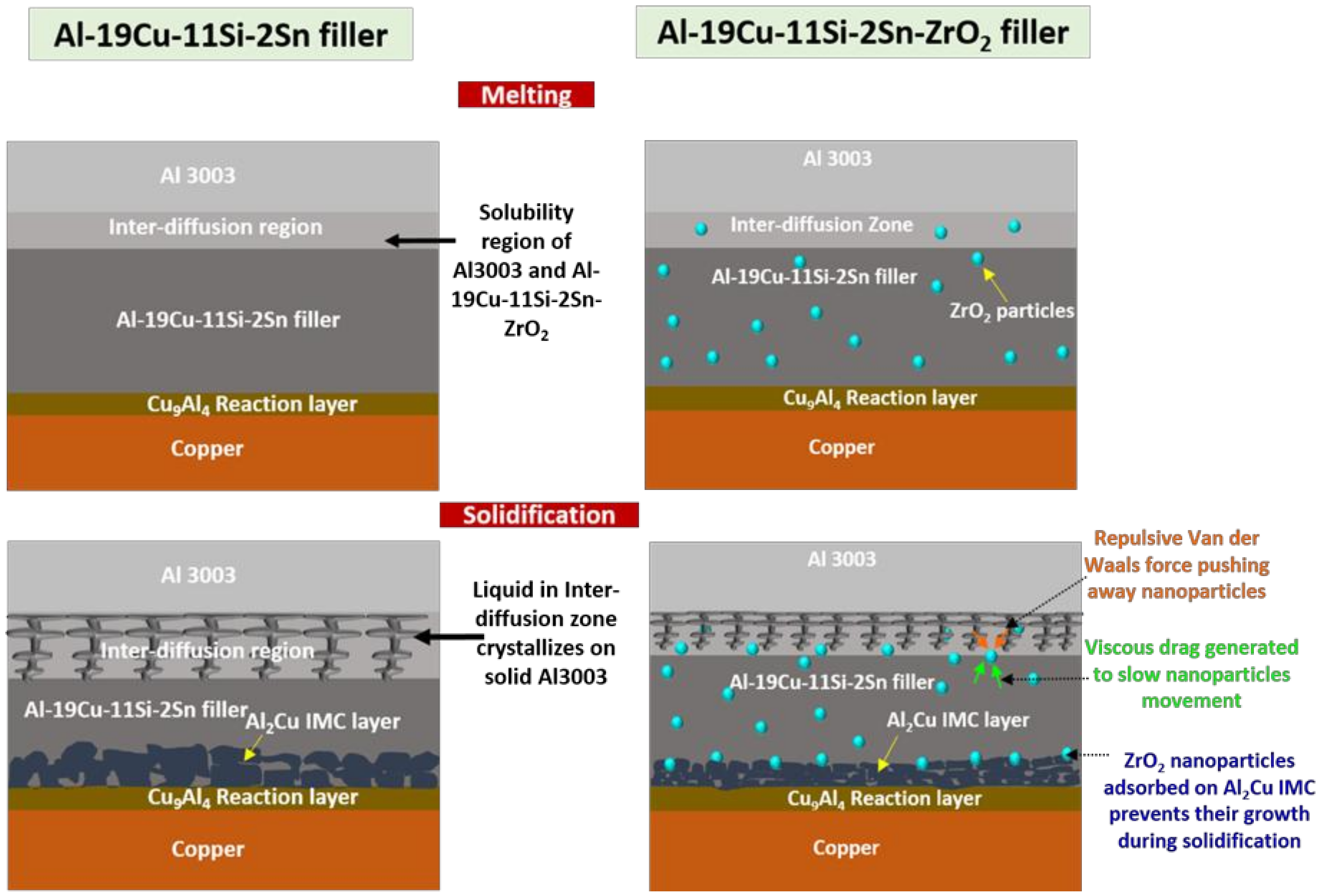
3.3.1. Interface Analysis
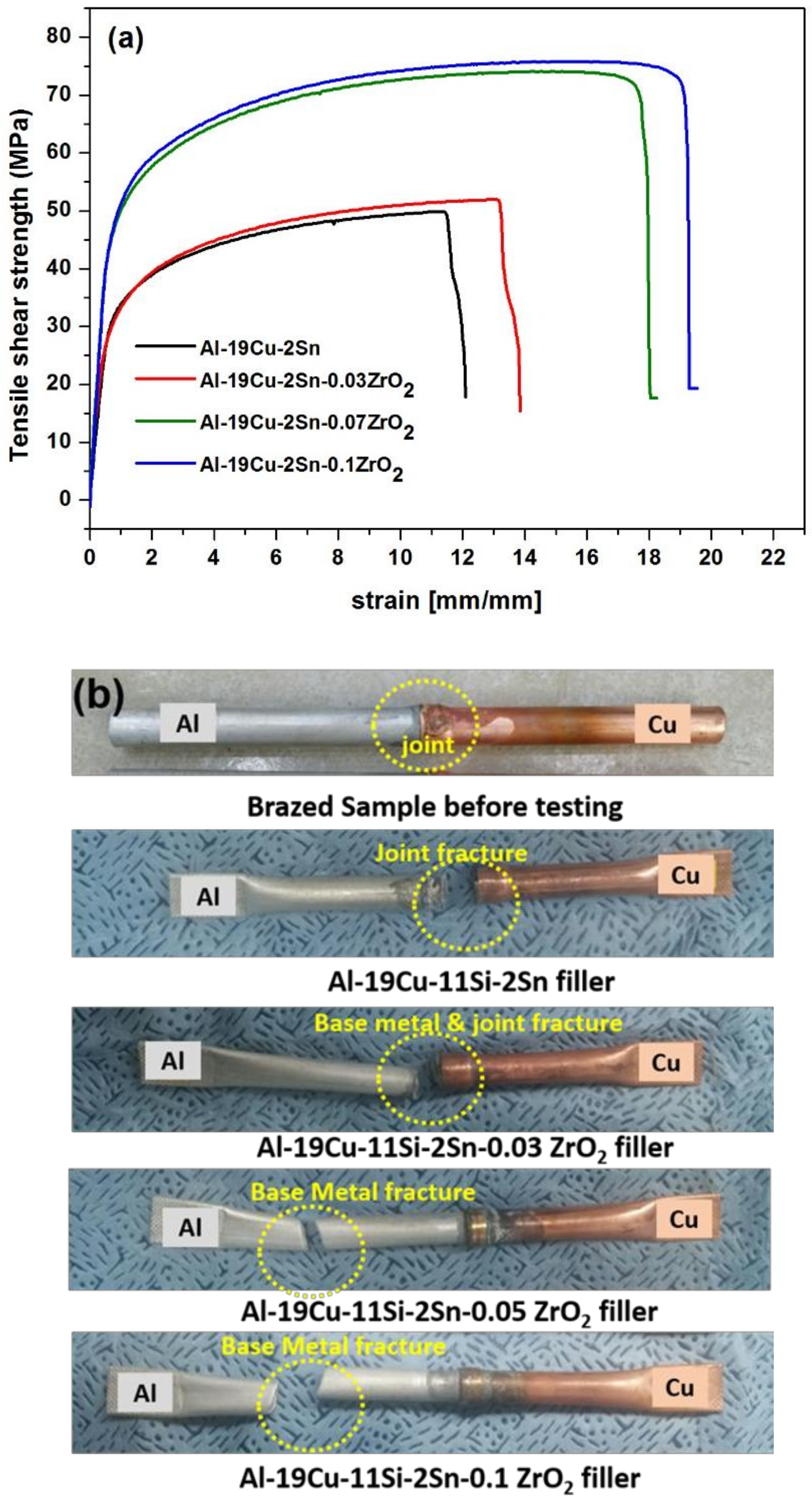
3.3.2. Tensile Properties of the Brazed Joint
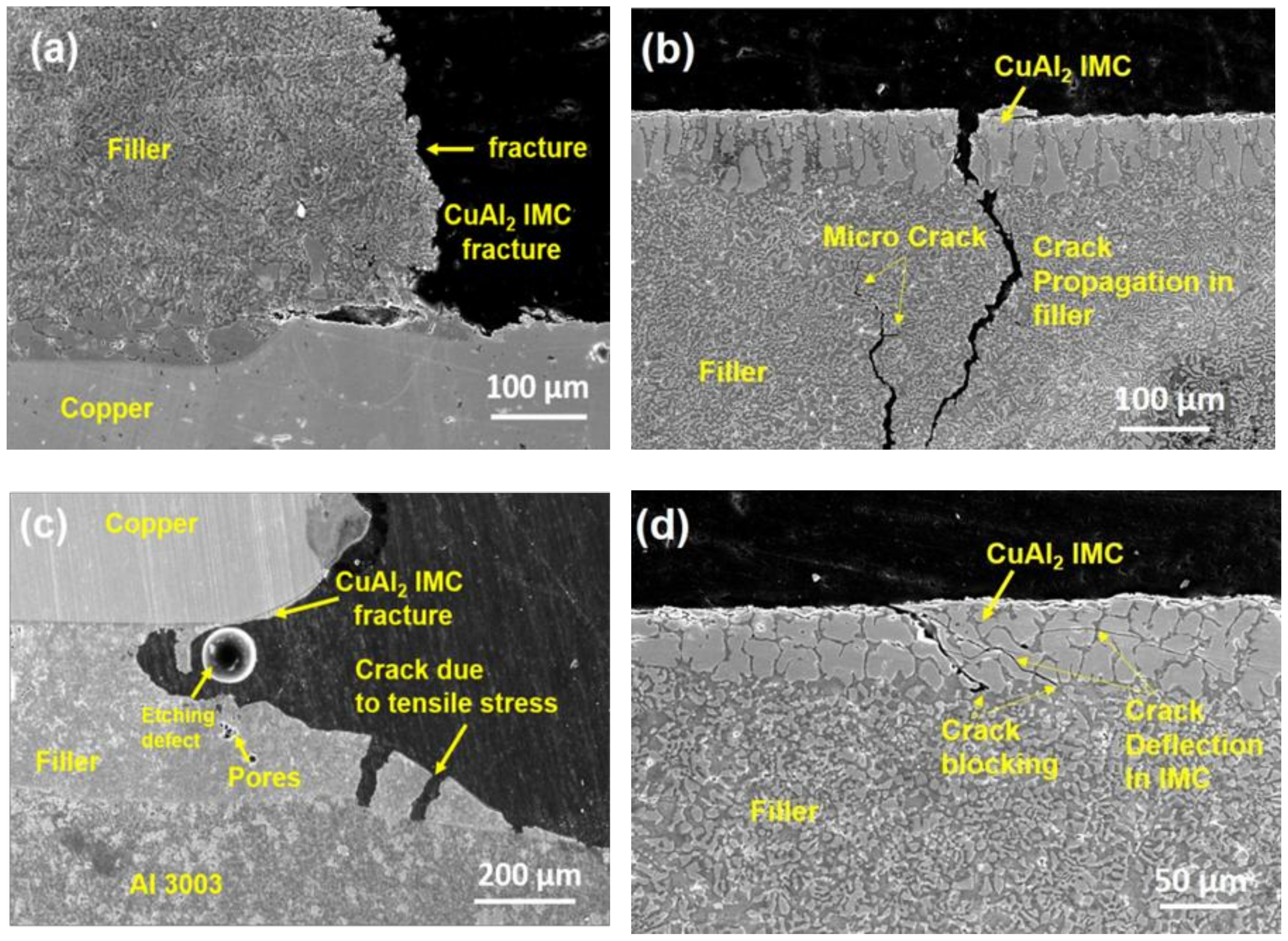
3.3.3. Fracture Analysis of the Brazed Joint
4. Conclusions
- (1)
- SEM analysis shows a significant size reduction in Si particles and CuAl2 IMC with the addition of ZrO2 nanomaterials. This phenomenon was explained using ZrO2 nanomaterial-induced phase growth control during solidification of the Al-19Cu-11Si-2Sn filler alloy. TEM confirms the presence of ZrO2 nanomaterials along the surface of Si particles and CuAl2 IMC justifying phase growth control of nanomaterials.
- (2)
- Addition of ZrO2 nanomaterials increases the spreading ratio of filler on Cu and Al substrates. Furthermore, ZrO2 nanomaterials added to Al-19Cu-11Si-2Sn filler showed better mechanical properties in terms of microhardness and tensile properties.
- (3)
- The joints with varying input power and brazing time demonstrated a good bonding for 40 kW input power for 6 and 8 seconds.
- (4)
- Interface analysis of joint brazed at 40 kW, 8 seconds with Al-19Cu-11Si-2Sn and Al-19Cu-11Si-2Sn-ZrO2 demonstrated the formation of continuous Cu9Al4 reaction layer and discontinuous Al2Cu IMC on the Cu side, and formation of an inter-diffusion layer on the Al3003 side. However, a thin IMC and inter-diffusion layer is formed using Al-19Cu-11Si-2Sn-ZrO2 filler. ZrO2 nanomaterials in the filler are responsible for interface modification by surface adsorption phenomenon.
- (5)
- The tensile test results of the brazed joint justified the importance of ZrO2 nano-reinforced Al-19Cu-11Si-2Sn filler to join Al and Cu joints. Tensile strength increased linearly with the concentration of ZrO2 nanomaterials in filler alloy. Joints with 0.05 and 0.1 wt. % ZrO2 added filler shows base metal fracture confirming sound joint.
- (6)
- Fracture analysis of the brazed joint shows that ZrO2 nanomaterials adsorbed on Al2Cu IMC block the crack propagation along the interface which leads to crack branching. In filler without ZrO2 nanomaterials, cracks propagated through the filler metal leading to fracture.
Author Contributions
Funding
Conflicts of Interest
References
- Voigt, C.; Ditscherlein, L.; Werzner, E.; Zienert, T.; Nowak, R.; Peuker, U.; Sobczak, N.; Aneziris, C.G. Wettability of AlSi7Mg alloy on alumina, spinel, mullite and rutile and its influence on the aluminum melt filtration efficiency. Mater. Des. 2018, 150, 75–85. [Google Scholar] [CrossRef]
- Hussain, T.; McCartney, D.G.; Shipway, P.H. Bonding between aluminium and copper in cold spraying: Story of asymmetry. Mater. Sci. Technol. 2012, 28, 1371–1378. [Google Scholar] [CrossRef]
- Gueydan, A.; Domengès, B.; Hug, E. Study of the intermetallic growth in copper-clad aluminum wires after thermal aging. Intermetallics 2014, 50, 34–42. [Google Scholar] [CrossRef]
- Balasundaram, R.; Patel, V.K.; Bholen, S.D.; Chen, D.L. Effect of zinc interlayer on ultrasonic spot welded aluminum-to-copper joints. Mater. Sci. Eng. A 2014, 607, 277–286. [Google Scholar] [CrossRef]
- Lee, K.S.; Kwon, Y.N. Solid-state bonding between Al and Cu by vacuum hot pressing. Trans. Nonferrous Met. Soc. China 2013, 23, 341–346. [Google Scholar] [CrossRef]
- Abbasi, M.; Taheri, A.K.; Salehi, M.T. Growth rate of intermetallic compounds in Al/Cu bimetal produced by cold roll welding process. J. Alloy. Compd. 2001, 319, 233–241. [Google Scholar] [CrossRef]
- Galvão, I.; Verdera, D.; Gesto, D.; Loureiro, A.; Rodrigues, D.M. Influence of aluminium alloy type on dissimilar friction stir lap welding of aluminium to copper. J. Mater. Process. Technol. 2013, 213, 1920–1928. [Google Scholar] [CrossRef] [Green Version]
- Gorka, U.; Jose, M.P.; Luis, N.L.; Aritz, A. Combination of friction drilling and form tapping processes on dissimilar materials for making nutless joints. Proc. IMechE Part B: J. Eng. Manuf. 2016, 1, 1–14. [Google Scholar]
- Labari, C.B.; Goñi, A.A.; Azpilicueta, P.B.; Peinado, M.; Carrasquilla, J. Study and selection of the most appropriate filler materials for an Al/Cu brazing joint in cooling circuits. Mater. Manuf. Process. 2011, 26, 236–241. [Google Scholar] [CrossRef]
- Srinivas, V.; Singh, A.K.; Krishna, G.; Reddy, M. Vacuum brazing of dissimilar tubular component of AA2219 and AISI 304 by a low melting Al-18Ag-20Cu-5Si-0.2Zn braze alloy. J. Mater. Process. Technol. 2018, 252, 1–12. [Google Scholar]
- Tsao, L.C.; Weng, W.P.; Cheng, M.D.; Tsao, C.W.; Chuang, T.H. Brazeability of a 3003 Aluminum alloy with Al-Si-Cu-based filler metals. J. Mater. Eng. Perform. 2002, 11, 360–364. [Google Scholar] [CrossRef]
- Humpston, G.; Sangha, S.P.S.; Jacobson, D.M. New filler metals process and for fluxless brazing of aluminum engineering alloys. Mater. Sci. Technol. 1995, 11, 1161–1168. [Google Scholar] [CrossRef]
- Chang, S.Y.; Tsao, L.C.; Li, T.Y.; Chuang, T.H. Joining 6061 Aluminium alloys with Al-Si-Cu filler metals. J. Alloy. Compd. 2009, 488, 174–180. [Google Scholar] [CrossRef]
- Boucherit, A.; Fenoel, M.N.A.; Taillard, R. Effect of Zn interlayer on dissimilar FSSW of Al and Cu. Mater. Des. 2017, 124, 87–99. [Google Scholar] [CrossRef]
- Suzuki, K.; Kagayama, M.; Takeuchi, Y. Eutectic phase equilibrium of Al-Si-Zn system and its applicability for lower temperature brazing. J. Jpn. Inst. Light Met. 1993, 43, 533–538. [Google Scholar] [CrossRef]
- Chen, L.Y.; Xu, J.Q.; Choi, H.; Konishi, H.; Jin, S.; Li, X.C. Rapod control of phase growth by nanoparticles. Nat. Commun. 2014, 5, 1–9. [Google Scholar]
- Tan, A.T.; Tan, A.W.; Yusof, F. Influence of nanoparticle addition on the formation and growth of intermetallic compounds (IMCs) in Cu/Sn–Ag–Cu/Cu solder joint during different thermal conditions. Sci. Technol. Adv. Mater. 2015, 16, 1–18. [Google Scholar]
- Wang, P.; Gao, Z.; Niu, J. Micro–nano filler metal foil on vacuum brazing of SiCp/Al composites. Appl. Phys. A 2016, 122, 1–11. [Google Scholar] [CrossRef]
- Sharma, A.; Roh, M.H.; Jung, J.P. Effect of La2O3 nanoparticles on the brazeability, microstructure, and mechanical properties of Al-11Si-20Cu alloy. J. Mater. Eng. Perform. 2016, 25, 3538–3545. [Google Scholar] [CrossRef]
- Sharma, A.; Roh, M.H.; Jung, D.H.; Jung, J.P. Effect of ZrO2 nanoparticles on the microstructure of Al-Si-Cu filler for low-temperature Al brazing applications. Metall. Mater. Trans. A 2016, 47, 510–521. [Google Scholar] [CrossRef]
- Sharma, A.; Lim, D.U.; Jung, J.P. Microstructure and brazeability of SiC nanoparticles reinforced Al–9Si–20Cu produced by induction melting. Mater. Sci. Technol. 2016, 32, 773–779. [Google Scholar] [CrossRef]
- Mahallawi, I.S.; Shash, A.Y.; Amer, A.E. Nano-reinforced cast Al-Si alloys with Al2O3, TiO2, and ZrO2 nanoparticles. Metals 2015, 5, 802–821. [Google Scholar] [CrossRef]
- Nowacki, J.; Moraneic, K. Evaluation of methods of soldering AlSi and AlSi–SiC particle composite Al foams. J. Mater. Eng. Perform. 2015, 24, 426–433. [Google Scholar] [CrossRef]
- Cao, Z.; Stevens, M.J.; Dobrynin, A.V. Adhesion and wetting of nanoparticles on soft surfaces. Macromolecules 2014, 47, 3203–3209. [Google Scholar] [CrossRef]
- Japanese industrial standard, JIS Z-3197; Japan. 2012.
- Sharma, A.; Sohn, H.R.; Jung, J.P. Effect of graphene nanoplatelets on wetting, microstructure, and tensile characteristics of Sn-3.0Ag-0.5Cu (SAC) alloy. Metall. Mater. Trans. A 2016, 47, 494–503. [Google Scholar] [CrossRef]
- ASTM E-8. Standard Test Methods for Tension Testing of Metallic Materials; STM International: West Conshohocken, PA, USA, 2011.
- Awe, S.A.; Seifeddine, S.; Jarfors, A.E.W.; Lee, Y.C.; Dahle, A.K. Development of new Al-Cu-Si alloys for high temperature performance. Adv. Mater. Lett. 2017, 8, 695–701. [Google Scholar] [CrossRef]
- Okayasu, M.; Ohkura, Y.; Takeuchi, S.; Takasu, S.; Ohfuji, H.; Shiraishi, T. A study of the mechanical properties of an Al-Si-Cu alloy (ADC12) produced by various casting processes. Mater. Sci. Eng. A 2012, 543, 185–192. [Google Scholar] [CrossRef]
- Chen, L.Y.; Xu, J.Q.; Li, X.C. Controlling phase growth during solidification by nanoparticles. Mater. Res. Lett. 2015, 3, 43–49. [Google Scholar] [CrossRef]
- Kui, W.; Haiyan, J.; Qudong, W.; Bing, Y.; Wenjiang, D. A novel method to achieve grain refinement in aluminum. Metall. Mater. Trans. A 2016, 47, 4788–4794. [Google Scholar]
- Ozsoy, I.B.; Li, G.; Choi, H.; Zhao, H. Shape effects on nanoparticle engulfment for metal matrix nanocomposites. J. Cryst. Growth 2015, 422, 62–68. [Google Scholar] [CrossRef]
- Xu, J.Q.; Chen, L.Y.; Choi, H.; Li, X.C. Theoretical study and pathways for nanoparticle capture during solidification of metal melt. J. Phys. Condens. Matter. 2012, 24, 255304–255314. [Google Scholar] [CrossRef] [PubMed]
- Stefanescu, D.M. Science and Engineering of Casting Solidification, 2nd ed.; Springer: Berlin, Germany, 2009. [Google Scholar]
- Gao, K.; Li, S.; Xu, L.; Fu, H. Effect of sample size on intermetallic Al2Cu microstructure and orientation evolution during directional solidification. J. Cryst. Growth 2014, 394, 89–96. [Google Scholar] [CrossRef]
- Dahle, A.K.; Nogita, K.; Zindel, J.W.; Mcdonald, S.D.; Hogan, L.M. Eutectic nucleation and growth in hypoeutectic Al-Si alloys at different strontium levels. Metall. Mater. Trans. A 2001, 32, 949–960. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Song, J.L.; Hu, W.X.; Li, J.C.; Xiong, S.M. Morphology transition of the primary silicon particles in a hypereutectic A390 alloy in high pressure die casting. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, H. Intermetallic formation at interface of Al/Cu clad fabricated by hydrostatic extrusion and its properties. J. Nanosci. Nanotechnol. 2015, 11, 8589–8592. [Google Scholar]
- Wu, J.; Xue, S.; Wang, J.; Wu, M.; Wang, J. Effects of α-Al2O3 nanoparticles-doped on microstructure and properties of Sn–0.3Ag–0.7Cu low-Ag solder. J. Mater. Sci. Mater. Electron. 2018, 29, 7372–7387. [Google Scholar] [CrossRef]
- Choi, S.; Awaji, H. Nanocomposites – a new material design concept. Sci. Technol. Adv. Mater. 2005, 6, 2–10. [Google Scholar] [CrossRef]
- Tang, F.; Anderson, I.E.; Herold, T.G.; Prask, H. Pure Al matrix composites produced by vacuum hot pressing: Tensile properties and strengthening mechanisms. Mater. Sci. Eng. A 2004, 383, 362–373. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.L. Consideration of Orowan strengthening effect in particulate- reinforced metal matrix nanocomposites: A model for predicting their yield strength. Scripta Mater. 2006, 54, 1321–1326. [Google Scholar] [CrossRef]
- Scudino, S.; Liu, G.; Prashanth, K.G.; Bartusch, B.; Surreddi, K.B.; Murty, B.S. Mechanical properties of Al-based metal matrix composites reinforced with Zr based glassy particles produced by powder metallurgy. Acta Mater. 2009, 57, 2029–2039. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, S.J.; Choi, D.Y.; Jung, J.P. Effect of brazing current and speed on the bead characteristics, microstructure, and mechanical properties of the arc brazed galvanized steel sheets. J. Mater. Process. Technol. 2017, 249, 212–220. [Google Scholar] [CrossRef]
- Nakagawa, H.; Lee, C.H.; North, T.H. Modeling of base metal dissolution behavior during transient liquid-phase brazing. Metall. Trans. A 1991, 22, 543–555. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; He, P.; Feng, J.; Sekulic, D.P. Dissolution of TiAl alloy during high temperature brazing. J. Mater. Sci. 2013, 48, 5249–5252. [Google Scholar] [CrossRef]
- Tsao, L.C.; Chang, S.Y.; Lee, C.I.; Huang, C.H. Effects of nano-Al2O3 additions on microstructure development and hardness of Sn3.5Ag0.5Cu solder. Mater. Des. 2010, 31, 4831–4835. [Google Scholar] [CrossRef]
- Ritchie, O. Mechanisms of fatigue crack propagation in ductile and brittle solids. Int. J. Fracture 1999, 100, 55–83. [Google Scholar] [CrossRef]
- Kumar, S.; Curtin, W.A. Crack interaction with microstructure. Mater. Today 2007, 10, 34–44. [Google Scholar] [CrossRef]
- He, M.Y.; Evans, A.G.; Hutchinson, J.W. Crack deflection at an interface between dissimilar elastic materials: Role of residual stresses. Int. J. Solids Struct. 1994, 31, 3443–3455. [Google Scholar] [CrossRef]
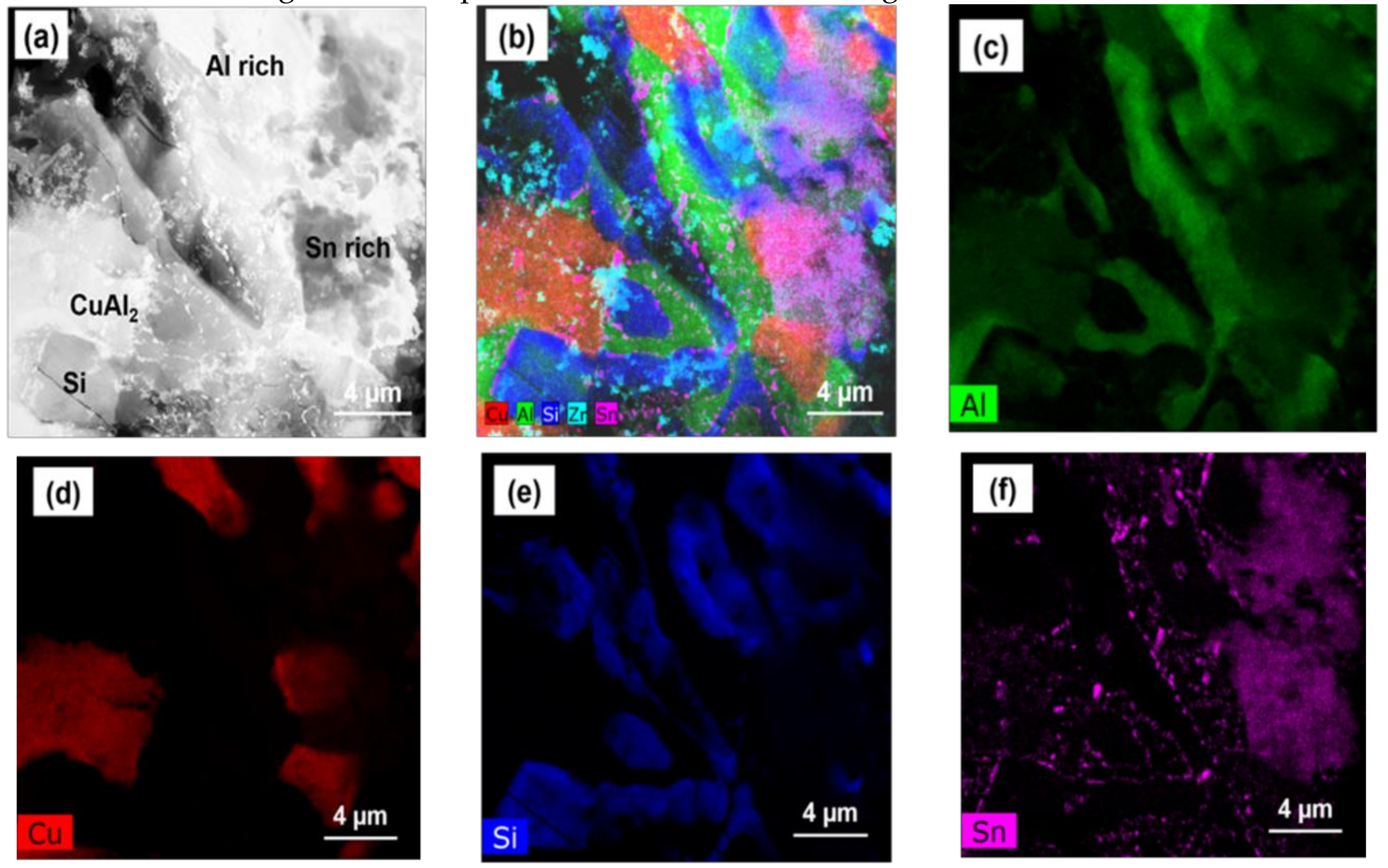
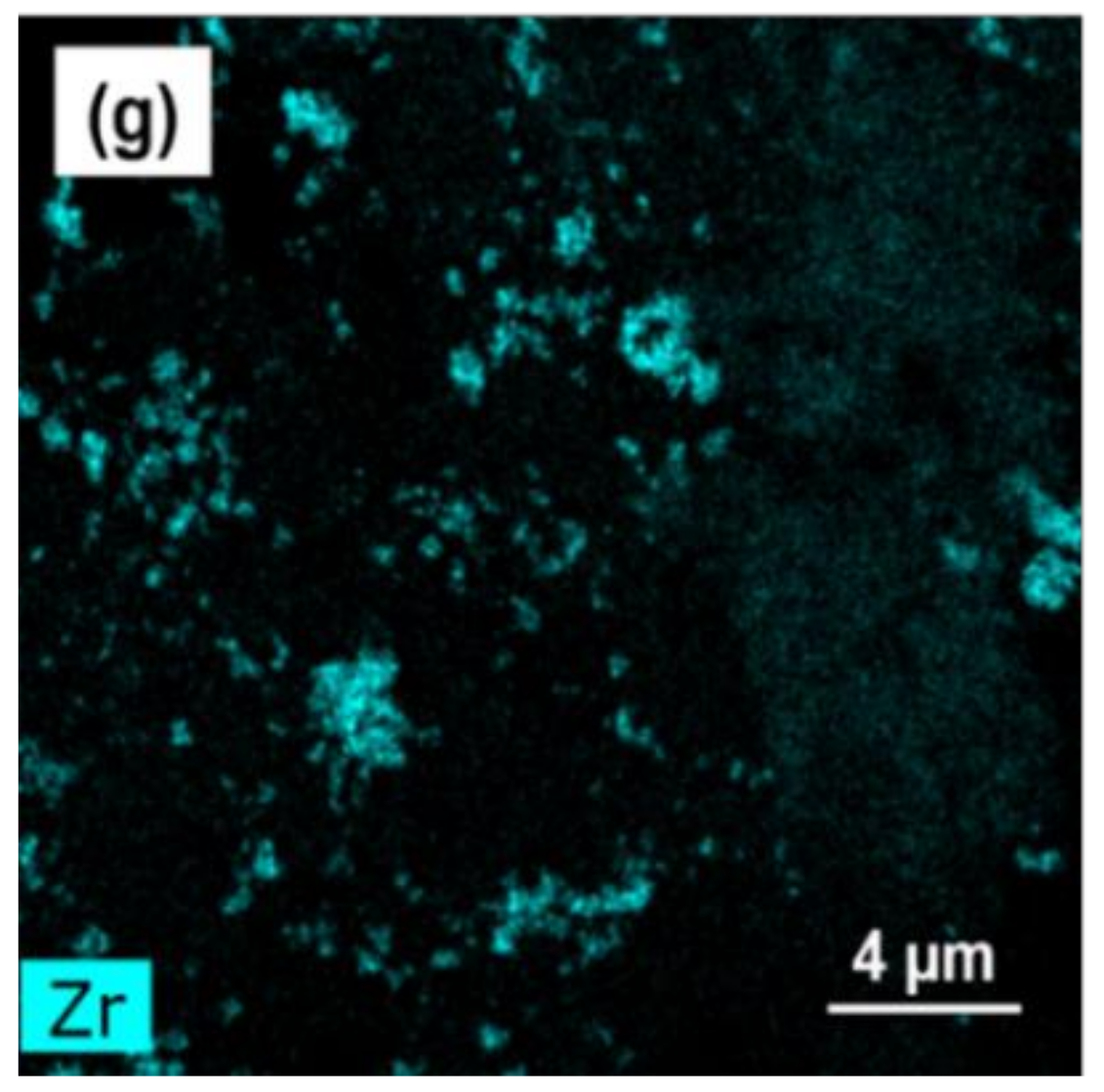


| Mark | Composition (at. %) | Phase | |||
|---|---|---|---|---|---|
| Al | Si | Cu | Sn | ||
| A | - | 100 | - | - | Si phase |
| B | 65.3 | - | 34.7 | - | CuAl2 IMC |
| C | 92.3 | 1.4 | 6.0 | 0.3 | Al rich phase |
| D | 2.5 | 0.4 | 3.2 | 93.9 | Sn rich phase |
| S.N0 | Samples | Micro Hardness (HV) | Tensile Properties | Quality Index(MPa) | ||
|---|---|---|---|---|---|---|
| Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | % Elongation | ||||
| 1 | Al-19Cu-11Si-2Sn | 89.4 ± 1.2 | 98.2 ± 1.1 | 124.7 ± 2.3 | 1.9 ± 0.2 | 167.2 |
| 2 | Al-19Cu-11Si-2Sn-0.03ZrO2 | 93.4 ± 0.9 | 103.4 ± 2.1 | 132.4 ± 1.1 | 2.2 ± 0.3 | 182.9 |
| 3 | Al-19Cu-11Si-2Sn-0.05ZrO2 | 107.8 ± 1.3 | 117.3 ± 1.7 | 157.4 ± 4.1 | 2.9 ± 0.1 | 225.6 |
| 4 | Al-19Cu-11Si-2Sn-0.1ZrO2 | 108.1 ± 1.4 | 123.4 ± 1.4 | 162.3 ± 3.3 | 2.9 ± 0.2 | 231.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, D.-H.; Rajendran, S.H.; Jung, J.-P. Effect of ZrO2 Nanomaterials on Wettability and Interfacial Characteristics of Al-19Cu-11Si-2Sn Filler Metal for Low Temperature Al to Cu Dissimilar Brazing. Nanomaterials 2018, 8, 784. https://doi.org/10.3390/nano8100784
Jung D-H, Rajendran SH, Jung J-P. Effect of ZrO2 Nanomaterials on Wettability and Interfacial Characteristics of Al-19Cu-11Si-2Sn Filler Metal for Low Temperature Al to Cu Dissimilar Brazing. Nanomaterials. 2018; 8(10):784. https://doi.org/10.3390/nano8100784
Chicago/Turabian StyleJung, Do-Hyun, Sri Harini Rajendran, and Jae-Pil Jung. 2018. "Effect of ZrO2 Nanomaterials on Wettability and Interfacial Characteristics of Al-19Cu-11Si-2Sn Filler Metal for Low Temperature Al to Cu Dissimilar Brazing" Nanomaterials 8, no. 10: 784. https://doi.org/10.3390/nano8100784





