Design, Synthesis, and Structure-Property Relationships of Er3+-Doped TiO2 Luminescent Particles Synthesized by Sol-Gel
Abstract
:1. Introduction
2. Results and Discussion
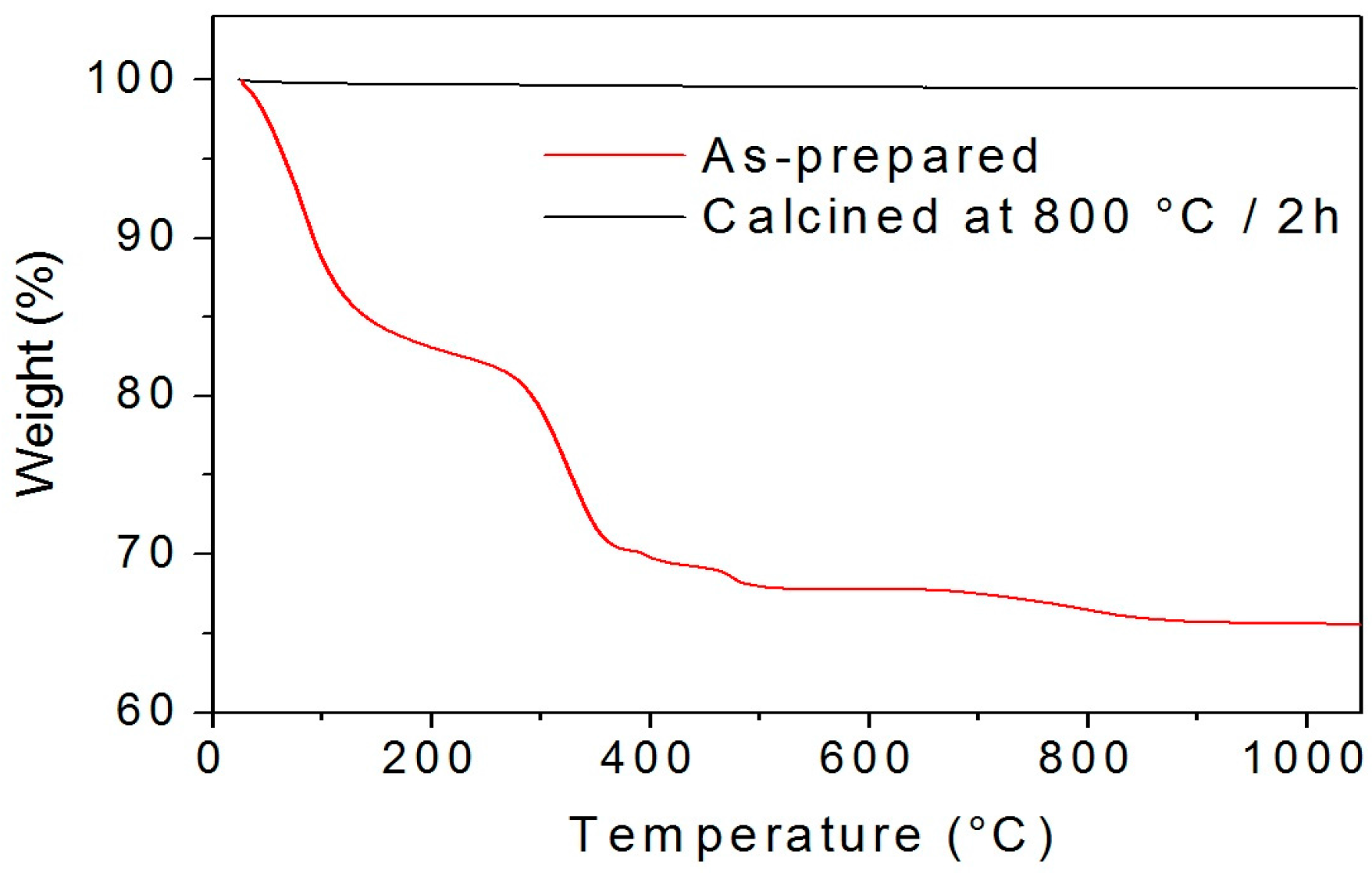
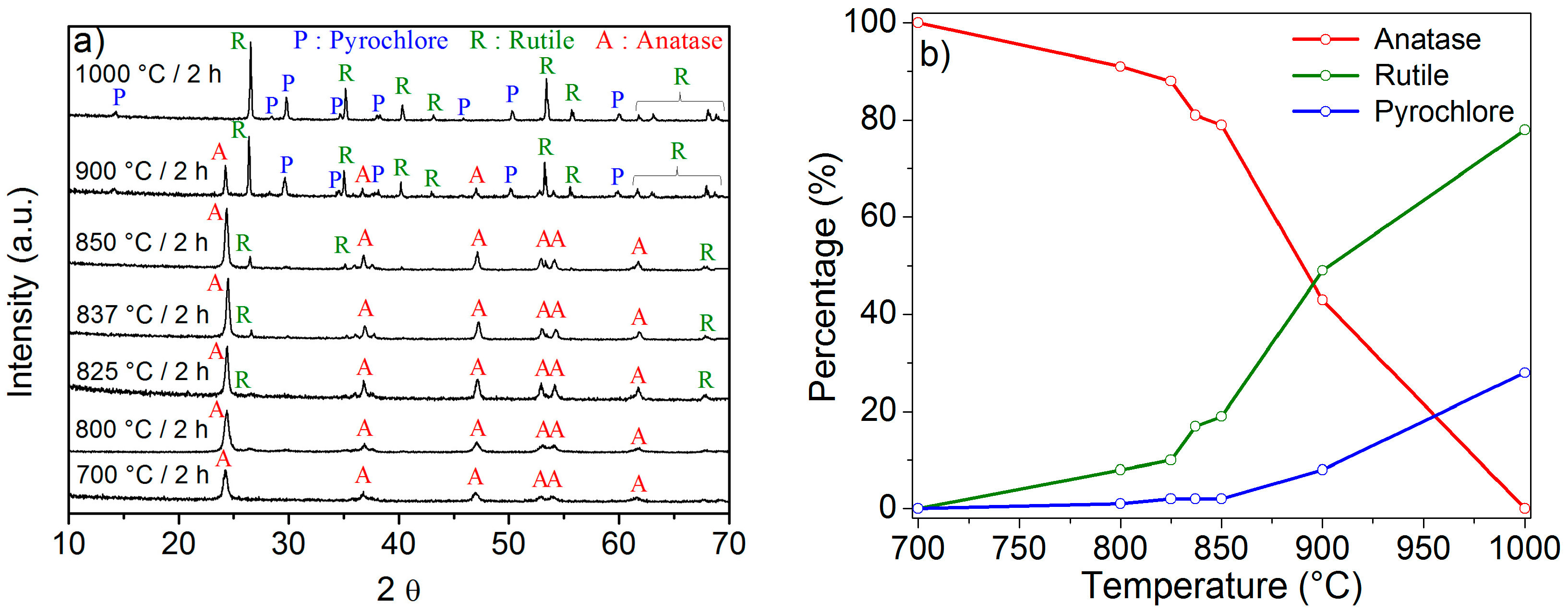
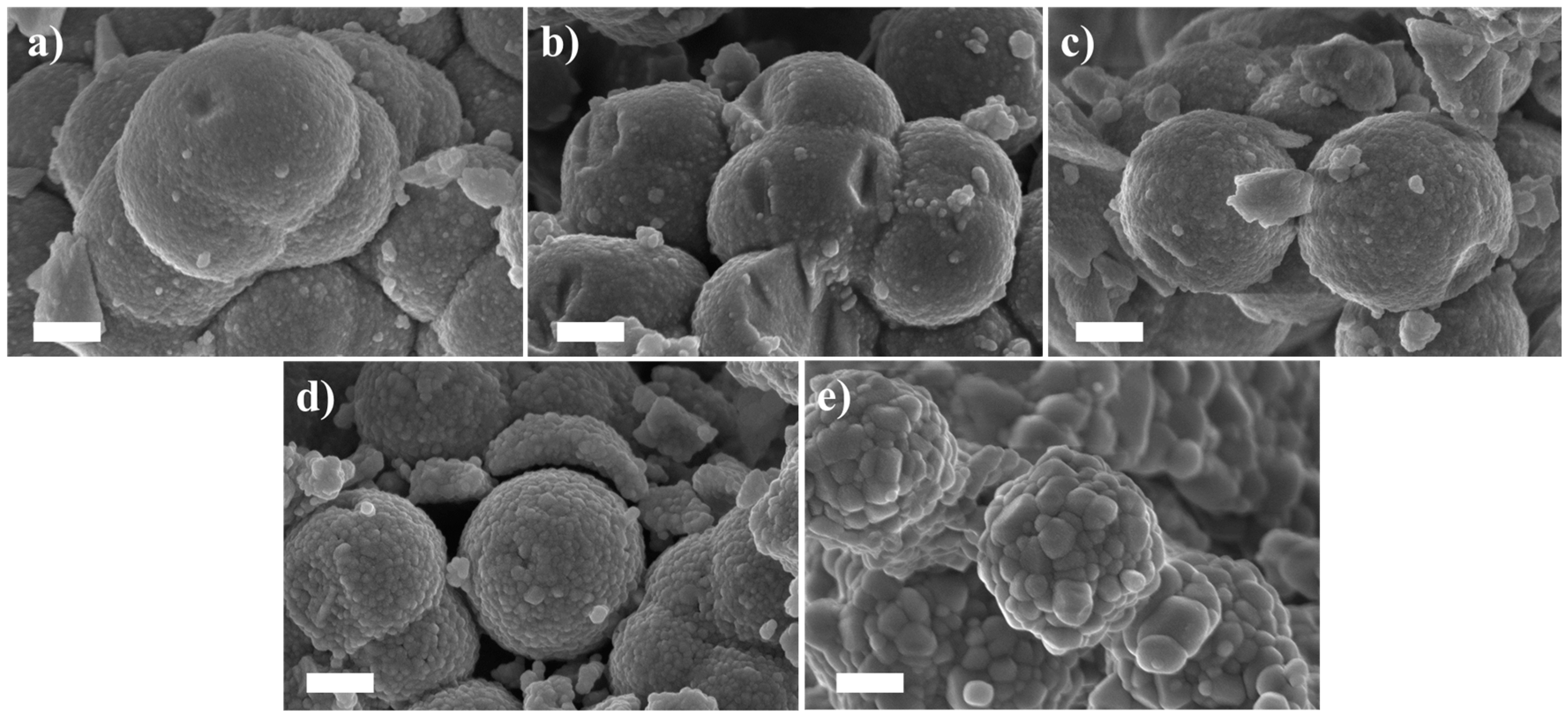
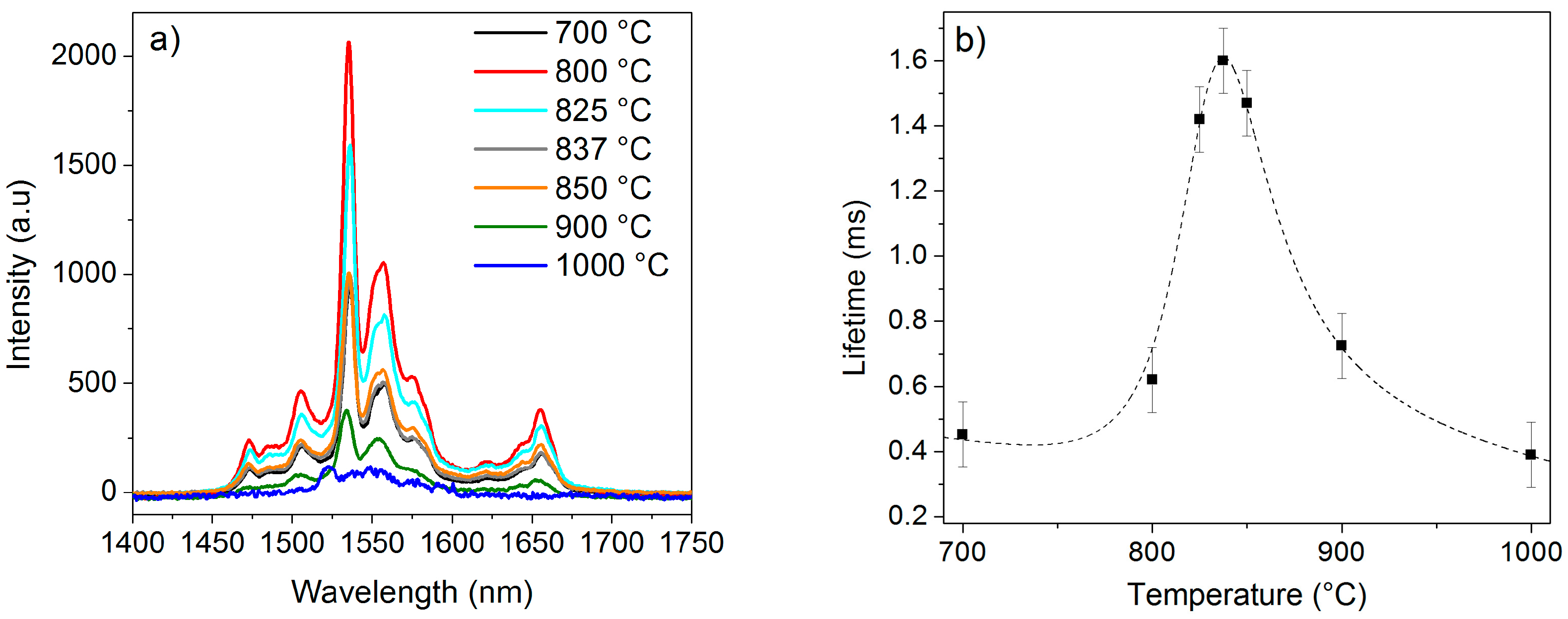
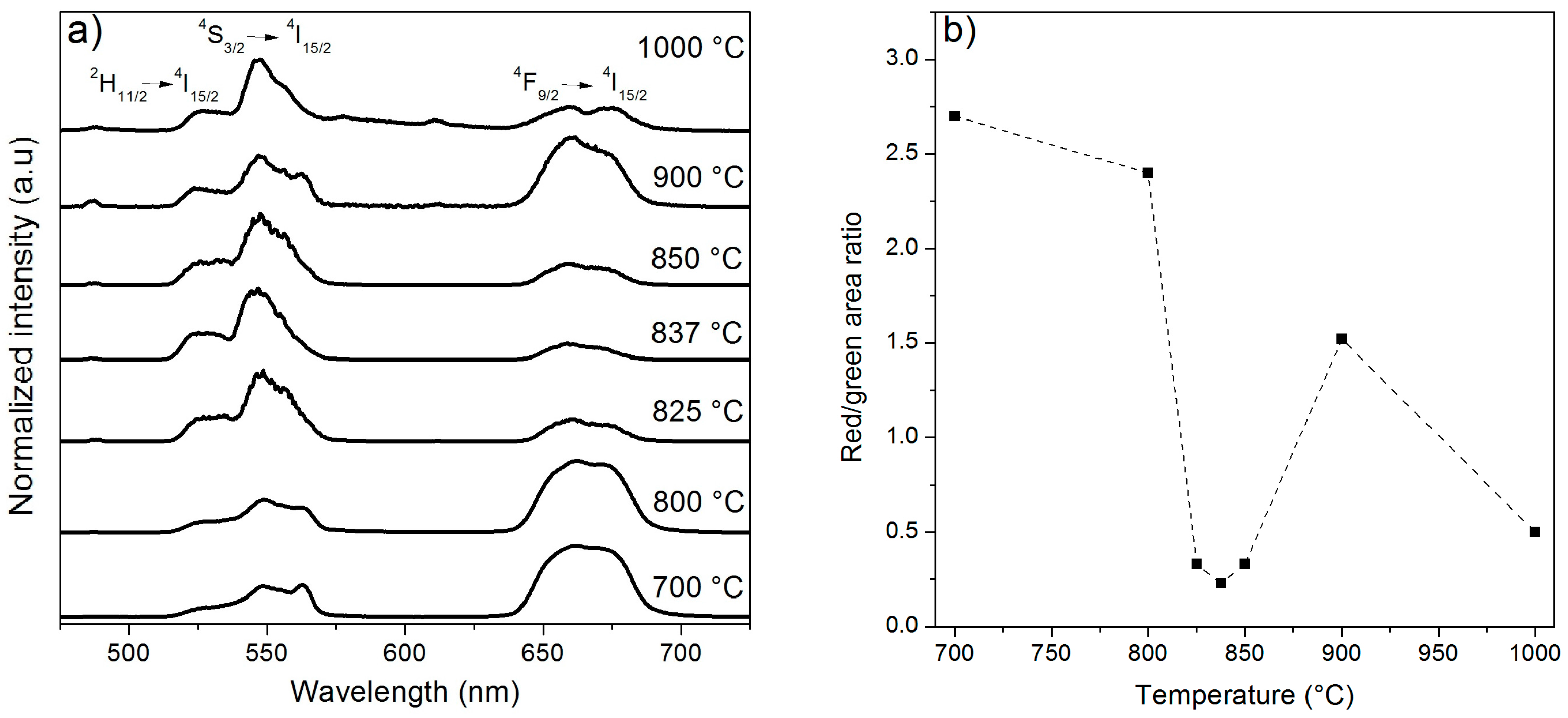
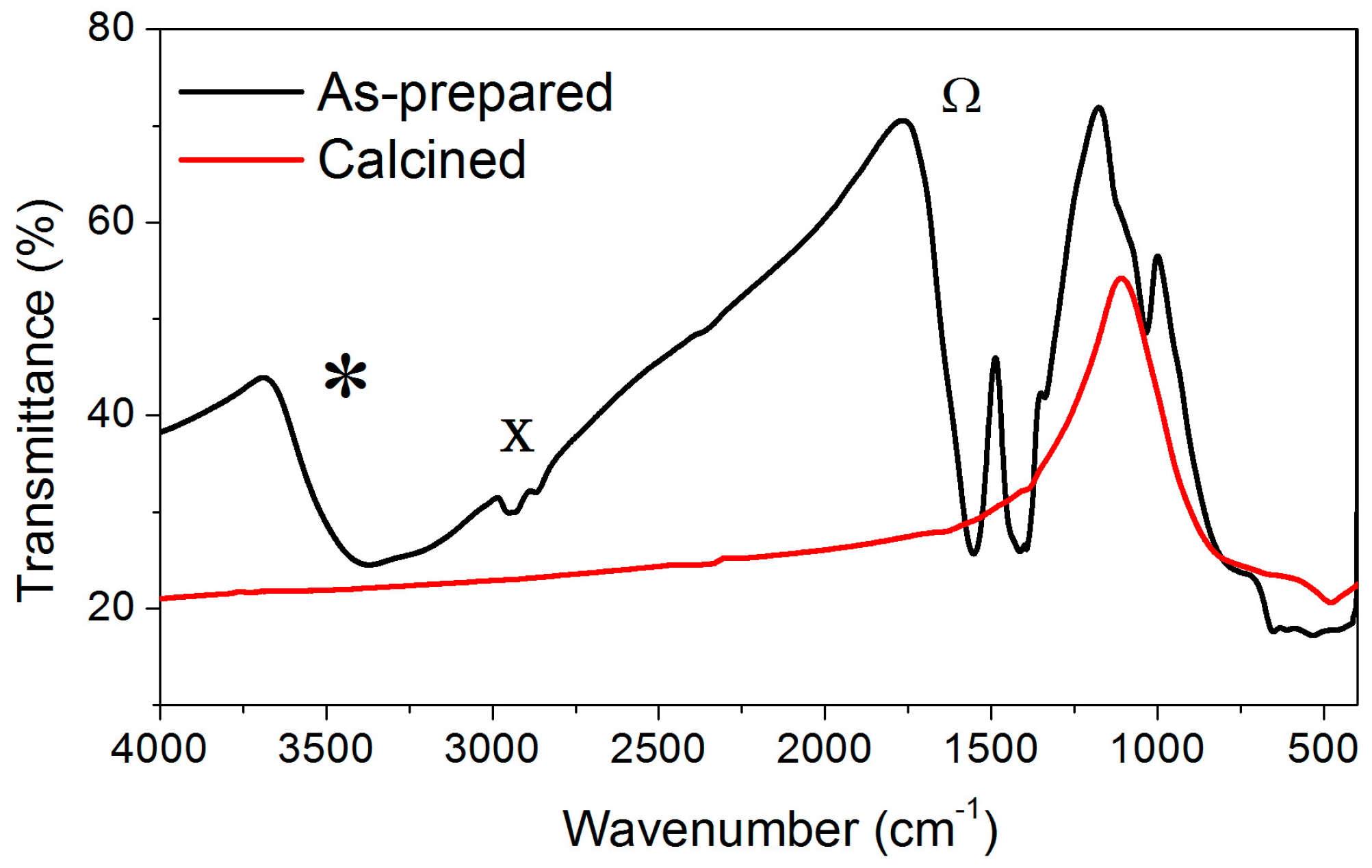
2.1. Effect of the Calcination Temperature on the Morphological, Structural, and Luminescence Properties of the TiO2 Particles
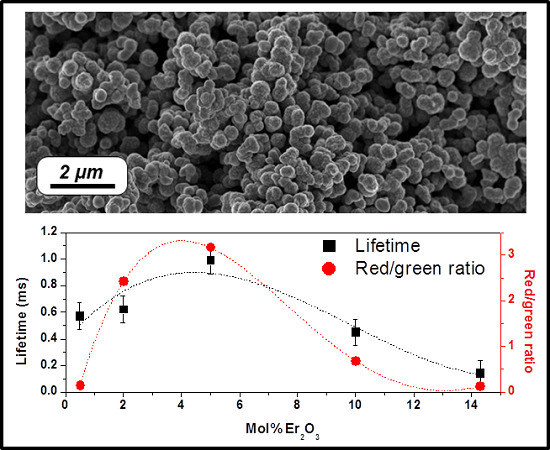
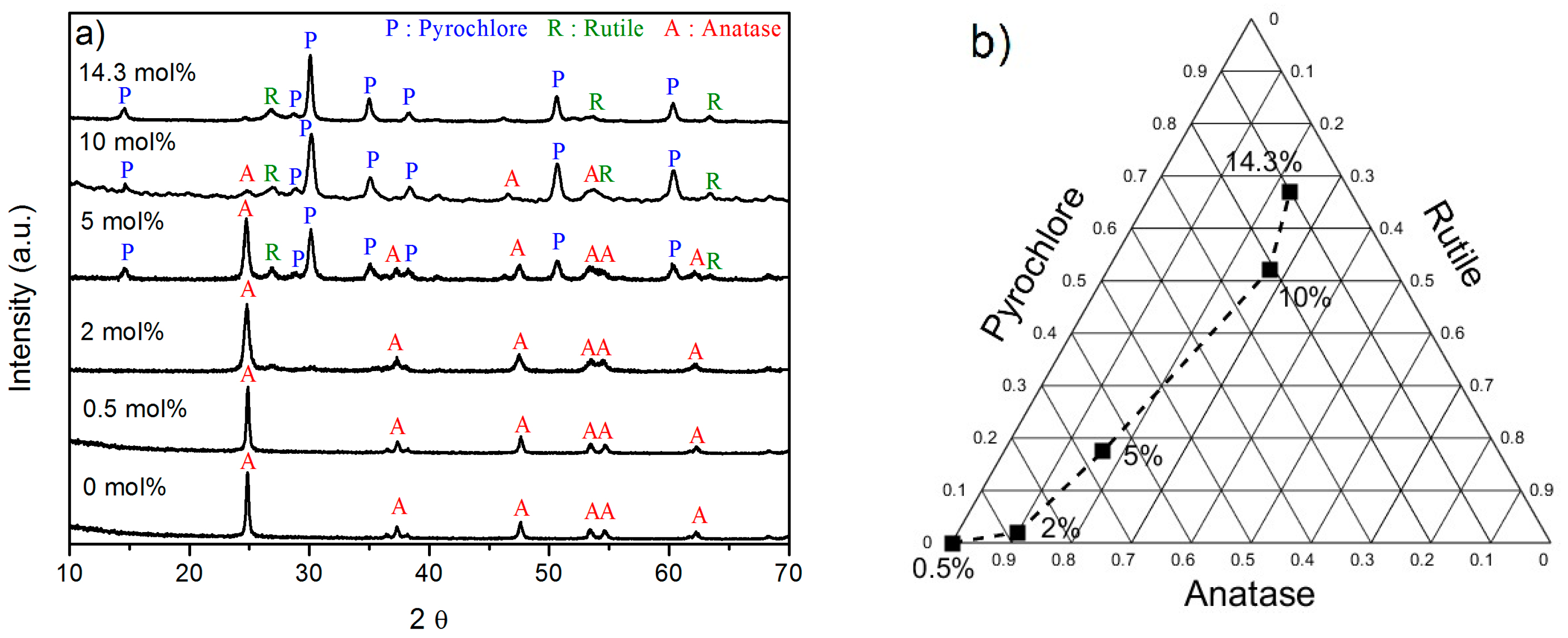
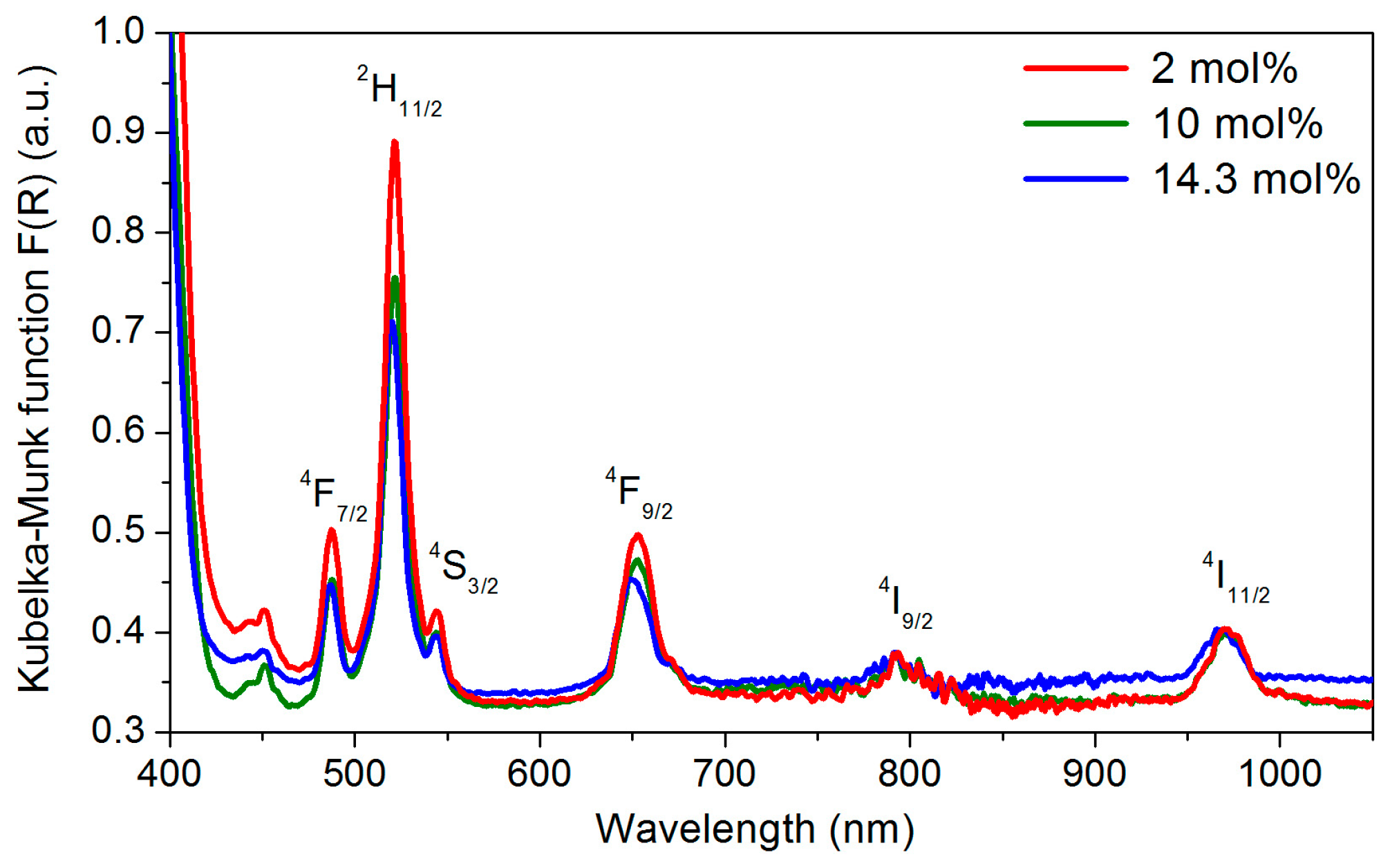
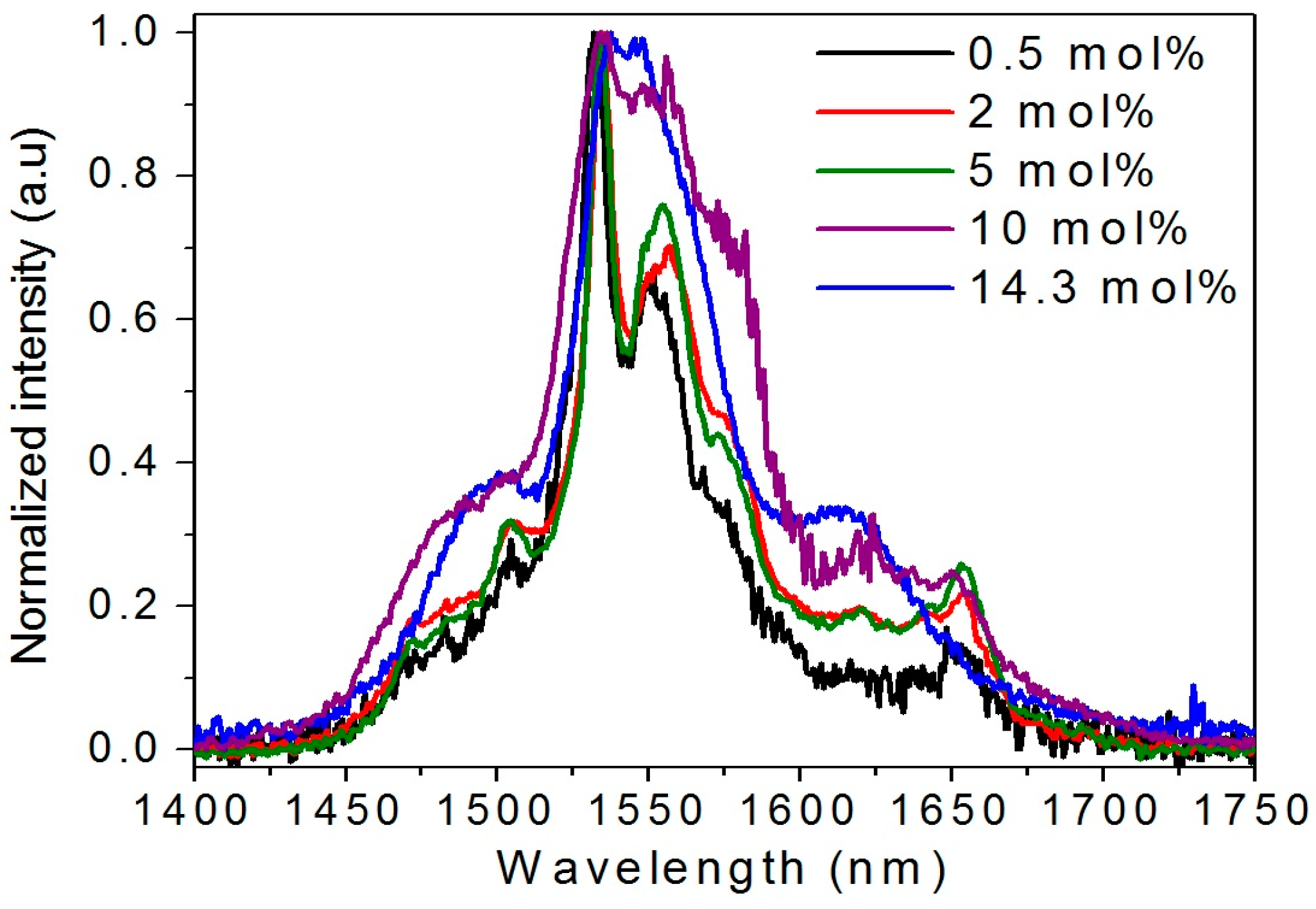
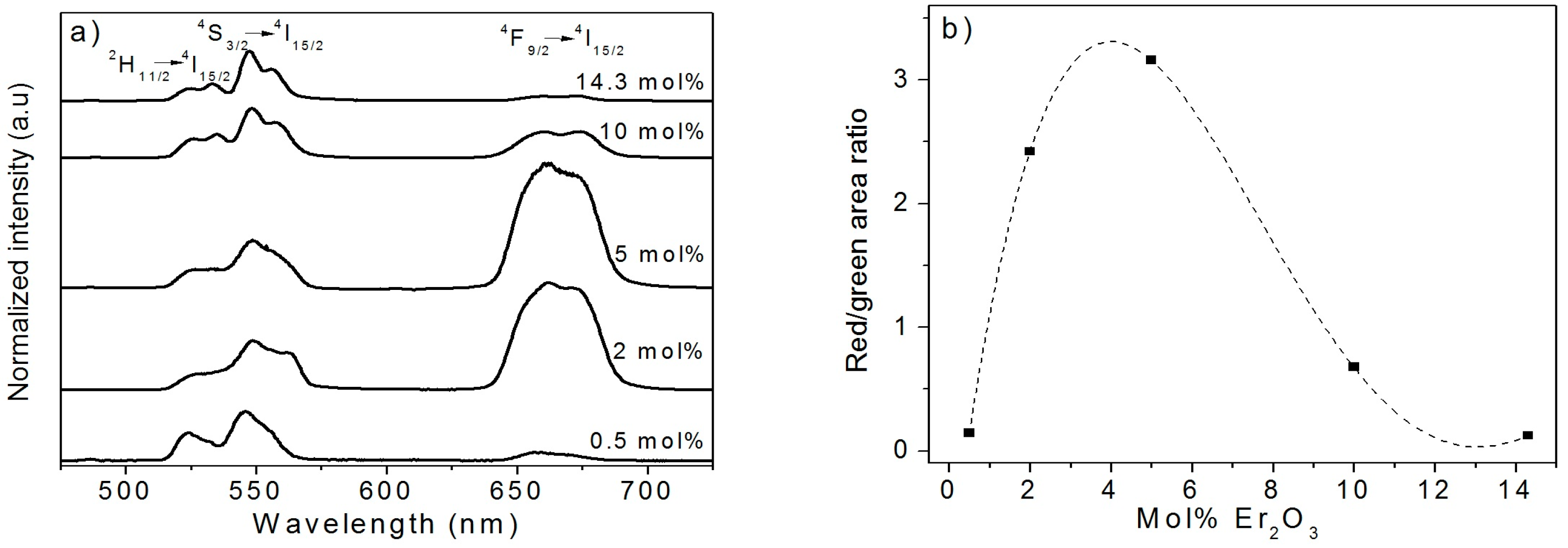
2.2. Effect of the Concentration of Er2O3 on the Morphological, Structural, and Luminescence Properties of the TiO2 Particles
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yin, Z.F.; Wu, L.; Yang, H.G.; Su, Y.H. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844–4858. [Google Scholar] [CrossRef] [PubMed]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Vasilaki, E.; Vernardou, D.; Kenanakis, G.; Vamvakaki, M.; Katsarakis, N. TiO2/WO3 photoactive bilayers in the UV–Vis light region. Appl. Phys. A 2017, 123, 231. [Google Scholar] [CrossRef]
- Vernardou, D.; Vlachou, K.; Spanakis, E.; Stratakis, E.; Katsarakis, N.; Kymakis, E.; Koudoumas, E. Influence of solution chemistry on the properties of hydrothermally grown TiO2 for advanced applications. Catal. Today 2009, 144, 172–176. [Google Scholar] [CrossRef]
- Suchea, M.; Christoulakis, S.; Tudose, I.V.; Vernardou, D.; Lygeraki, M.I.; Anastasiadis, S.H.; Kitsopoulos, T.; Kiriakidis, G. Pure and Nb2O5-doped TiO2 amorphous thin films grown by dc magnetron sputtering at room temperature: Surface and photo-induced hydrophilic conversion studies. Mater. Sci. Eng. B 2007, 144, 54–59. [Google Scholar] [CrossRef]
- Johannsen, S.R.; Roesgaard, S.; Julsgaard, B.; Ferreira, R.A.S.; Chevallier, J.; Balling, P.; Ram, S.K.; Nylandsted Larsen, A. Influence of TiO2 host crystallinity on Er3+ light emission. Opt. Mater. Express 2016, 6, 1664–1678. [Google Scholar] [CrossRef]
- Bahtat, A.; Bouazaoui, M.; Bahtat, M.; Mugnier, J. Fluorescence of Er3+ ions in TiO2 planar waveguides prepared by a sol-gel process. Opt. Commun. 1994, 111, 55–60. [Google Scholar] [CrossRef]
- Mignotte, C. Structural characterization for Er3+-doped oxide materials potentially useful as optical devices. Appl. Surf. Sci. 2004, 226, 355–370. [Google Scholar] [CrossRef]
- Lopez-Iscoa, P.; Petit, L.; Massera, J.; Janner, D.; Boetti, N.G.; Pugliese, D.; Fiorilli, S.; Novara, C.; Giorgis, F.; Milanese, D. Effect of the addition of Al2O3, TiO2 and ZnO on the thermal, structural and luminescence properties of Er3+-doped phosphate glasses. J. Non-Cryst. Solids 2017, 460, 161–168. [Google Scholar] [CrossRef]
- Miniscalco, W.J. Erbium-doped glasses for fiber amplifiers at 1500 nm. J. Lightwave Technol. 1991, 9, 234–250. [Google Scholar] [CrossRef]
- Bahtat, A.; Bouazaoui, M.; Bahtat, M.; Garapon, C.; Jacquier, B.; Mugnier, J. Up-conversion fluorescence spectroscopy in Er3+:TiO2 planar waveguides prepared by a sol–gel process. J. Non-Cryst. Solids 1996, 202, 16–22. [Google Scholar] [CrossRef]
- Johannsen, S.R.; Rosenfeld Lauridsen, L.; Julsgaard, B.; Neuvonen, P.T.; Ram, S.K.; Nylandsted Larsen, A. Optimization of Er3+-doped TiO2-thin films for infrared light up-conversion. Thin Solid Films 2014, 550, 499–503. [Google Scholar] [CrossRef]
- de Wild, J.; Meijerink, A.; Rath, J.K.; van Sark, W.G.J.H.M.; Schropp, R.E.I. Upconverter solar cells: materials and applications. Energy Environ. Sci. 2011, 4, 4835–4848. [Google Scholar] [CrossRef]
- Trupke, T.; Green, M.A.; Würfel, P. Improving solar cell efficiencies by up-conversion of sub-band-gap light. J. Appl. Phys. 2002, 92, 4117–4122. [Google Scholar] [CrossRef]
- Fischer, S.; Goldschmidt, J.C.; Löper, P.; Bauer, G.H.; Brüggemann, R.; Krämer, K.; Biner, D.; Hermle, M.; Glunz, S.W. Enhancement of silicon solar cell efficiency by upconversion: Optical and electrical characterization. J. Appl. Phys. 2010, 108, 044912. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Z.; Liu, X.; Li, Z.; Wu, X.; Jiang, J.; Li, M.; Zhu, Q.; Zhou, W. Ti3+ self-doped blue TiO2(B) single-crystalline nanorods for efficient solar-driven photocatalytic performance. ACS Appl. Mater. Interfaces 2016, 8, 26851–26859. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.K.; Tiwari, R.S.; Srivastava, O.N.; Sinha, A.S.K. Synthesis of TiO2 nanoribbons and its application in photoelectrochemical water splitting for hydrogen production. Int. J. Nanosci. 2011, 10, 723–726. [Google Scholar] [CrossRef]
- Li, Y.-F.; Liu, Z.-P. Particle size, shape and activity for photocatalysis on titania anatase nanoparticles in aqueous surroundings. J. Am. Chem. Soc. 2011, 133, 15743–15752. [Google Scholar] [CrossRef] [PubMed]
- Scolan, E.; Sanchez, C. Synthesis and characterization of surface-protected nanocrystalline titania particles. Chem. Mater. 1998, 10, 3217–3223. [Google Scholar] [CrossRef]
- Chemseddine, A.; Moritz, T. Nanostructuring titania: control over nanocrystal structure, size, shape, and organization. Eur. J. Inorg. Chem. 1999, 1999, 235–245. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, X. Wet-chemical preparation of TiO2-based composites with different morphologies and photocatalytic properties. Nanomaterials 2017, 7, 310. [Google Scholar] [CrossRef] [PubMed]
- Galliano, P.; De Damborenea, J.J.; Pascual, M.J.; Durán, A. Sol-gel coatings on 316L steel for clinical applications. J. Sol-Gel Sci. Technol. 1998, 13, 723–727. [Google Scholar] [CrossRef]
- Mohammed, A.J.; Kadhum, A.A.H.; Ba-Abbad, M.M.; Al-Amiery, A.A. Optimization of solar photocatalytic degradation of chloroxylenol using TiO2, Er3+/TiO2, and Ni2+/TiO2 via the Taguchi orthogonal array technique. Catalysts 2016, 6, 163. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, S.; Long, J.; Wu, T.; Fan, Z. The synthesis of a core-shell photocatalyst material YF3:Ho3+@TiO2 and investigation of its photocatalytic properties. Materials 2017, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Braun, P.V. Hydrothermal synthesis of Er-doped luminescent TiO2 nanoparticles. Chem. Mater. 2003, 15, 1256–1263. [Google Scholar] [CrossRef]
- Keddie, J.L.; Norton, L.J.; Kramer, E.J.; Giannelis, E.P. Neutron reflectometry characterization of interface width between sol-gel titanium dioxide and silicon dioxide thin films. J. Am. Ceram. Soc. 1993, 76, 2534–2538. [Google Scholar] [CrossRef]
- Langlet, M.; Coutier, C.; Fick, J.; Audier, M.; Meffre, W.; Jacquier, B.; Rimet, R. Sol–gel thin film deposition and characterization of a new optically active compound: Er2Ti2O7. Opt. Mater. 2001, 16, 463–473. [Google Scholar] [CrossRef]
- Yang, J.; Mei, S.; Ferreira, J.M.F. Hydrothermal synthesis of TiO2 nanopowders from tetraalkylammonium hydroxide peptized sols. Mater. Sci. Eng. C 2001, 15, 183–185. [Google Scholar] [CrossRef]
- Ovenstone, J.; Yanagisawa, K. Effect of hydrothermal treatment of amorphous titania on the phase change from anatase to rutile during calcination. Chem. Mater. 1999, 11, 2770–2774. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Ovenstone, J. Crystallization of anatase from amorphous titania using the hydrothermal technique: effects of starting material and temperature. J. Phys. Chem. B 1999, 103, 7781–7787. [Google Scholar] [CrossRef]
- Cacciotti, I.; Bianco, A.; Pezzotti, G.; Gusmano, G. Synthesis, thermal behaviour and luminescence properties of rare earth-doped titania nanofibers. Chem. Eng. J. 2011, 166, 751–764. [Google Scholar] [CrossRef]
- Mignotte, C. EXAFS studies on erbium-doped TiO2 and ZrO2 sol–gel thin films. J. Non-Cryst. Solids 2001, 291, 56–77. [Google Scholar] [CrossRef]
- Howard, C.J.; Sabine, T.M.; Dickson, F. Structural and thermal parameters for rutile and anatase. Acta Crystallogr. Sect. B: Struct. Sci. 1991, 47, 462–468. [Google Scholar] [CrossRef]
- Chen, X.-C.; Zhou, J.-P.; Wang, H.-Y.; Xu, P.-S.; Pan, G.-Q. In situ high temperature X-ray diffraction studies of ZnO thin film. Chin. Phys. B 2011, 20, 096102. [Google Scholar] [CrossRef]
- Patra, A.; Friend, C.S.; Kapoor, R.; Prasad, P.N. Fluorescence upconversion properties of Er3+-doped TiO2 and BaTiO3 nanocrystallites. Chem. Mater. 2003, 15, 3650–3655. [Google Scholar] [CrossRef]
- Zhao, J.-G.; Zhang, W.-Y.; Ma, Z.-W.; Xie, E.-Q.; Zhao, A.-K.; Liu, Z.-J. Structure and photoluminescence properties of Er3+-doped TiO2–SiO2 powders prepared by sol–gel method. Chin. Phys. B 2011, 20, 087701. [Google Scholar] [CrossRef]
- Ting, C.-C.; Chen, S.-Y.; Hsieh, W.-F.; Lee, H.-Y. Effects of yttrium codoping on photoluminescence of erbium-doped TiO2 films. J. Appl. Phys. 2001, 90, 5564–5569. [Google Scholar] [CrossRef]
- He, C.; Tian, B.; Zhang, J. Synthesis of thermally stable and highly ordered bicontinuous cubic mesoporous titania–silica binary oxides with crystalline framework. Microporous Mesoporous Mater. 2009, 126, 50–57. [Google Scholar] [CrossRef]
- Li, J.-G.; Wang, X.-H.; Kamiyama, H.; Ishigaki, T.; Sekiguchi, T. RF plasma processing of Er-doped TiO2 luminescent nanoparticles. Thin Solid Films 2006, 506–507, 292–296. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 96th ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781482260977. [Google Scholar]
- Reszczyńska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Visible light activity of rare earth metal doped (Er3+, Yb3+ or Er3+/Yb3+) titania photocatalysts. Appl. Catal. B 2015, 163, 40–49. [Google Scholar] [CrossRef]
- Bahtat, A.; Bouderbala, M.; Bahtat, M.; Bouazaoui, M.; Mugnier, J.; Druetta, M. Structural characterisation of Er3+ doped sol–gel TiO2 planar optical waveguides. Thin Solid Films 1998, 323, 59–62. [Google Scholar] [CrossRef]
- Lopez-Iscoa, P.; Salminen, T.; Hakkarainen, T.; Petit, L.; Janner, D.; Boetti, N.G.; Lastusaari, M.; Pugliese, D.; Paturi, P.; Milanese, D. Effect of partial crystallization on the structural and luminescence properties of Er3+-doped phosphate glasses. Materials 2017, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Tobaldi, D.M.; Tucci, A.; Škapin, A.S.; Esposito, L. Effects of SiO2 addition on TiO2 crystal structure and photocatalytic activity. J. Eur. Ceram. Soc. 2010, 30, 2481–2490. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Quimby, R.S.; Miniscalco, W.J.; Thompson, B. Clustering in erbium-doped silica glass fibers analyzed using 980 nm excited-state absorption. J. Appl. Phys. 1994, 76, 4472–4478. [Google Scholar] [CrossRef]
- Golding, P.S.; Jackson, S.D.; King, T.A.; Pollnau, M. Energy transfer processes in Er3+-doped and Er3+,Pr3+-codoped ZBLAN glasses. Phys. Rev. B Condens. Matter 2000, 62, 856–864. [Google Scholar] [CrossRef]
- Hubbard, C.R.; Snyder, R.L. RIR-measurement and use in quantitative XRD. Powder Diffr. 1988, 3, 74–77. [Google Scholar] [CrossRef]
- Sandoval, C.; Kim, A.D. Deriving Kubelka–Munk theory from radiative transport. J. Opt. Soc. Am. A 2014, 31, 628–636. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Iscoa, P.; Pugliese, D.; Boetti, N.G.; Janner, D.; Baldi, G.; Petit, L.; Milanese, D. Design, Synthesis, and Structure-Property Relationships of Er3+-Doped TiO2 Luminescent Particles Synthesized by Sol-Gel. Nanomaterials 2018, 8, 20. https://doi.org/10.3390/nano8010020
Lopez-Iscoa P, Pugliese D, Boetti NG, Janner D, Baldi G, Petit L, Milanese D. Design, Synthesis, and Structure-Property Relationships of Er3+-Doped TiO2 Luminescent Particles Synthesized by Sol-Gel. Nanomaterials. 2018; 8(1):20. https://doi.org/10.3390/nano8010020
Chicago/Turabian StyleLopez-Iscoa, Pablo, Diego Pugliese, Nadia G. Boetti, Davide Janner, Giovanni Baldi, Laeticia Petit, and Daniel Milanese. 2018. "Design, Synthesis, and Structure-Property Relationships of Er3+-Doped TiO2 Luminescent Particles Synthesized by Sol-Gel" Nanomaterials 8, no. 1: 20. https://doi.org/10.3390/nano8010020