Stability and Synergistic Effect of Polyaniline/TiO2 Photocatalysts in Degradation of Azo Dye in Wastewater
Abstract
:1. Introduction
2. Materials and Methods
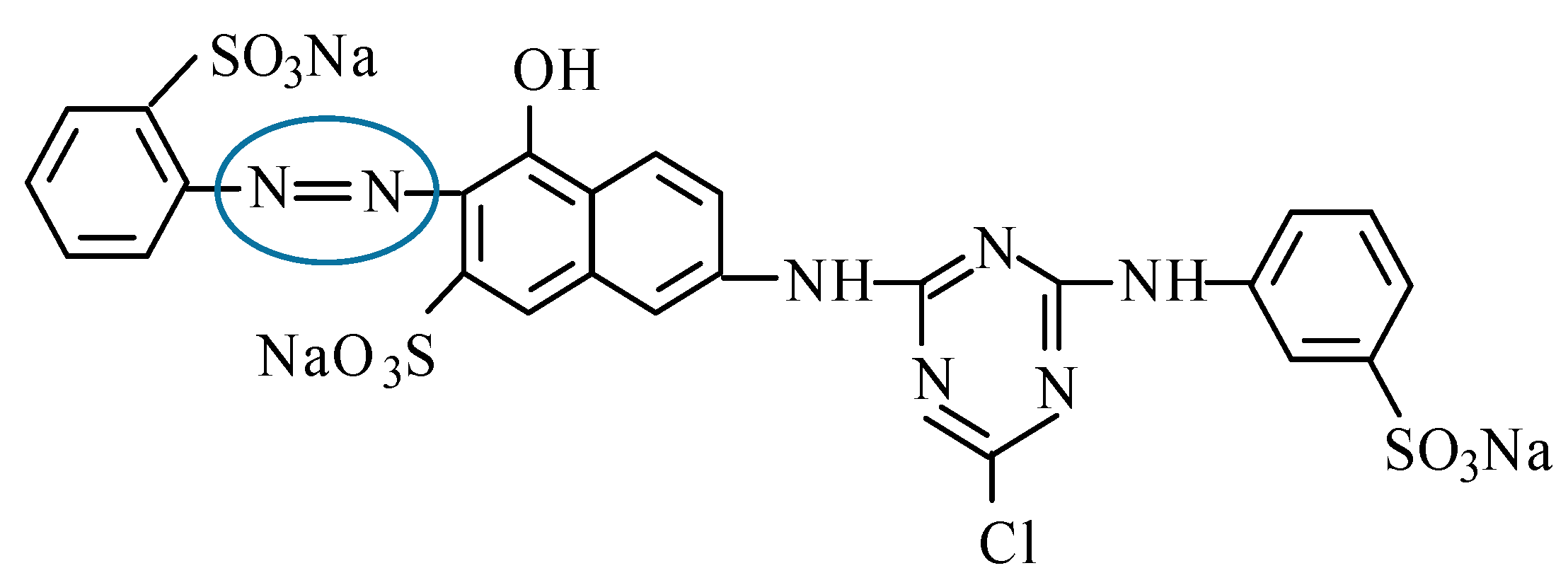
2.1. Materials
2.2. PANI/TiO2 Photocatalyst Preparation
2.3. Characterizations
2.4. Photocatalytic Activity Test
3. Results and Discussion
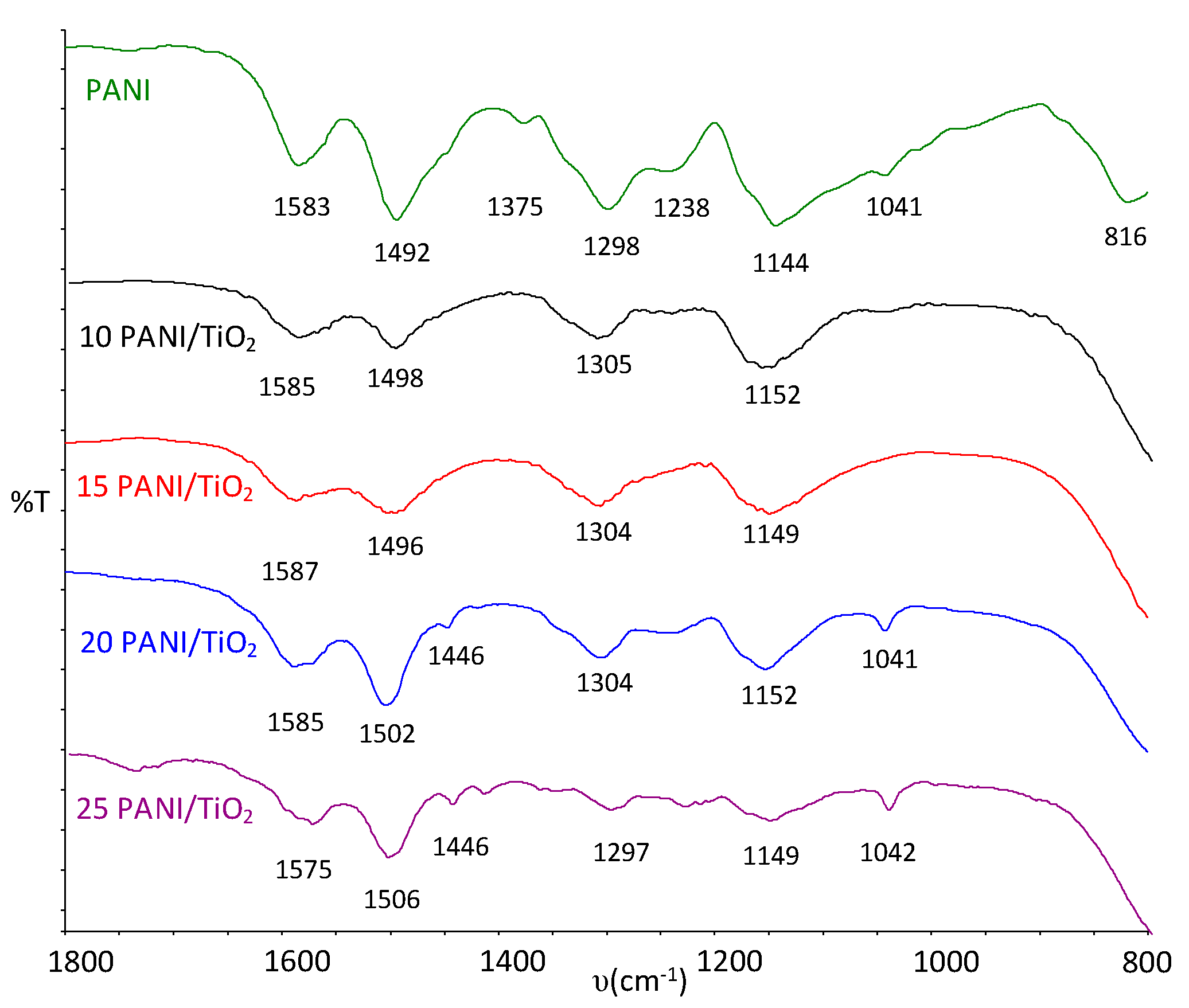
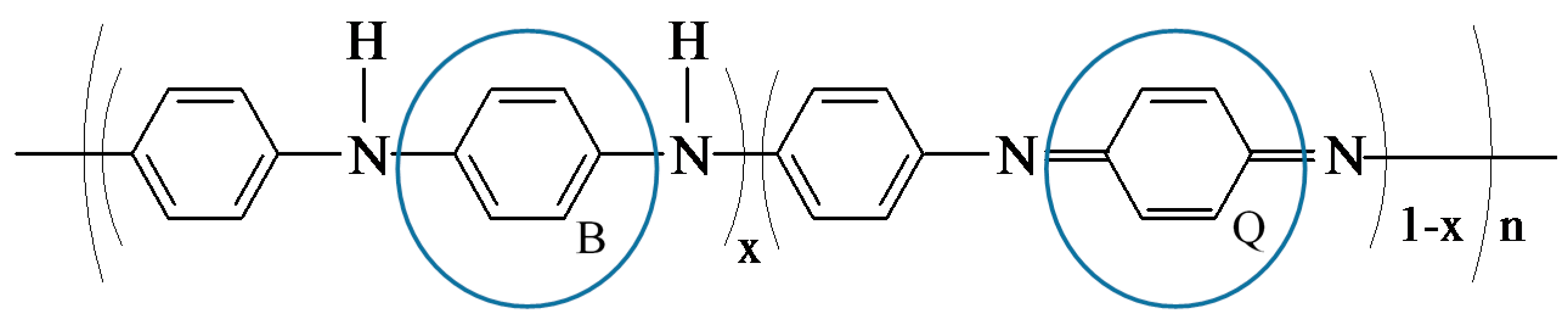
3.1. FT-IR Analyses of Photocatalysts
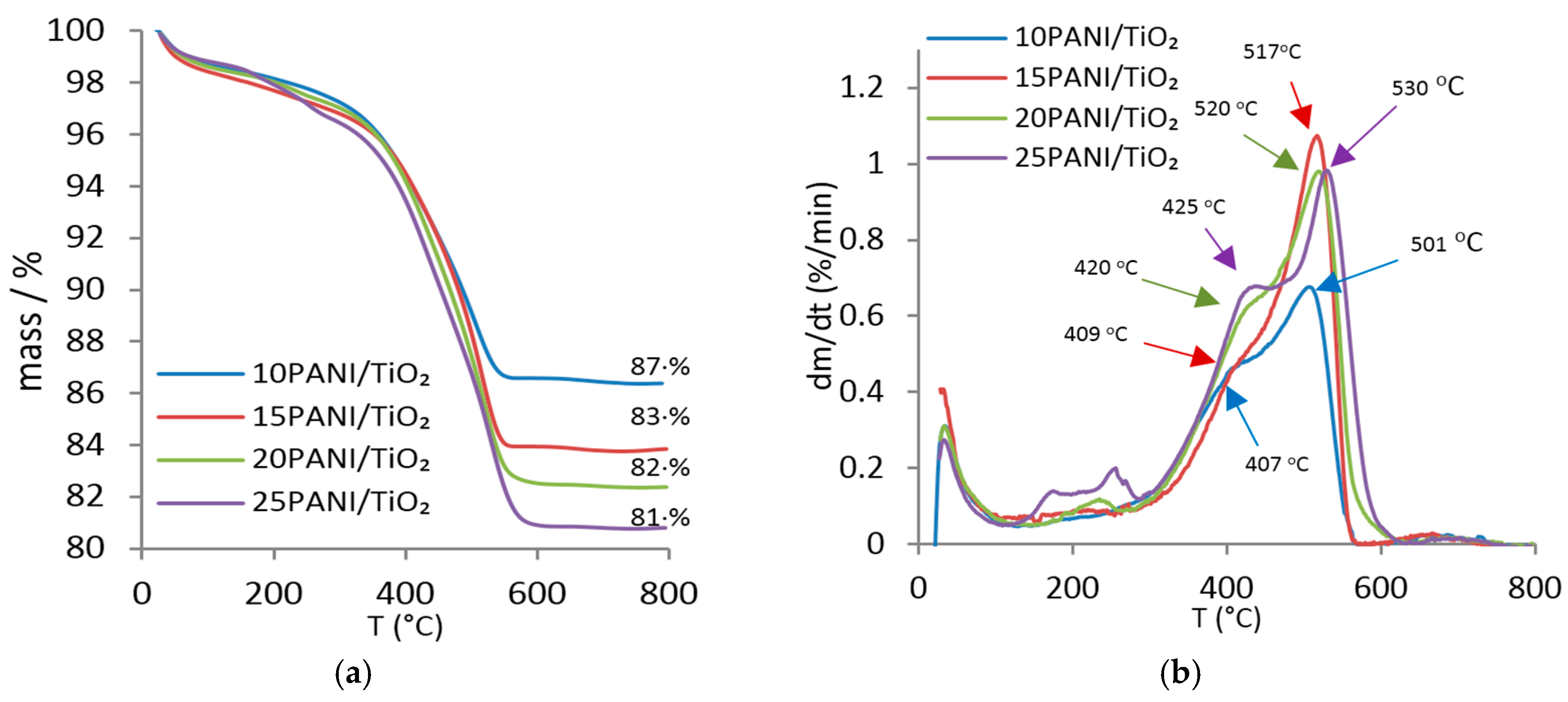
3.2. TG Analyses of Photocatalysts
3.3. Conductivity of Photocatalysts
3.4. UV-Vis Spectra of the Photocatalysts
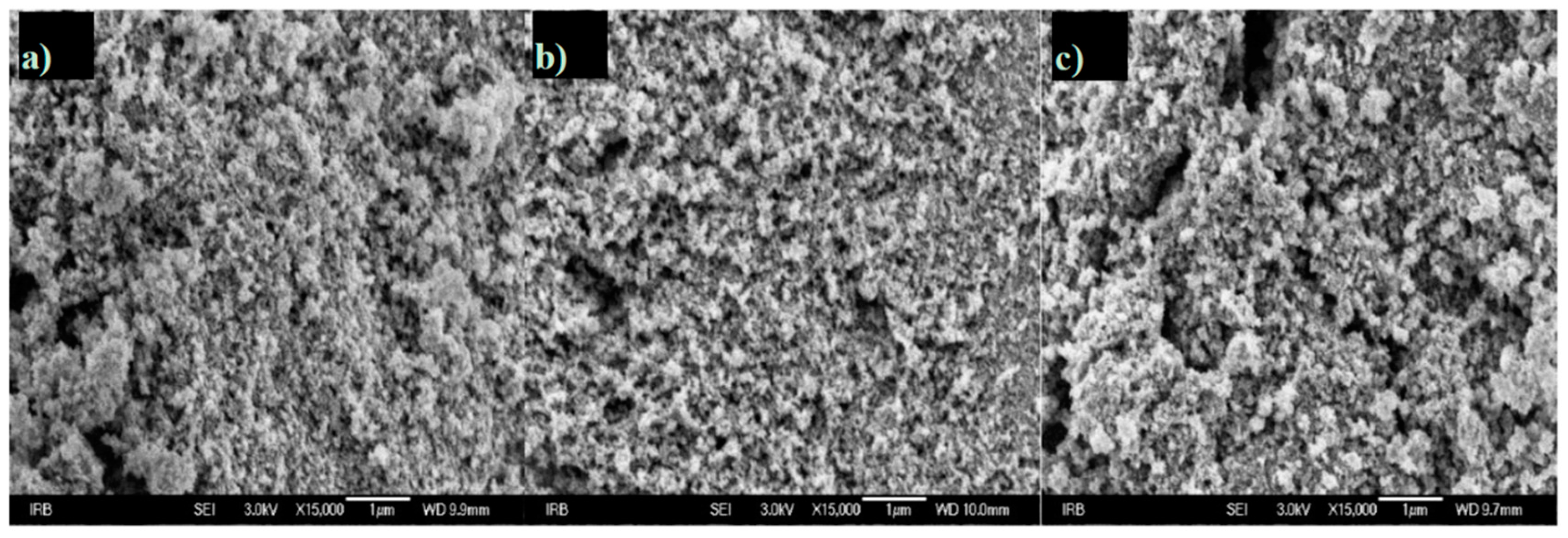
3.5. FE-SEM and TEM Analyses
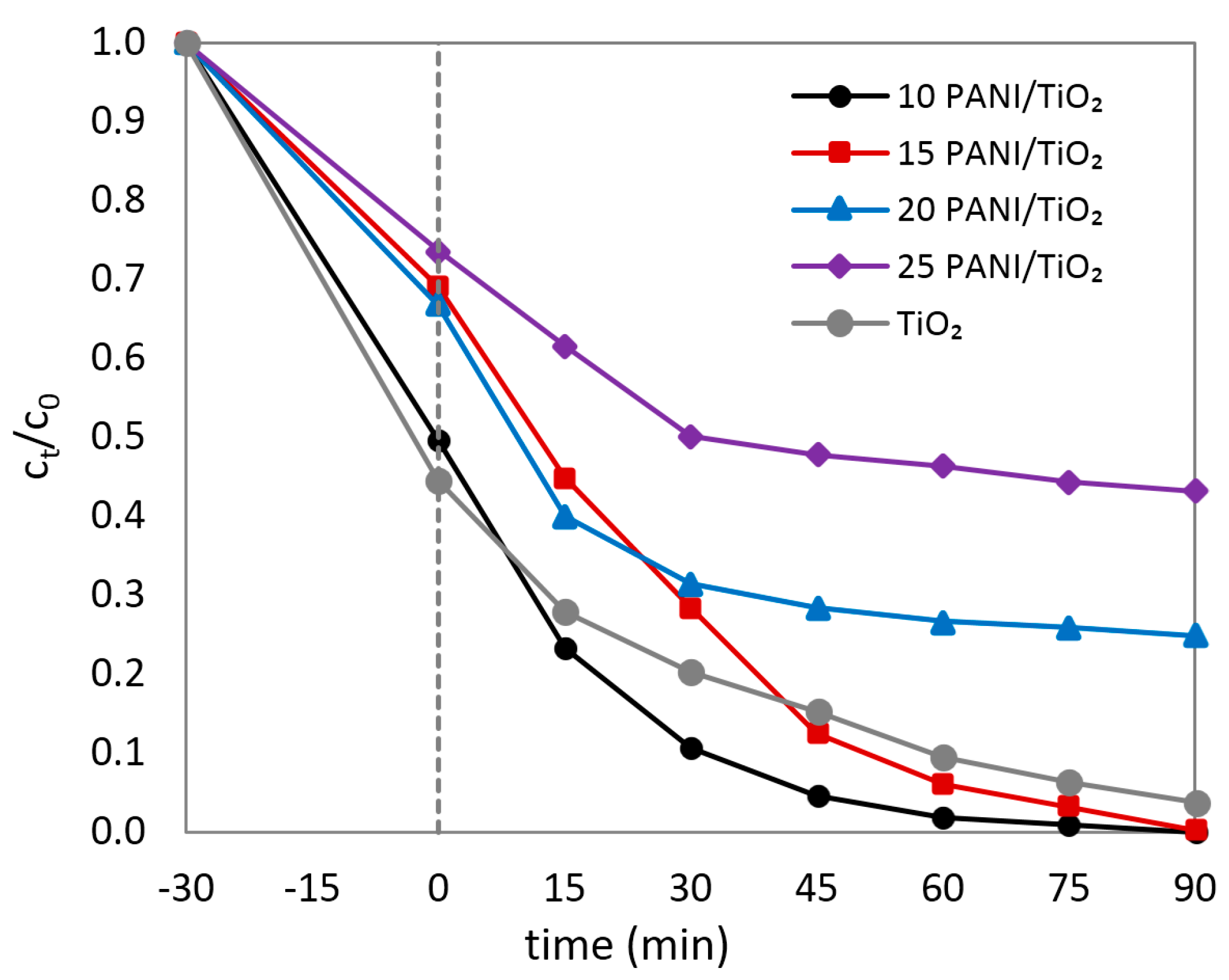
3.6. Photocatalysis under UVA
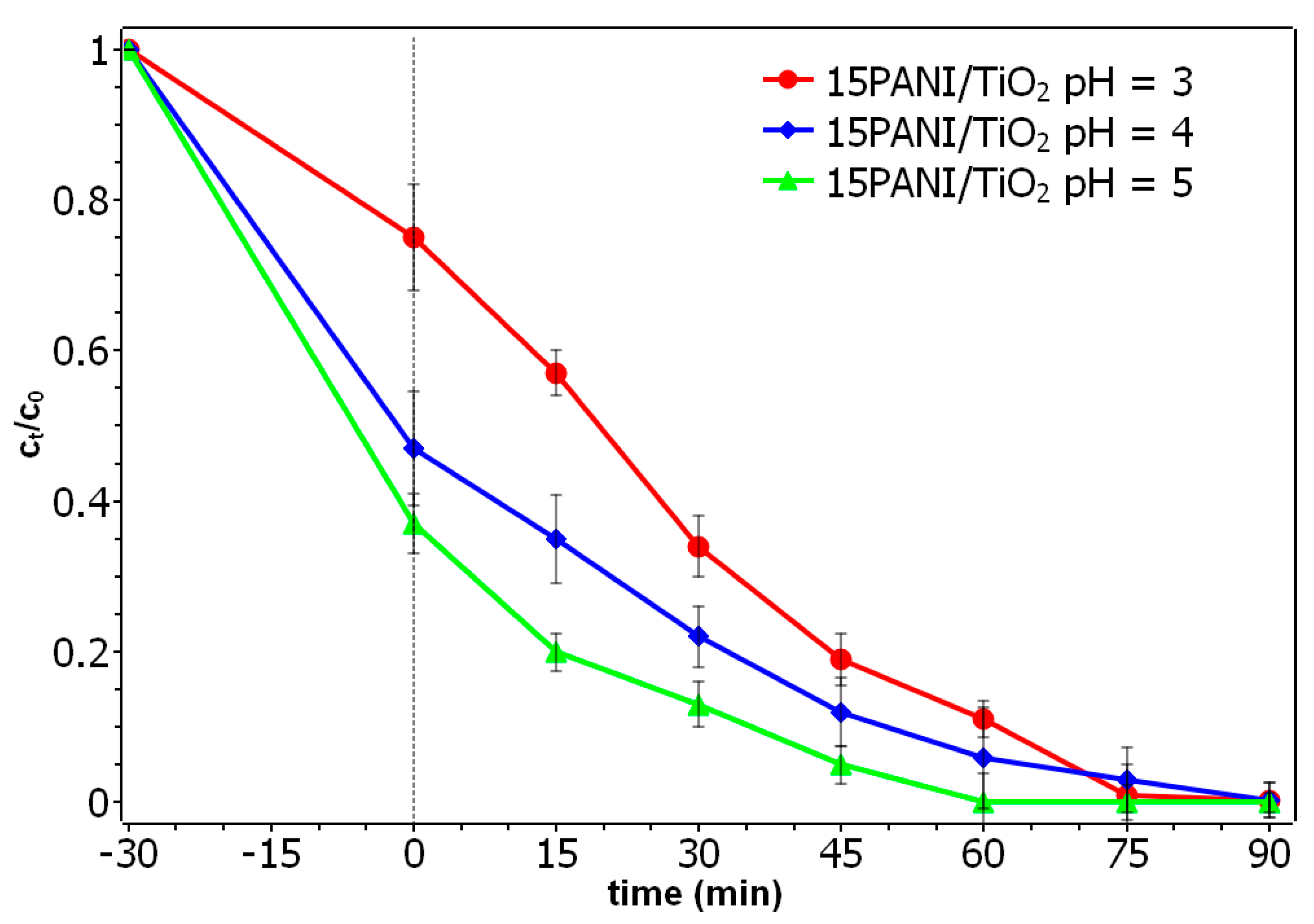
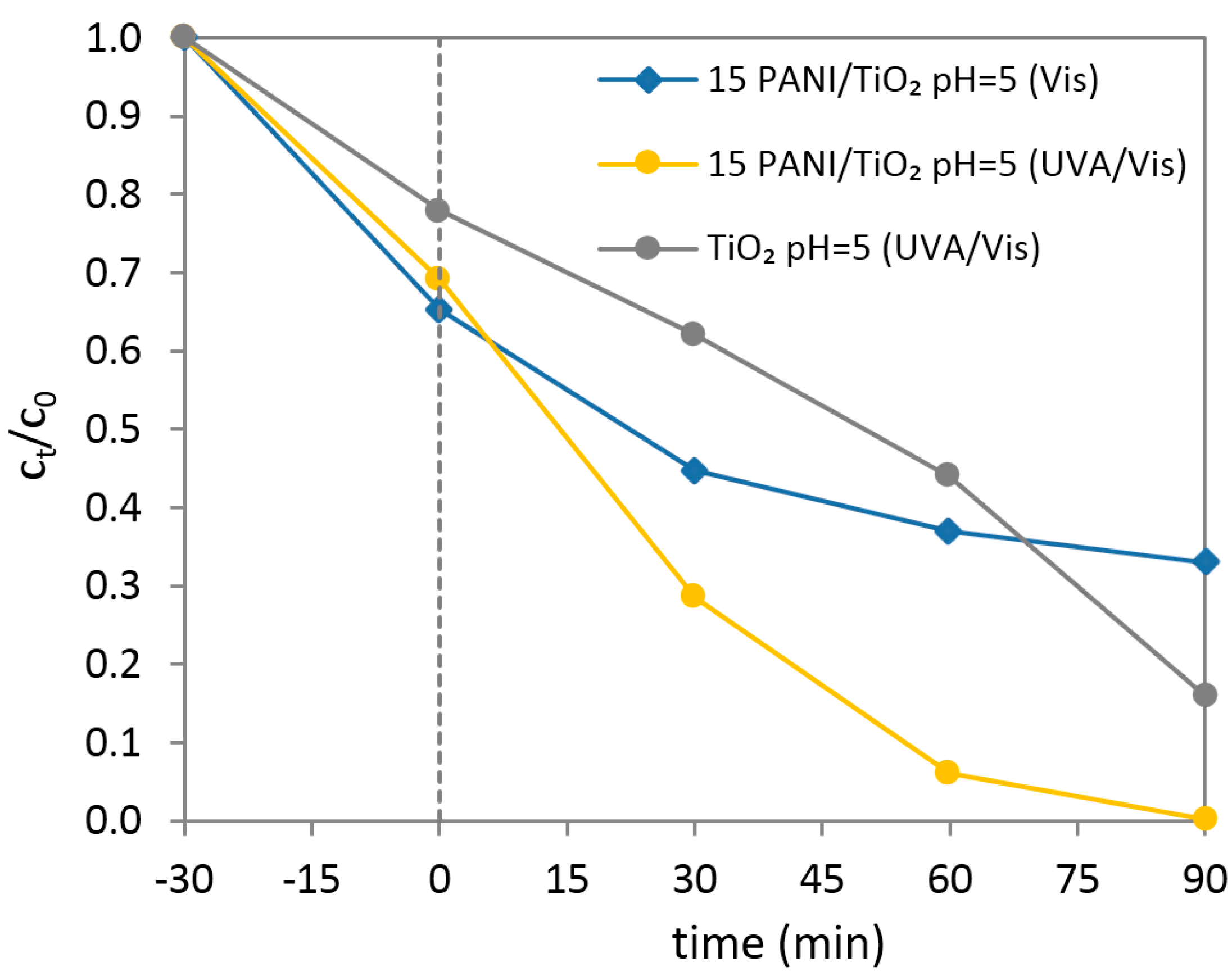
3.7. Photocatalysis and Effect of pH Solution
3.8. Photocatalysis under Solar Irradiation
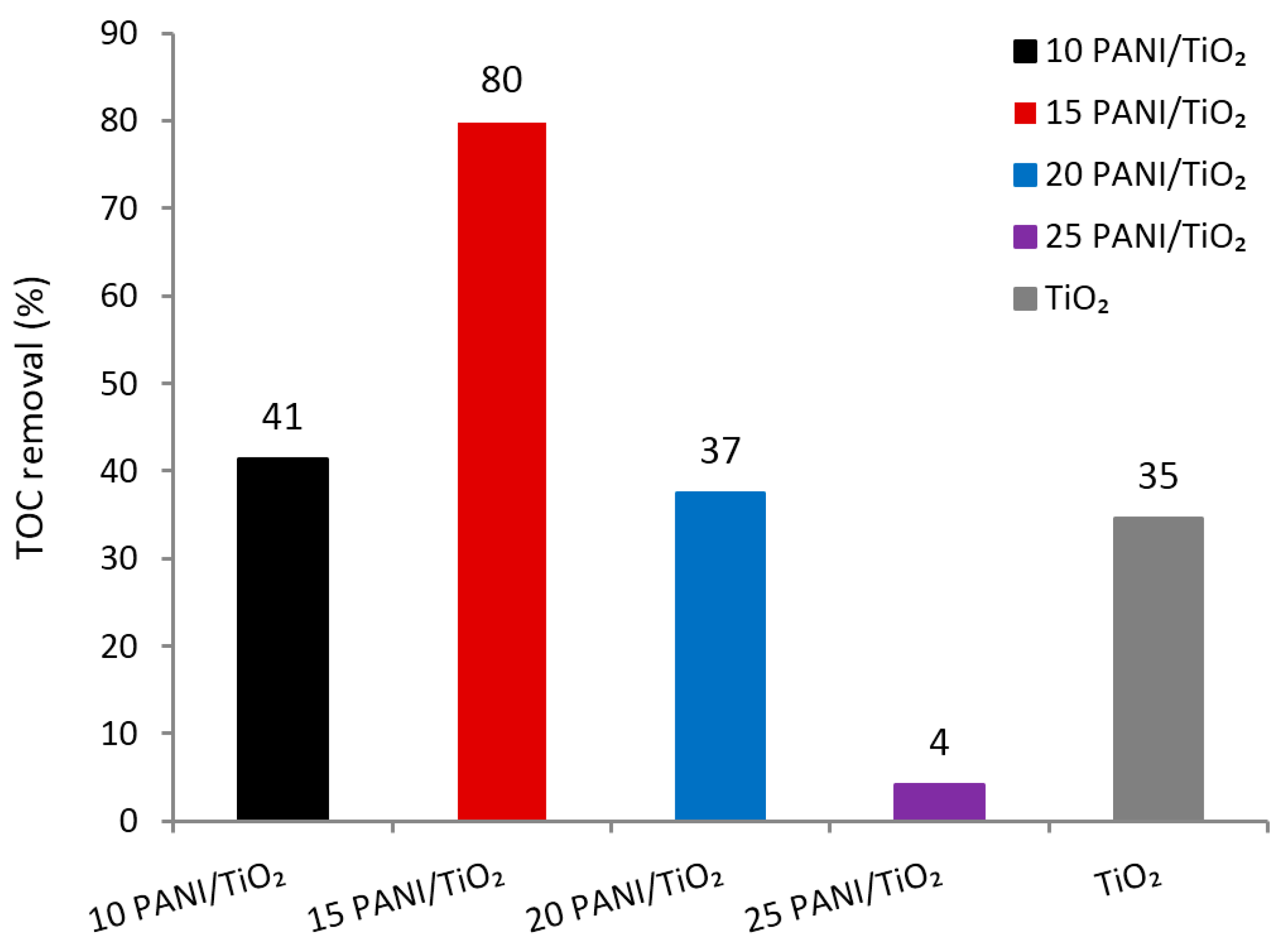
3.9. Stability of Photocatalysts
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Azbar, N.; Yonar, T.; Kestioglu, K. Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 2004, 55, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.Q.; Yates, J.T. Photocatalysis on TiO2 surfaces—Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Gupta, S.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Kumar, S.; Isaacs, M.A.; Trofimovaite, R.; Durndell, L.; Parlett, C.M.A.; Douthwaite, R.E.; Coulson, B.; Cockett, M.C.R.; Wilson, K.; Lee, A.F. P25@CoAl layered double hydroxide heterojunction nanocomposites for CO2 photocatalytic reduction. Appl. Catal. B Environ. 2017, 209, 394–404. [Google Scholar] [CrossRef]
- Chen, Q.F.; Shi, W.M.; Xu, Y.; Wu, D.; Sun, Y.H. Visible-light-responsive Ag-Si codoped anatase TiO2 photocatalyst with enhanced thermal stability. Mater. Chem. Phys. 2011, 125, 825–832. [Google Scholar] [CrossRef]
- Yang, X.; Ma, F.Y.; Li, K.X.; Guo, Y.G.; Hu, J.L.; Li, W.; Huo, M.X.; Guo, Y.H. Mixed phase titania nanocomposite codoped with metallic silver and vanadium oxide: New efficient photocatalyst for dye degradation. J. Hazard. Mater. 2010, 175, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; He, S.; Zhang, Q.Z.; Yin, Z.; Zhang, Y.X.; Xu, X.H. Synthesis of dual-phase titanate/anatase with controllable morphology doped with nitrogen from a bola-amphiphile amine surfactant as template. Ceram. Int. 2015, 41, 5916–5925. [Google Scholar] [CrossRef]
- Salem, M.A.; Al-Ghonemiy, A.F.; Zaki, A.B. Photocatalytic degradation of Allura red and Quinoline yellow with Polyaniline/TiO2 nanocomposite. Appl. Catal. B Environ. 2009, 91, 59–66. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Lin, Y.M.; Li, D.Z.; Hu, J.H.; Xiao, G.C.; Wang, J.X.; Li, W.J.; Fu, X.Z. Highly Efficient Photocatalytic Degradation of Organic Pollutants by PANI-Modified TiO2 Composite. J. Phys. Chem. C 2012, 116, 5764–5772. [Google Scholar] [CrossRef]
- Lee, H.U.; Lee, S.C.; Choi, S.; Son, B.; Lee, S.M.; Kim, H.J.; Lee, J. Efficient visible-light induced photocatalysis on nanoporous nitrogen-doped titanium dioxide catalysts. Chem. Eng. J. 2013, 228, 756–764. [Google Scholar] [CrossRef]
- Umar, M.; Aziz, H.A. Photocatalytic Degradation of Organic Pollutants in Water. In Organic Pollutants—Monitoring, Risk and Treatment; InTech: Rijeka, Croatia, 2013; Chapter 8. [Google Scholar]
- Sapurina, I.Y.; Shishov, M.A. Oxidative Polymerization of Aniline: Molecular Synthesis of Polyaniline and the Formation of Supramolecular Structures. In New Polymers for Special Applications; De Souza Gomes, A., Ed.; InTech: Brussels, Belgium, 2012. [Google Scholar]
- Zic, M. Influence of the OPDA additions on impedance response and on anion exchange property of the modified PANI. J. Electroanal. Chem. 2010, 647, 43–52. [Google Scholar] [CrossRef]
- Rokovic, M.K.; Jurisic, A.; Zic, M.; Duic, L.; Schauperl, Z. Manipulation of Polymer Layer Characteristics by Electrochemical Polymerization from Mixtures of Aniline and ortho-Phenylenediamine Monomers. J. Appl. Polym. Sci. 2009, 113, 427–436. [Google Scholar] [CrossRef]
- Kraljic, M.; Zic, M.; Duic, L. O-phenlylenediamine-containing polyaniline coatings for corrosion protection of stainless steel. Bull. Electrochem. 2004, 20, 567–570. [Google Scholar]
- Trchova, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef]
- Quillard, S.; Berrada, K.; Louarn, G.; Lefrant, S.; Lapkowski, M.; Pron, A. In-situ raman-spectroscopic studies of the electrochemical-behavior of polyaniline. New J. Chem. 1995, 19, 365–374. [Google Scholar]
- Asturias, G.E.; Macdiarmid, A.G.; McCall, R.P.; Epstein, A.J. The oxidation-state of emeraldine base. Synth. Met. 1989, 29, E157–E162. [Google Scholar] [CrossRef]
- Erdem, B.; Hunsicker, R.A.; Simmons, G.W.; Sudol, E.D.; Dimonie, V.L.; El-Aasser, M.S. XPS and FTIR surface characterization of TiO2 particles used in polymer encapsulation. Langmuir 2001, 17, 2664–2669. [Google Scholar] [CrossRef]
- Nabid, M.R.; Golbabaee, M.; Moghaddam, A.B.; Dinarvand, R.; Sedghi, R. Polyaniline/TiO2 Nanocomposite: Enzymatic Synthesis and Electrochemical Properties. Int. J. Electrochem. Sci. 2008, 3, 1117–1126. [Google Scholar]
- Li, X.W.; Chen, W.; Bian, C.Q.; He, J.B.; Xu, N.; Xue, G. Surface modification of TiO2 nanoparticles by polyaniline. Appl. Surf. Sci. 2003, 217, 16–22. [Google Scholar] [CrossRef]
- Luo, K.; Shi, N.L.; Sun, C. Thermal transition of electrochemically synthesized polyaniline. Polym. Degrad. Stab. 2006, 91, 2660–2664. [Google Scholar] [CrossRef]
- Ding, L.L.; Wang, X.W.; Gregory, R.V. Thermal properties of chemically synthesized polyaniline (EB) powder. Synth. Met. 1999, 104, 73–78. [Google Scholar] [CrossRef]
- Li, J.; Liu, G.; Zhang, W.; Cheng, W.; Xu, H.; Ding, S. Competitive detection of pregnancy-associated plasma protein-A in serum using functional single walled carbon nanotubes/chitosan-based electrochemical immunosensor. J. Electroanal. Chem. 2013, 708, 95–100. [Google Scholar] [CrossRef]
- Mizoguchi, K.; Kume, K. Metallic temperature-dependence in the conducting polymer, polyaniline—Spin dynamics study by esr. Solid State Commun. 1994, 89, 971–975. [Google Scholar] [CrossRef]
- Mizoguchi, K.; Nechtschein, M.; Travers, J.P.; Menardo, C. Spin dynamics study in polyaniline. Synth. Met. 1989, 29, E417–E424. [Google Scholar] [CrossRef]
- Zic, M. The influence of the PANI structure on the conductive mechanism and on the electrical equivalent circuit analysis. J. Electroanal. Chem. 2009, 635, 29–38. [Google Scholar] [CrossRef]
- Ganesan, R.; Gedanken, A. Organic-inorganic hybrid materials based on polyaniline/TiO2 nanocomposites for ascorbic acid fuel cell systems. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; Wang, H.T.; Zhou, D.X.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z.X. Stability and Aggregation of Metal Oxide Nanoparticles in Natural Aqueous Matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, D.; Madras, G.; Radhakrishnan, S.; Patil, S. Adsorption of sulfonated dyes by polyaniline emeraldine salt and its kinetics. J. Phys. Chem. B 2008, 112, 10153–10157. [Google Scholar] [CrossRef] [PubMed]
- Macdiarmid, A.G.; Epstein, A.J. The concept of secondary doping as applied to polyaniline. Synth. Met. 1994, 65, 103–116. [Google Scholar] [CrossRef]
- Macdiarmid, A.G.; Epstein, A.J. Secondary doping in polyaniline. Synth. Met. 1995, 69, 85–92. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.W. Conducting Polyaniline Nanowire and Its Applications in Chemiresistive Sensing. Nanomaterials 2013, 3, 498–523. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Truppi, A.; Petronella, F.; Placido, T.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Visible-Light-Active TiO2-Based Hybrid Nanocatalysts for Environmental Applications. Catalysts 2017, 7. [Google Scholar] [CrossRef]






| Sample | 10PANI/TiO2 | 15PANI/TiO2 | 20PANI/TiO2 | 25PANI/TiO2 | PANI |
|---|---|---|---|---|---|
| m (PANI)/% | 10 | 15 | 20 | 25 | - |
| m (TiO2)/g | 0.8 | 0.8 | 0.8 | 0.8 | 0 |
| V (An)/mL | 0.392 | 0.588 | 0.784 | 0.979 | 0.392 |
| m (APS)/g | 0.245 | 0.367 | 0.490 | 0.612 | 0.245 |
| V (H2SO4)/mL | 0.055 | 0.055 | 0.055 | 0.055 | 0.055 |
| Final pH | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Sample | 10PANI/TiO2 | 15PANI/TiO2 | 20PANI/TiO2 | 25PANI/TiO2 |
|---|---|---|---|---|
| Conductivity/S cm −1 | 2.76 × 10−5 | 3.74 × 10−5 | 6.33 × 10−5 | 1.70 × 10−6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilja, V.; Novaković, K.; Travas-Sejdic, J.; Hrnjak-Murgić, Z.; Kraljić Roković, M.; Žic, M. Stability and Synergistic Effect of Polyaniline/TiO2 Photocatalysts in Degradation of Azo Dye in Wastewater. Nanomaterials 2017, 7, 412. https://doi.org/10.3390/nano7120412
Gilja V, Novaković K, Travas-Sejdic J, Hrnjak-Murgić Z, Kraljić Roković M, Žic M. Stability and Synergistic Effect of Polyaniline/TiO2 Photocatalysts in Degradation of Azo Dye in Wastewater. Nanomaterials. 2017; 7(12):412. https://doi.org/10.3390/nano7120412
Chicago/Turabian StyleGilja, Vanja, Katarina Novaković, Jadranka Travas-Sejdic, Zlata Hrnjak-Murgić, Marijana Kraljić Roković, and Mark Žic. 2017. "Stability and Synergistic Effect of Polyaniline/TiO2 Photocatalysts in Degradation of Azo Dye in Wastewater" Nanomaterials 7, no. 12: 412. https://doi.org/10.3390/nano7120412





