Pnma-BN: Another Boron Nitride Polymorph with Interesting Physical Properties
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. Structural Properties
3.2. Stability
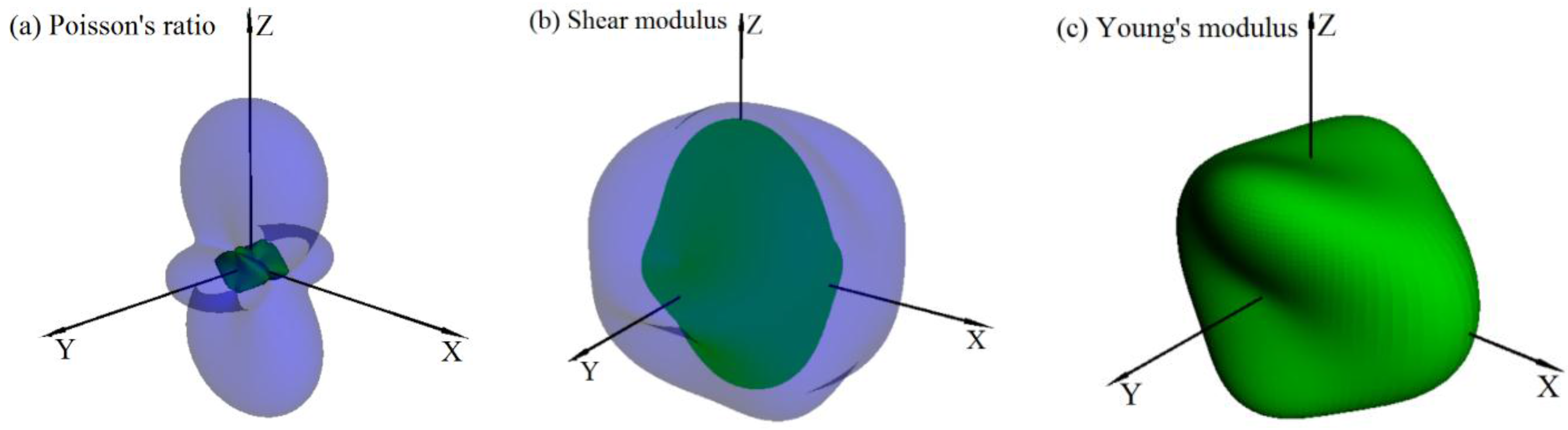
3.3. Mechanical and Anisotropic Properties
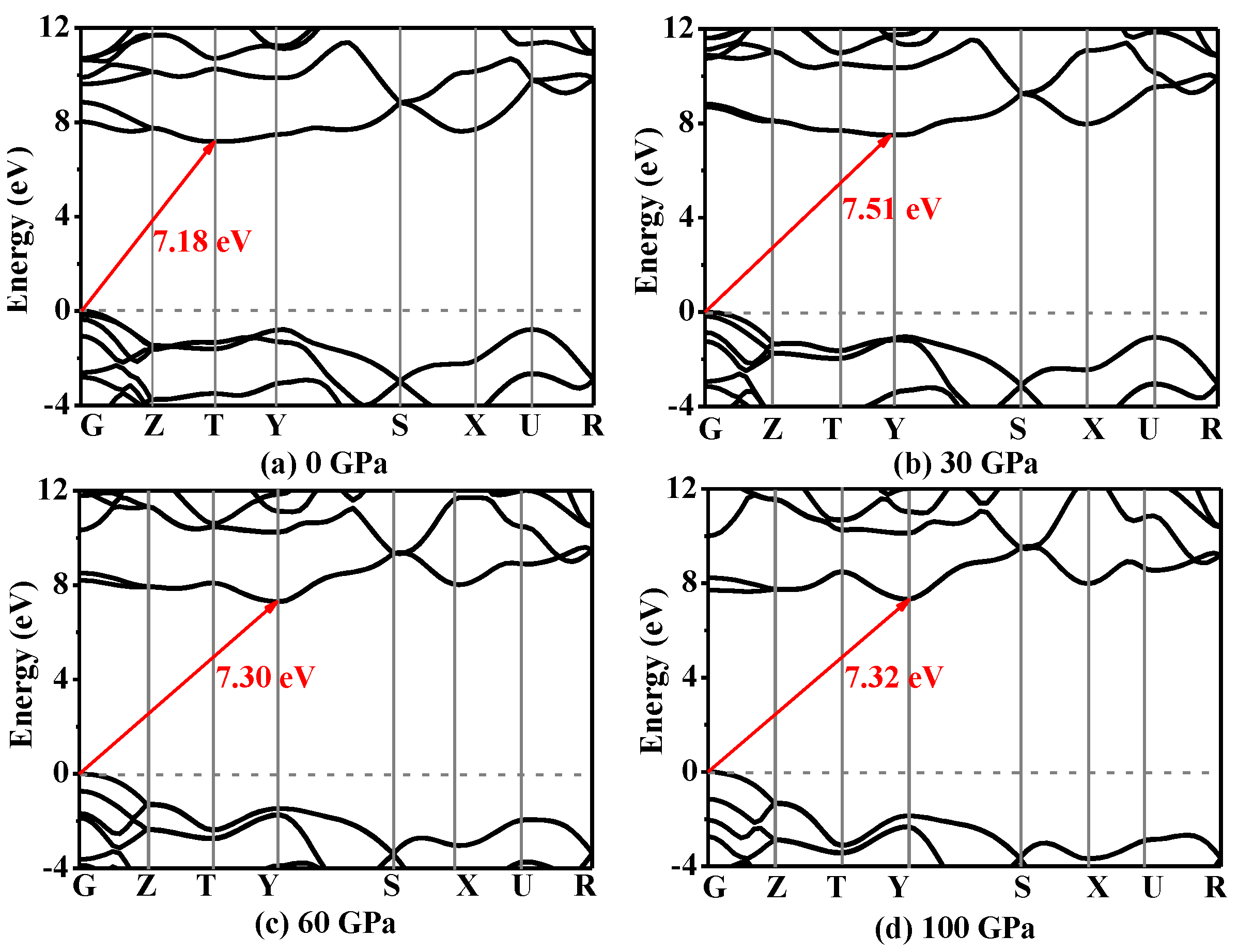
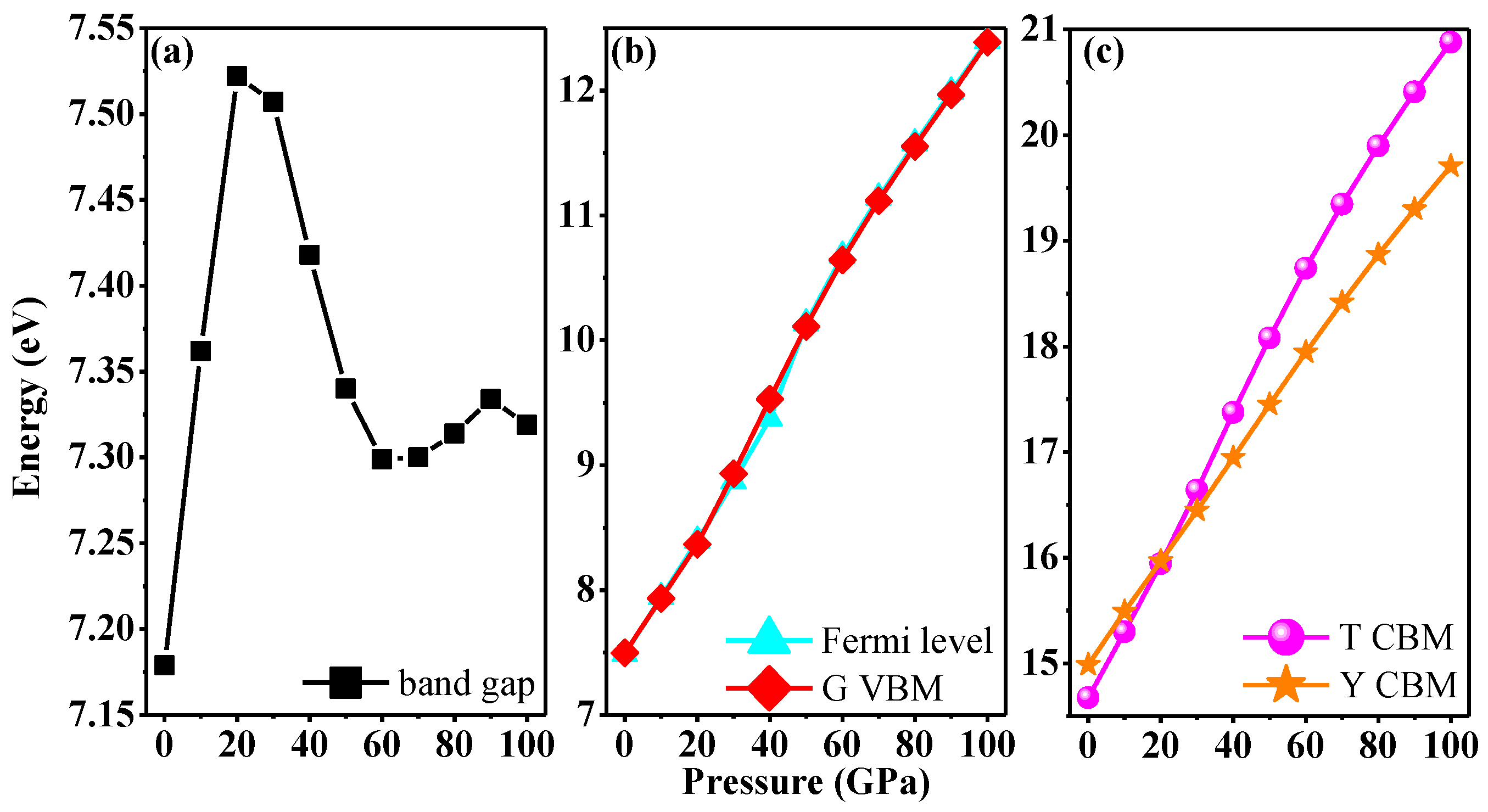
3.4. Electronic Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tian, Y.J.; Xu, B.; Yu, D.L.; Ma, Y.M.; Wang, Y.B.; Jiang, Y.B.; Hu, W.T.; Tang, C.C.; Gao, Y.F.; Luo, K.; et al. Ultrahard nanotwinned cubic boron nitride. Nature 2013, 493, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.G.; Wei, Q.; Yan, H.Y.; Zhao, Y.R.; Wang, H. A novel superhard tetragonal carbon mononitride. J. Phys. Chem. C 2014, 118, 3202–3208. [Google Scholar] [CrossRef]
- Wang, X.L. Polymorphic phases of sp3-hybridized superhard CN. J. Chem. Phys. 2012, 137, 184506. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.Y.; Wei, Q.; Yan, H.Y.; Zhang, M.G.; Zhang, D.Y.; Zhang, J.Q. A new potential superhard phase of OsN2. Acta Phys. Pol. A 2014, 126, 740–746. [Google Scholar] [CrossRef]
- Yan, H.Y.; Zhang, M.G.; Wei, Q.; Guo, P. Ab initio studies of ternary semiconductor BeB2C2. Comput. Mater. Sci. 2013, 68, 174–180. [Google Scholar] [CrossRef]
- Pease, R.S. An X-ray study of boron nitride. Acta Crystallogr. 1952, 5, 356–361. [Google Scholar] [CrossRef]
- Bundy, P.; Wentorf, R.H., Jr. Direct transformation of hexagonal boron nitride to denser forms. J. Chem. Phys. 1963, 38, 1144–1149. [Google Scholar] [CrossRef]
- Thomas, J., Jr.; Weston, N.E.; Oconnor, T.E. Turbostratic boron nitride, thermal transformation to ordered-layer-lattice boron nitride. J. Am. Chem. Soc. 1963, 84, 4619–4622. [Google Scholar] [CrossRef]
- Mirkarimi, P.B.; McCarty, K.F.; Medlin, D.L. Review of advances in cubic boron nitride film synthesis. Mater. Sci. Eng. R-Rep. 1997, 21, 47–100. [Google Scholar] [CrossRef]
- Paine, R.T.; Narula, C.X. Synthetic routes to boron nitride. Chem. Rev. 1990, 90, 73–91. [Google Scholar] [CrossRef]
- Bosak, A.; Serrano, J.; Krisch, M.; Watanabe, K.; Taniquchi, T.; Kanda, H. Lateral adsorption geometry and site-specific electronic structure of a large organic chemisorbate on a metal surface. Phys. Rev. B 2006, 73, 041402. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [PubMed]
- Chopra, N.G.; Luyken, R.J.; Cherrey, K.; Crespi, V.H.; Cohen, M.L.; Louie, S.G.; Zettl, A. Boron Nitride Nanotubes. Science 1995, 269, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhao, J.J.; Ahuja, R. A novel superhard BN polymorph under cold compression of h-BN. J. Phys. Condens. Matter. 2013, 25, 122204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Wang, Y.C.; Lv, J.; Zhu, C.Y.; Li, Q.; Zhang, M.; Li, Q.; Ma, Y.M. First-principles structural design of superhard materials. J. Chem. Phys. 2013, 138, 114101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Wang, Q.; Kawazoe, Y.; Jena, P.R. Three-dimensional metallic boron nitride. J. Am. Chem. Soc. 2013, 135, 18216–18221. [Google Scholar] [CrossRef] [PubMed]
- He, C.Y.; Sun, L.Z.; Zhang, C.X.; Peng, X.Y.; Zhang, K.W.; Zhong, J.X. Z-BN: A novel superhard boron nitride phase. Phys. Chem. Chem. Phys. 2012, 14, 10967–10971. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, B.F. Predicted a novel high-pressure superhard boron nitride phase. J. Alloy Compd. 2014, 598, 54–56. [Google Scholar] [CrossRef]
- Niu, C.Y.; Wang, J.T. Three-dimensional three-connected tetragonal BN: Ab initio calculations. Phys. Lett. A 2014, 378, 2303–2307. [Google Scholar] [CrossRef]
- Germaneau, E.; Su, G.; Zheng, Q.R. New boron nitride structures B4N4: A first-principles random searching application. J. Phys. Condens. Matter. 2013, 25, 125504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Lu, M.C.; Zhu, L.; Zhu, L.L.; Li, Y.D.; Zhang, M.; Li, Q. Orthorhombic BN: A novel superhard image boron nitride polymorph. Phys. Lett. A 2014, 378, 741–744. [Google Scholar] [CrossRef]
- Wen, B.; Zhao, J.J.; Melnikc, R.; Tian, Y.J. Body-centered tetragonal B2N2: A novel sp3 bonding boron nitride polymorph. Phys. Chem. Chem. Phys. 2011, 13, 14565–14570. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wu, X.; Yang, J.; Zeng, X.C. Unusual metallic microporous boron nitride networks. J. Phys. Chem. Lett. 2013, 4, 3484–3488. [Google Scholar] [CrossRef]
- Dai, J.; Wu, X.; Yang, J.; Zeng, X.C. Porous boron nitride with tunable pore size. J. Phys. Chem. Lett. 2014, 5, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.B.; Kim, T.; Liu, X.D.; Ma, J.M.; Zheng, W.J. Ionothermal Synthesis of Turbostratic Boron Nitride Nanoflakes at Low Temperature. J. Phys. Chem. C 2009, 113, 9135–9140. [Google Scholar] [CrossRef]
- Li, Y.W.; Hao, J.; Liu, H.Y.; Lu, S.Y.; Tse, J.S. High-energy density and superhard nitrogen-rich B-N compounds. Phys. Rev. Lett. 2015, 115, 105502. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Zunger, A. Self-interaction correction to density-functional approximations for many-electron systems. Phys. Rev. B 1981, 23, 5048–5079. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of crystals with the Quasi-Newton method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892R–7895R. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Fan, Q.Y.; Chai, C.C.; Wei, Q.; Yang, Y.T.; Yang, Q.; Chen, P.Y.; Xing, M.J.; Zhang, J.Q.; Yao, R.H. Prediction of novel phase of silicon and Si–Ge alloys. J. Solid State Chem. 2016, 233, 471–483. [Google Scholar] [CrossRef]
- Fan, Q.Y.; Chai, C.C.; Wei, Q.; Yang, Y.T.; Qiao, L.P.; Zhao, Y.B.; Zhou, P.K.; Xing, M.J.; Zhang, J.Q.; Yao, R.H. Mechanical and electronic properties of Ca1-xMgxO alloys. Mater. Sci. Semicond. Process 2015, 40, 676–684. [Google Scholar] [CrossRef]
- Voigt, W. Lehrburch der Kristallphysik; Teubner: Leipzig, Germany, 1928. [Google Scholar]
- Reuss, A. Berechnung der Fließgrenze von Mischkristallen auf Grund der Plastizitätsbedingung für Einkristalle. J. Appl. Math. Mech. 1929, 9, 49–58. (In German) [Google Scholar] [CrossRef]
- Hill, R. The elastic behaviour of a crystalline aggregate. Phys. Soc. Lond. Sect. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Doll, K.; Schön, J.C.; Jansen, M. Structure prediction based on ab initio simulated annealing for boron nitride. Phys. Rev. B 2008, 78, 144110. [Google Scholar] [CrossRef]
- Fan, Q.Y.; Wei, Q.; Yan, H.Y.; Zhang, M.G.; Zhang, Z.X.; Zhang, J.Q.; Zhang, D.Y. Elastic and electronic properties of Pbca-BN: First-principles calculations. Comput. Mater. Sci. 2014, 85, 80–87. [Google Scholar] [CrossRef]
- Fan, Q.Y.; Wei, Q.; Chai, C.C.; Yan, H.Y.; Zhang, M.G.; Lin, Z.Z.; Zhang, Z.X.; Zhang, J.Q.; Zhang, D.Y. Structural, mechanical, and electronic properties of P3m1-BCN. J. Phys. Chem. Solids 2015, 79, 89–96. [Google Scholar] [CrossRef]
- Petrescu, M.L. Boron nitride theoretical hardness compared to carbon polymorphs. Diamond Relat. Mater. 2004, 13, 1848. [Google Scholar] [CrossRef]
- Wu, Z.J.; Zhao, E.J.; Xiang, H.P.; Hao, X.F.; Liu, X.J.; Meng, J. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys. Rev. B 2007, 76, 054115. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Duan, Y.H.; Sun, Y.; Peng, M.J.; Zhou, S.G. Anisotropic elastic properties of the Ca–Pb compounds. J. Alloy. Compd. 2014, 595, 14–21. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Niu, H.; Li, D.; Li, Y. Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef]
- Lyakhov, A.O.; Oganov, A.R. Evolutionary search for superhard materials: Methodology and applications to forms of carbon and TiO2. Phys. Rev. B 2011, 84, 092103. [Google Scholar] [CrossRef]
- Xing, M.J.; Li, B.H.; Yu, Z.T.; Chen, Q. Monoclinic C2/m-20 carbon: A novel superhard sp3 carbon allotrope. RSC Adv. 2016, 6, 32740–32745. [Google Scholar] [CrossRef]
- Marmier, A.; Lethbridge, Z.A.D.; Walton, R.I.; Smith, C.W.; Parker, S.C.; Evans, K.E. ElAM: A computer program for the analysis and representation of anisotropic elastic properties. Comput. Phys. Commun. 2010, 181, 2102–2115. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.C.; Liu, Y.; Li, D.J.; Zeng, X.Q.; Xu, C.S. First-principles study of structural and electronic properties of C14-type Laves phase Al2Zr and Al2Hf. Comput. Mater. Sci. 2014, 83, 27–34. [Google Scholar] [CrossRef]
- Ranganathan, S.I.; Ostoja-Starzewski, M. Universal elastic anisotropy index. Phys. Rev. Lett. 2008, 101, 055504. [Google Scholar] [CrossRef] [PubMed]
- Anderson, O.L. A simplified method for calculating the debye temperature from elastic constants. J. Phys. Chem. Solids. 1963, 24, 909–917. [Google Scholar] [CrossRef]
- Panda, K.B.; Ravi, K.S. Determination of elastic constants of titanium diboride (TiB2) from first principles using FLAPW implementation of the density functional theory. Comput. Mater. Sci. 2006, 35, 134–150. [Google Scholar] [CrossRef]
- Anderson, O.L. Physical Acoustics; Academic Press: New York, NY, USA, 1965; Volume III. [Google Scholar]
- Grimvall, G. Thermophysical Properties of Materials; North-Holland: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Adachi, S. Handbook on Physical Properties of Semiconductors; Springer: Boston, MA, USA, 2004. [Google Scholar]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Fan, Q.; Chai, C.; Wei, Q.; Yang, Y. Two novel silicon phases with direct band gaps. Phys. Chem. Chem. Phys. 2016, 18, 12905–12913. [Google Scholar] [CrossRef] [PubMed]
- Ohba, N.; Miwa, K.; Nagasako, N.; Fukumoto, A. First-principles study on structural, dielectric, and dynamical properties for three BN polytypes. Phys. Rev. B 2001, 63, 115207. [Google Scholar] [CrossRef]
- Watanabe, K.; Taniguchi, T.; Kanda, H. Direct-bandgap properties and evidence for ultraviolet lasing of hexagonal boron nitride single crystal. Nat. Mater. 2004, 3, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Chai, C.; Wei, Q.; Yang, J.; Zhou, P.; Zhang, D.; Yang, Y. A new phase of GaN. J. Chem. 2016, 2016, 8612892. [Google Scholar] [CrossRef]
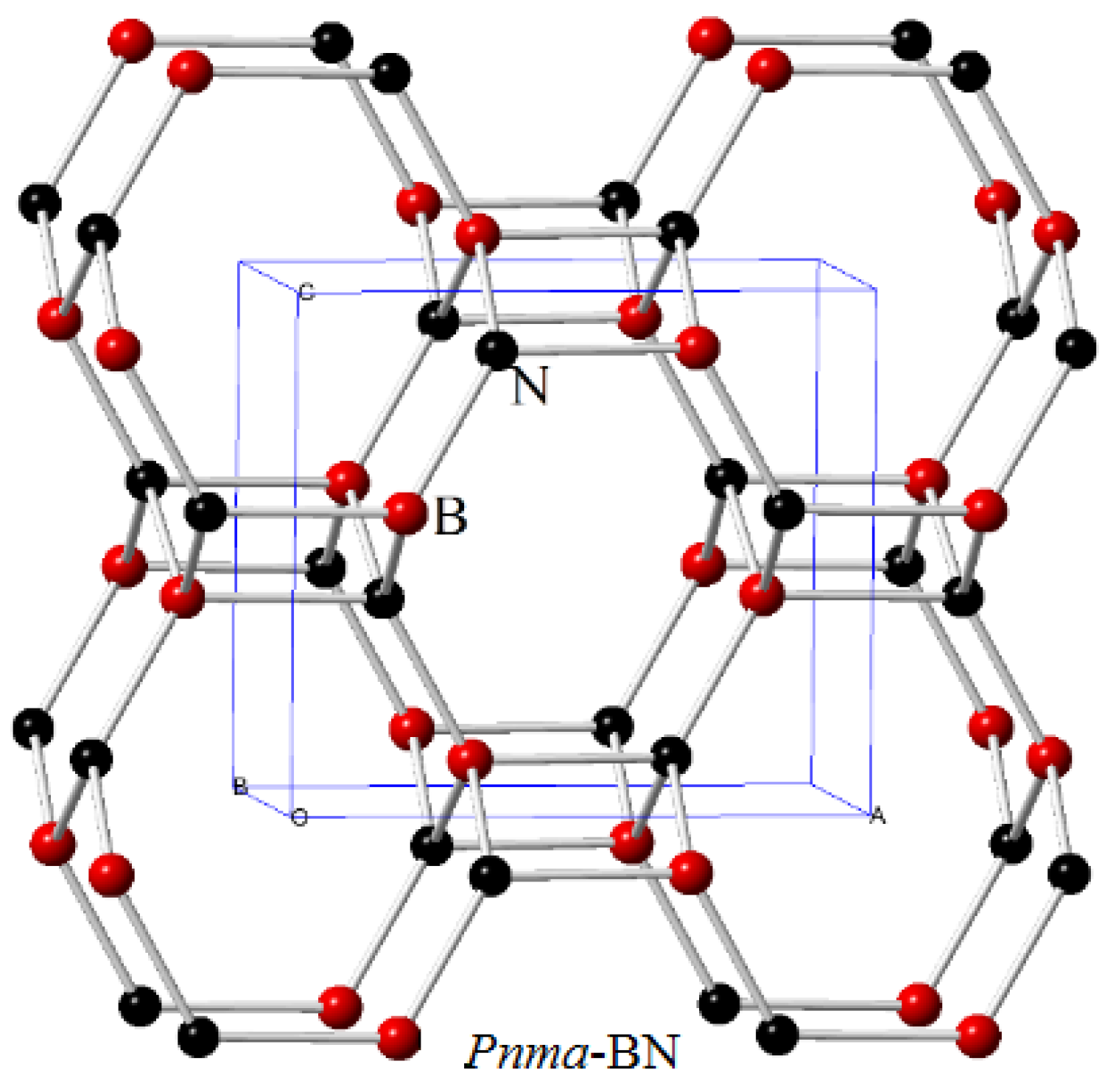
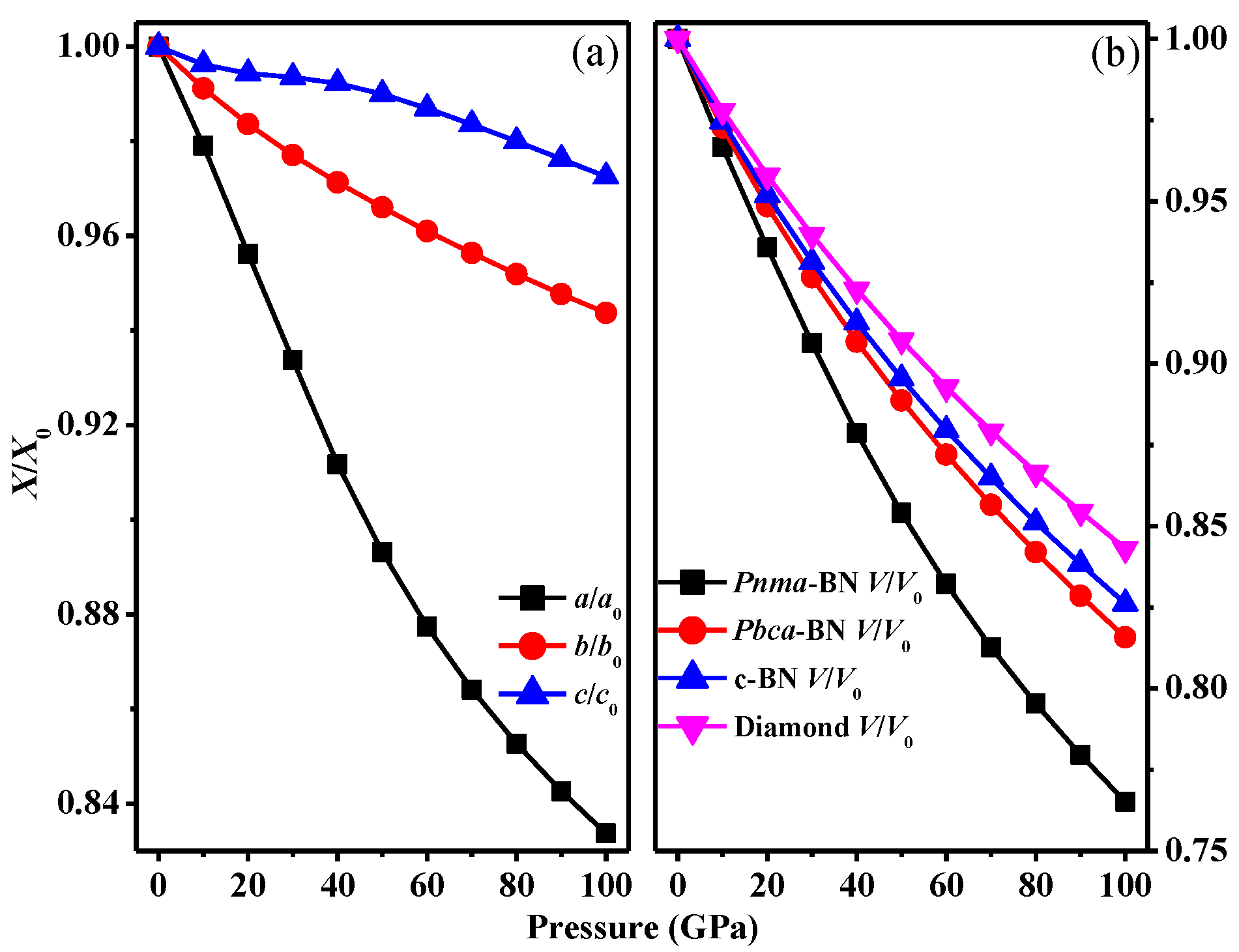
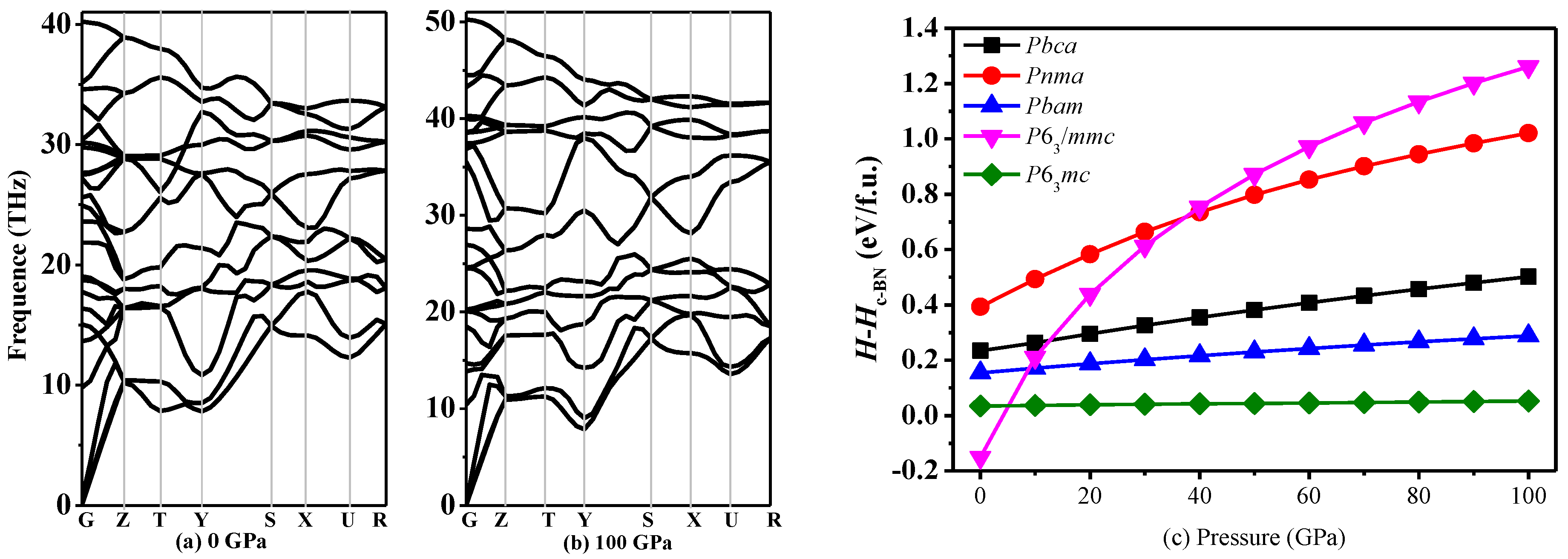
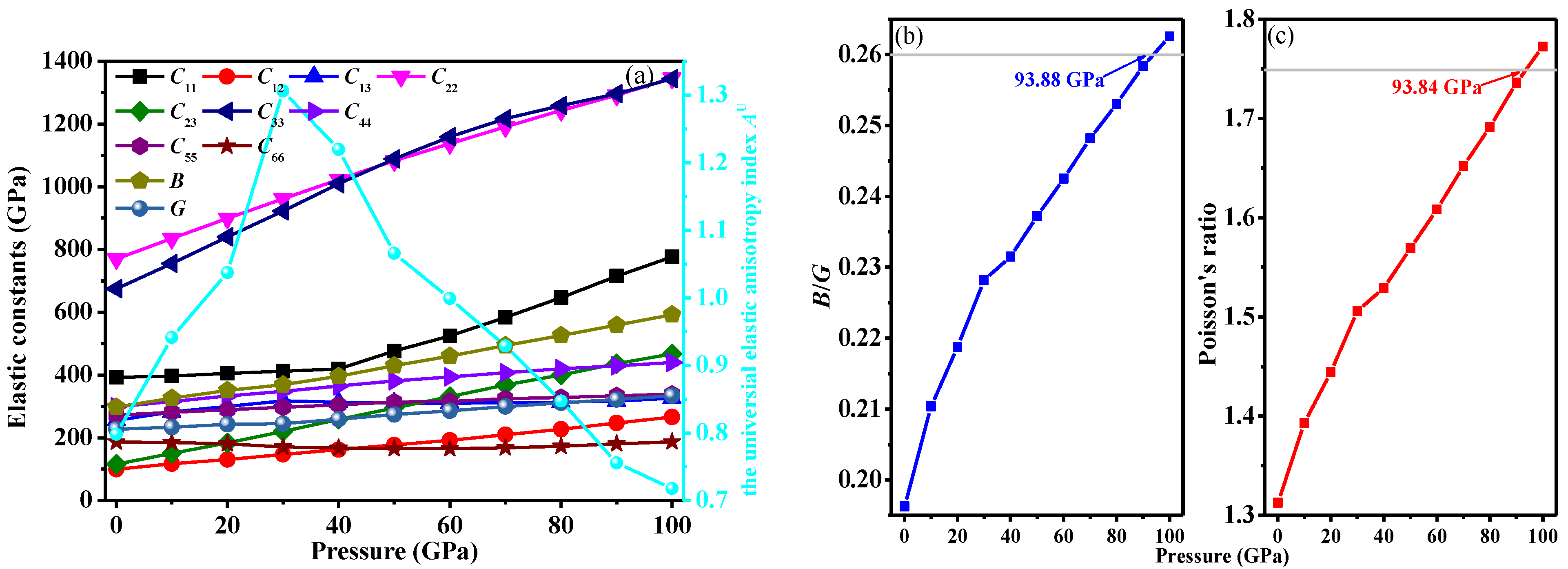

| Space Group | Methods | a (Å) | b (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|---|
| Pnma | GGA | 4.8900 | 2.5890 | 4.2835 | 13.5574 |
| LDA | 4.7954 | 2.5569 | 4.2432 | 13.0068 | |
| LDA 1 | 4.7600 | 2.5800 | 4.2900 | 13.1712 | |
| Pbca | GGA | 5.0987 | 4.4216 | 4.3981 | 12.3940 |
| GGA 2 | 5.1103 | 4.4336 | 4.3992 | 12.4591 | |
| LDA | 5.0412 | 4.3794 | 4.3316 | 11.9538 | |
| LDA 2 | 5.0458 | 4.3800 | 4.3392 | 11.9873 | |
| GGA | 3.6258 | 11.9166 | |||
| GGA 3 | 3.6224 | 11.8835 | |||
| LDA | 3.5692 | 11.3672 | |||
| LDA 3 | 3.5764 | 11.4364 | |||
| Experiment 4 | 3.6200 | 11.8595 |
| Materials | p | C11 | C12 | C13 | C22 | C23 | C33 | C44 | C55 | C66 | B | G | E | v |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pnma-BN | 0 | 392 | 99 | 256 | 770 | 116 | 675 | 299 | 272 | 187 | 298 | 227 | 543 | 0.196 |
| 0 1 | 403 | 107 | 273 | 824 | 132 | 730 | 316 | 282 | 187 | 318 | 236 | 568 | 0.202 | |
| 10 | 397 | 117 | 282 | 836 | 150 | 756 | 316 | 281 | 185 | 326 | 234 | 566 | 0.210 | |
| 20 | 405 | 131 | 300 | 899 | 184 | 840 | 333 | 289 | 181 | 351 | 243 | 592 | 0.219 | |
| 30 | 412 | 147 | 318 | 961 | 220 | 923 | 348 | 297 | 171 | 369 | 245 | 602 | 0.228 | |
| 40 | 420 | 162 | 316 | 1023 | 258 | 1010 | 365 | 305 | 167 | 396 | 259 | 638 | 0.232 | |
| 50 | 477 | 177 | 311 | 1083 | 295 | 1089 | 381 | 314 | 165 | 430 | 274 | 678 | 0.237 | |
| 60 | 524 | 192 | 310 | 1138 | 331 | 1159 | 394 | 315 | 166 | 460 | 286 | 711 | 0.242 | |
| 70 | 584 | 209 | 312 | 1191 | 368 | 1217 | 408 | 325 | 168 | 494 | 299 | 746 | 0.248 | |
| 80 | 646 | 227 | 314 | 1243 | 400 | 1259 | 420 | 329 | 173 | 526 | 311 | 779 | 0.253 | |
| 90 | 716 | 247 | 317 | 1292 | 437 | 1296 | 430 | 334 | 181 | 559 | 322 | 810 | 0.258 | |
| 100 | 777 | 266 | 326 | 1347 | 468 | 1344 | 440 | 339 | 187 | 592 | 334 | 843 | 0.263 | |
| Pbca-BN | 0 | 769 | 145 | 133 | 870 | 105 | 716 | 307 | 255 | 340 | 340 | 312 | 717 | 0.148 |
| 0 2 | 772 | 135 | 139 | 885 | 92 | 716 | 312 | 257 | 357 | 344 | 316 | 718 | 0.140 | |
| c-BN | 0 | 788 | 160 | 443 | 369 | 386 | 859 | 0.112 | ||||||
| 0 2 | 779 | 165 | 446 | 370 | 384 | 856 | 0.120 |
| Surface | Poisson’s Ratio v | Shear Modulus (GPa) | Young’s Modulus (GPa) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| vmax | vmin | vmax/vmin | Gmax | Gmin | Gmax/Gmin | Emax | Emin | Emax/Emin | |
| (001) | 0.366 | 0.074 | 4.945 | 298.60 | 186.70 | 1.599 | 740.15 | 291.12 | 2.534 |
| (010) | 0.635 | 0.069 | 9.203 | 298.60 | 126.04 | 2.369 | 658.13 | 291.12 | 2.261 |
| (100) | 0.635 | 0.044 | 14.431 | 298.60 | 186.70 | 1.599 | 740.15 | 504.52 | 1.467 |
| (111) | 0.494 | 0.045 | 10.978 | 307.33 | 126.04 | 2.439 | 649.49 | 389.52 | 1.667 |
| All | 0.635 | 0.010 | 63.500 | 310.85 | 126.04 | 2.466 | 740.15 | 291.12 | 2.534 |
| p | ρ | vp | vs | vm | ΘD |
|---|---|---|---|---|---|
| 0 | 3.040 | 14057 | 8642 | 9537 | 1502 |
| 10 | 3.144 | 14244 | 8627 | 9534 | 1518 |
| 20 | 3.248 | 14415 | 8649 | 9567 | 1540 |
| 30 | 3.354 | 14402 | 8547 | 9465 | 1540 |
| 40 | 3.460 | 14639 | 8653 | 9585 | 1576 |
| 50 | 3.559 | 14949 | 8774 | 9727 | 1614 |
| 60 | 3.653 | 15176 | 8848 | 9815 | 1643 |
| 70 | 3.740 | 15449 | 8941 | 9924 | 1674 |
| 80 | 3.822 | 15688 | 9020 | 10018 | 1702 |
| 90 | 3.899 | 15920 | 9087 | 10098 | 1728 |
| 100 | 3.973 | 16158 | 9169 | 10194 | 1755 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Han, Z.; Liu, X.; Yu, X.; Wang, D.; Tian, Y. Pnma-BN: Another Boron Nitride Polymorph with Interesting Physical Properties. Nanomaterials 2017, 7, 3. https://doi.org/10.3390/nano7010003
Ma Z, Han Z, Liu X, Yu X, Wang D, Tian Y. Pnma-BN: Another Boron Nitride Polymorph with Interesting Physical Properties. Nanomaterials. 2017; 7(1):3. https://doi.org/10.3390/nano7010003
Chicago/Turabian StyleMa, Zhenyang, Zheng Han, Xuhong Liu, Xinhai Yu, Dayun Wang, and Yi Tian. 2017. "Pnma-BN: Another Boron Nitride Polymorph with Interesting Physical Properties" Nanomaterials 7, no. 1: 3. https://doi.org/10.3390/nano7010003
APA StyleMa, Z., Han, Z., Liu, X., Yu, X., Wang, D., & Tian, Y. (2017). Pnma-BN: Another Boron Nitride Polymorph with Interesting Physical Properties. Nanomaterials, 7(1), 3. https://doi.org/10.3390/nano7010003





